| Contents | Previous | Next |
The key principles of panic disorder treatment reviewed in this section include the following:
Because the most frightening aspects of panic disorder are the somatic symptoms and the cultural stigma of mental illness, many patients with panic attacks believe they have a severe physical illness (Katon 1984). The first stage in the treatment of panic disorder in primary care is to negotiate explanatory models of illness with the patient (Katon and Kleinman 1980). To elicit patients' beliefs about their illness, Kleinman and colleagues (1978) recommended initially asking them several open-ended questions about their view of the illness:
If these do not elicit the necessary information, then the physician should inquire specifically about the patient's beliefs concerning each of five issues:
To elicit such information, physicians must demonstrate warmth, empathy, and persistence, and they must be nonjudgmental. Most of all, physicians must have a genuine interest in the meaning the sickness has for the patients and must make explicit to the patients their intent to draw on this essential information in constructing an appropriate treatment plan.
Patients with panic disorder are often convinced that they have a cardiac or neurologic disorder and that their anxiety or nervousness is secondary to their physical symptoms. Studies that support this patient perception have shown that, during a panic attack, fear and anxiety are late symptoms following autonomic symptoms such as dyspnea, palpitations, chest pain, and hot or cold flashes (Katerdnahl 1988). Indeed, evidence suggests that generalized anxiety and avoidance behavior usually follow the onset of panic attacks, rather than preceding the severe episodic anxiety (Uhde et al. 1985).
When the physician tells patients with panic attacks that they have a severe anxiety disorder, they often feel that the physician does not believe that they have "real" physical symptoms (Katon 1986). It is very helpful to educate these patients about the biologic research on panic disorder. Explaining that panic disorder results from a dysfunction of the sympathetic nervous system, in which bursts of catecholamines are released into the peripheral circulation causing symptoms such as tachycardia, chest pain, dyspnea, and dizziness, often decreases patient defensiveness. A further analogy about panic attacks being quite similar to the fight-or-flight response may help. Thus, the patient is provided with the following explanation: "If you were walking down a dark street and heard a sudden sound, your heart might begin to race, your respiratory rate might increase, you would feel tense, shaky, warm, sweaty, and you would be prepared to either flee or fight for your life. These attacks you are having are set off by dysregulation of the same area of the brain that controls the fight-or-flight response, but you are having this alarm or danger response at inappropriate times when there is no actual danger." This explanation also leads naturally to a discussion of the effectiveness of medications that dampen psychophysiologic activation of the autonomic nervous system.
Patients often describe the anxiety attacks by emphasizing that since their episodes began, stresses that they frequently coped with quite well now lead to symptoms, and that such attacks occur regardless of whether they are anxious or stressed by life circumstances. Here the physician can emphasize that panic disorder causes the alarm mechanisms in the brain to be hypersensitive (the phenomenon of sensitization) to any stresses the patient faces (Weekes 1976) and, in addition, will often discharge with no apparent stress present. Not only is the patient's autonomic nervous system sensitized to external stresses, but minor physiologic symptoms such as rapid heart rate or sweating induced by exercise may also provoke an alarm response.
The physician must also recognize that many patients are quite demoralized about their symptoms, and it is important to project an image of confidence in the diagnosis. Patients should be told that this is a common disorder afflicting an estimated 5 million Americans and that the physician has treated many cases effectively. A lack of fear of the symptoms on the physician's part, a thorough but reasonable and brief medical workup, and the willingness to tolerate a fairly large amount of dependency in the first few days, weeks, and occasionally months of this problem are all essential and desirable physician behaviors.
Recent double-blind, placebo-controlled studies have demonstrated that three classes of medications are effective for the treatment of panic disorder:
Preliminary results of a multinational study have demonstrated that imipramine, phenelzine, and alprazolam are all more effective than placebo in the treatment of panic disorder, and the three medications do not differ significantly in efficacy (Sheehan et al. 1980,1984). The physician should become familiar with one or two medications from each of these classes.
The goal of psychopharmacologic treatment is to block spontaneous panic attacks (Roy-Byrne et al. in press). Avoidance behavior and social phobias often develop secondary to these spontaneous attacks and, after pharmacologic blockade of the anxiety attacks is achieved, it is important to push patients to reexpose themselves to these phobic situations.
This advice is helpful in two ways. First, it helps determine whether complete pharmacologic blockade of the panic attacks has been achieved. If the patient still breaks through with occasional panic attacks, the dosage of medication should be increased until the patient has no further acute panic attacks. Emphasize to patients that they can and will still have generalized or free-floating anxiety and nervousness and anxiety in stressful situations, but they should not have the severe bursts of autonomic symptoms (tachycardia, dyspnea, chest pain, diaphoresis, dizziness).
The second main reason for patients to reexpose themselves to phobic situations is to reexperience the situation and realize that they no longer need to avoid it because their anxiety attacks have gone away. This knowledge frequently markedly decreases avoidance behavior, phobias, and anticipatory anxiety.
Make it clear to patients that they may have to enter phobic situations without having anxiety attacks several times before their anticipatory anxiety decreases. In some cases, the patient is remarkably recalcitrant to enter feared or phobic situations, and the addition of behavioral psychotherapeutic techniques may be necessary.
Patients with panic disorder are one of the most difficult groups of patients to treat with medications. All psychopharmacologic treatments have side effects, and these patients are already hyper-vigilant about bodily symptoms. Patients with panic disorder also have a strong sense of feeling out of control due to the dysregulation of their autonomic nervous system, and they are often desperately trying to control their lives by increasing exercise, constricting their social contacts to a minimum, and at times, using alcohol or sedative-hypnotic agents. The idea of taking a medication that will have central nervous system therapeutic and side effects frequently makes them feel even more out of control and frightened. These patients often take the prescribed medication for a few days and then stop, reporting that it causes symptoms like jitteriness, nervousness, and tachycardia—the very symptoms they presented initially. Several useful treatment strategies to increase compliance include the following:
In primary care medicine, the usual first pharmacologic treatment for panic disorder should be the tricyclic antidepressants. These medications are quite safe, and they are the most extensively studied medications in the treatment of panic disorder (Katon 1986). In addition, studies of primary care patients with panic disorder have found that about 50 percent of these patients have a concurrent major depression, and tricyclic antidepressants work effectively in the treatment of both disorders.
The best studied tricyclic antidepressant in the treatment of panic disorder is imipramine (Sheehan et al. 1980; Klein 1964; Zitrin 1983). Nine of ten double-blind placebo-controlled studies have found it to be significantly more effective than placebo (Roy-Byrne and Katon 1987). Uncontrolled studies have shown that desipramine, nortriptyline, amitriptyline, clomipramine (not yet released in the United States), and doxepin are also effective agents (Liebowitz et al. 1986; Gloger et al. 1981; Roy-Byrne et al. in press; Lydiard 1988).
Other antidepressant medications are either untested or have had negative results in patients with panic disorder. Trazodone and maprotiline have been found to be of questionable value in the treatment of panic disorder (Lydiard 1988). Fluoxetine has not yet been tested in panic disorder, and amoxapine should be reserved for patients with psychotic symptoms (it is metabolized in the body to an antipsychotic and antidepressant).
A useful strategy is to start the patient on a low dose, such as 25 mg of imipramine or desipramine, and to gradually increase it by 25 mg every 3 days. It helps to tell the patient that you would like to gradually increase the medication to 150 mg to 300 mg or until the panic attacks totally cease. Give the patient the every-3-day increase schedule, but also suggest that if problematic side effects occur, they can slow down the rate of increase.
About 1 in 10 people get an excitatory or stimulant-like effect with imipramine or desipramine. Tell patients that if this occurs, they may have started at too high a dosage. Then decrease the dosage to 10 mg of imipramine or desipramine and increase it by 10 mg increments every 3 days. If the patient still has stimulatory effects, change to a less noradrenergic drug, such as 25 mg of nortriptyline. An alternate strategy is to temporarily add a low dose of alprazolam—0.5 mg two or three times a day—to the tricyclic. This decreases the stimulatory side effect and allows a gradual increase in the tricyclic antidepressant dosage. For those rare patients who cannot tolerate this side effect, switch to a monoamine oxidase inhibitor or benzodiazepine.
Table 2 lists the tricyclic antidepressants and their common side effects (Katon and Roy-Byrne 1988). All available tricyclics have some degree of anticholinergic action; the possible side effects include dry mouth, constipation, blurry vision, urinary retention, sinus tachycardia, and memory dysfunction (Frazer and Conway 1974). Should a serious anticholinergic effect occur, switch to a tricyclic with a low anticholinergic agent such as desipramine. Sedation can also be a problem, especially with amitriptyline, doxepin, trimipramine, and trazodone. Should this occur, switching to a nonsedating tricyclic such as desipramine is often helpful. Other less common adverse effects of the antidepressants are weight gain and sexual dysfunction. Fluoxetine appears to have less propensity to cause weight gain, but it is currently untested in panic disorder (Wernicke 1985).
All tricyclics have significant effects on the heart (Bigger et al. 1978). They tend to slow both atrial and ventricular depolarization and cause an increase in PR, ORS, and QT intervals as well as a decrease in T-wave amplitude. The tricyclics all have quinidine-like effects on the heart, slowing conduction time through the bundle of His (Bigger et al. 1978). The tricyclics, like other Group I antiarrhythmics, can cause abnormally slow post-AV nodal conduction. Many patients with anxiety attacks complain of irregular heartbeats and are found to have premature ventricular contractions. It is not surprising that tricyclic therapy often strikingly decreases their ventricular irritability; ventricular ectopia is not a contraindication to tricyclic use (Bigger et al. 1977).
| Table 2. Pharmacologic properties of the polycyclic antidepressant | ||||||||
|
Potency of reuptake blockade |
Histamine1 | Anticholergic | Sedation | Orthostatic hypotension | ||||
| Serotonin | Norepinephrine | |||||||
| dosage (mg) | ||||||||
| Tertiary amines | ||||||||
| Doxepin (Sinequan) | *** | ** | 100-300 | Highest | Moderate | High | *** | |
| Amitriptyline (Elavil) | **** | ** | 100-300 | Mod-Hi | Highest | High | *** | |
| Imipramine (Tofranil) | **** | ** | 100-300 | Low | Moderate | Moderate | ** | |
| Trimipramine (Surmontil) | * | * | 100-300 | High | Moderate | High | *** | |
| Secondary amines | ||||||||
| Nortriptyline (Pamelor) | *** | *** | 50-125 | Low | Low | Moderate | * | |
| Protriptyline (Vivactil) | *** | **** | 20-60 | Low | High | Low | * | |
| Desipramine (Norpramin) | ** | **** | 100-300 | Low | Low | Low | * | |
| Amoxapine (Ascendin) | ** | *** | 100-300 | 7 | Low | Low | ** | |
| Tetracyclic | ||||||||
| Maprotiline (Ludiomil) | * | *** | 100-300 | Moderate | Low | Moderate | ** | |
| Triazolopyridine | ||||||||
| Trazodone (Desyrel) | *** | * | 150-500 | Low | Lowest | High | *** | |
| Bicyclic | ||||||||
| Fluoxetine (Prozac) | **** | 0 | 20-80 | ? | Lowest | Low | 0 | |
| Source: Katon and Roy-Byrne 1988. 0 = None, * = Slight, ** = Moderate, *** = Marked, **** = Pronounced |
||||||||
Because tricyclics cause slowing of conduction time through the bundle of His, the physician should be most cautious with panic disorder patients who have preexisting bundle-branch blocks on EKG (Bigger et al. 1978). In these patients, treatment should proceed cautiously with an initial 25 mg dosage of a tricyclic and increases of 25 mg every 5 to 7 days. These patients should be followed with serial electrocardiograms.
Several studies have found that tricyclic antidepressants do not decrease myocardial contractility in therapeutic dosages. Veith and colleagues (1982) used radionucleotide ventriculography to measure ventricular ejection fractions before and during maximum exercise in depressed patients who had heart disease and were being treated with therapeutic dosages of doxepin and imipramine. No adverse effects on left ventricular function were noted. Another study in a group of 21 depressed patients with left ventricular impairment found that ejection fraction was unchanged by nortriptyline (Roose et al. 1986).
The most common side effect that limits tricyclic dosage is orthostatic hypotension (Katon and Roy-Byrne 1988). Although tricyclic-induced ortho-static hypotension had been thought to be due to alpha-1 adrenergic blockade, the relative hypotensive effects of specific tricyclic drugs do not follow the alpha-1 receptor blocking pattern. A number of factors are associated with an increased likelihood of orthostatic hypotension, including:
The following guidelines are especially useful in medically ill geriatric patients to minimize serious sequelae secondary to orthostatic hypotension (Halaris et al. 1986-87).
Therapeutic monitoring of blood levels of tricyclics should be reserved for nonresponsive patients (Roy-Byrne et al. in press). Dosages of the traditional tricyclics (with the exception of nortriptyline and protriptyline) should be increased gradually until panic attacks have stopped. If 300 mg is reached and the patient still has anxiety attacks, a serum level 12 hours after the last dose should be drawn. If the serum level is below the therapeutic range, the dosage should be increased in 25- to 50-mg increments. Other reasons to draw a blood level include moderate to severe side effects and suspicion of noncompliance.
There is a large variability in blood levels of tricyclics, probably due to differences in absorption and metabolism of the drug (Halaris 1986). This is especially true in the elderly. Thus, serious side effects may suggest that serum levels are higher than needed, and lowering the dosage may enhance response and decrease side effects. Nortriptyline has a therapeutic window of 50 to 150 ng/ml, and serum levels below or above usually do not provide a therapeutic response for depression (Amsterdam et al. 1980).
The clinical implications of blood levels of imipramine and desipramine have been relatively well established and suggest a sigmoidal dose relationship. Preliminary studies in patients with panic disorder suggest that for imipramine, a serum level above 150 mg/ml is most effective, and for desipramine, a serum level above 125 mg/ml appears to be most effective (Lydiard 1988). Research is in progress to further delineate effective serum levels in the treatment of panic disorder.
The mechanism of action of the tricyclic antidepressants in panic disorder is unclear. The catecholamine theory of depression suggested a depletion of catecholamines, norepinephrine, and serotonin, and, therefore, a decrease in synaptic transmission in areas of the brain that control affective regulation (limbic system) (Schildkraut 1965). According to this theory, tricyclic antidepressants prevent neurotransmitters in the neurosynaptic cleft from being returned to the sending neuron, and thus more of these chemicals are available for synaptic transmission. However, maximal reuptake blockade occurs in the first week of treatment, whereas maximum therapeutic effects occur in the second and third weeks (Charney et al. 1981b). Some evidence suggests that 6-therapeutic efficacy results from changes tricyclics induce in the sensitivities of neurotransmitter receptors, whose time course correlates better with the onset of clinical improvement (Charney et al. 1981b). Imipramine has been shown to decrease the sensitivity of beta-adrenergic receptors and to decrease locus ceruleus activity in animals (Charney et al. 198 la). Imipramine also decreases plasma MHPG (a marker of central norepinephrine turnover), and this reduction is associated with a decrease in the signs and symptoms of panic attacks (Charney et al. 1984; Charney and Heninger 1986).
Successful Treatment of Panic Disorder With a Tricyclic AntidepressantMr. O was a 35-year-old engineer who presented with symptoms of headache, dizziness, rapid heart rate, dyspnea and paresthesias 3 weeks after a severe auto accident. A careful neurologic workup including physical examination, skull x rays, and CT scan proved negative. After describing increased nervousness and social phobic behavior (inability to be comfortable in meetings and conferences at work) his family physician referred him for psychiatric consultation. He was diagnosed as having panic disorder and started on imipramine 25 mg. Mr. O telephoned 3 days later and reported feeling “speeded up” on the medication as if he had drunk too much coffee. He was advised to continue imipramine, but at the lower dosage of 10 mg. This decreased the sense of feeling ”speeded up”. The medication was then gradually increased to 150 mg over 3 to 4 weeks, with complete amelioration of panic attacks. His social phobic behavior also rapidly disappeared, and he continued to go to meetings, finding he was no longer having anxiety attacks. |
The introduction of the high-potency benzodiazepine, alprazolam, has altered the perception that benzodiazepines are not effective in panic disorder. Alprazolam has been found to be as effective as imipramine and phenelzine in the treatment of panic disorder (Sheehan 1983; Sheehan et al. 1984; Ballenger et al. 1988). Two other high-potency benzodiazepines, clonazepam and lorazepam, also appear to be effective antipanic drugs (Charney et al. 1987; Tesar et al. 1987b). Moreover, a study of high-dosage diazepam (a mean of 30 mg/day) has found that it also markedly decreases panic attacks, suggesting that older studies may have suffered from too low a dosage as well as infrequent dosing (Dunner et al. 1986).
The benzodiazepines' therapeutic efficacy appears to be secondary to their ability to facilitate inhibitory neurotransmission via potentiation of the action of GABA (Insel et al. 1984; Skolnick and Paul 1983). Benzodiazepines bind stereo-specifically to receptors linked to GABA, which have their highest density in limbic brain areas controlling affective arousal and autonomic function. Also, benzodiazepine-GABA receptors are in the area of the locus ceruleus that inhibits the neuronal activity of this modulator of the autonomic nervous system (Marks and Tobena 1986).
Alprazolam and lorazepam have relatively short plasma half-lives (table 3) and, thus, require three to four daily dosages, whereas clonazepam and diazepam have relatively long half-lives usually requiring only two daily dosages. The advantages of benzodiazepines in the treatment of panic disorder are that they have a rapid onset of action and are well tolerated with few side effects. However, several disadvantages tend to deter the clinician from their use as a first-line antipanic drug (Roy-Byrne et al. in press). These disadvantages include:
Because of the above concerns, benzodiazepines should be reserved for patients with panic disorder who do not tolerate tricyclic antidepressants and who elect not to try monoamine oxidase inhibitors owing to concerns over the potential "cheese reaction" (see below). Another use of these agents is to prescribe them concurrently with a tricyclic in the first few weeks of therapy to try to decrease anxiety and the tendency to worry about antidepressant side effects. The benzodiazepines may also help decrease the initial stimulatory tricyclic effects. Despite the admonitions against their use in primary care as a first-line drug, the benzodiazepines are effective in panic disorder, and they are useful medications for those patients who do not tolerate tricyclics.
| Table 3. Classification of benzodiaze pines by plasma half-life | |||
| Generic | Brand | Half-life (hours) | |
| Very short | |||
| Triazolam | Halcion | 2-6 | |
| Short | |||
| Alprazolam | Xanax | 6-20 | |
| Lorazepam | Ativan | 9-22 | |
| Oxazepam | Serax | 6-24 | |
| Temazepam | Restoril | 5-20 | |
| Intermediate | |||
| Chlordiazepoxide | Librium | 7-46 | |
| Diazepam | Valium | 14-90 | |
| Clonazepam | Klonopin | 20-40 | |
| Long | |||
| Clorazepate | Tranxene | 30-200 | |
| Halazepam | Paxipam | 30-200 | |
| Prazepam | Verstran | 30-200 | |
| Very long | |||
| Flurazepam | Dalmane | 90-200 | |
Several types of patients should not be treated with benzodiazepines because of their likelihood to abuse them. Marks (1983) has shown that the majority of patients who abuse benzodiazepines have a history of polydrug or alcohol abuse. Thus, a history of polydrug or alcohol abuse should be a contraindication to the use of these agents. Patients with personality disorders and chronic benign pain conditions (back pain, headaches) also should not be treated with benzodiazepines. Finally, patients with first-degree relatives who abuse alcohol may be at increased risk for abuse of benzodiazepines. Thus, a complete individual psychiatric and family history is necessary before these agents are prescribed.
Benzodiazepines are well absorbed on an empty stomach and are oxidized by microsomal enzymes in the liver to active metabolites, which in turn are conjugated in the liver and excreted in the urine (Roy-Byrne et al. in press). The main side effects of benzodiazepines are sedation and psychomotor impairment (Greenblatt and Shader 1974). Fatigue, ataxia, slurred speech, and amnesia can also occur. Sedation often appears in the first few days of treatment and tends to decrease in 1 to 2 weeks. Psychomotor impairment as well as effects on recent memory may persist, at least to a subtle degree (Petersen and Ghoneim 1980). This impairment maybe more common in the first minutes to hours after a dose is taken. Patients must be warned about these more subtle effects, and family and friends may help provide objective evidence if decrements in psychomotor activity or new-learning ability appear. More frequent dosing at decreased dosages may decrease these side effects.
Patients with panic disorder should be started on 0.5 mg PO BID to TID of alprazolam, with increases of 0.5 mg every 2 or 3 days until the panic attacks have stopped. In psychopharmacology clinics, patients generally require 3 mg to 10 mg of alprazolam for effective treatment; however, in primary care, most patients respond at between 1.0 and 3.0 mg of alprazolam. This is probably because they are less severely ill than are patients who are referred to psychiatrists.
Among the other benzodiazepines found to be effective in panic, dosages equivalent to 0.5 mg of alprazolam are 0.25 of clonazepam, 5 mg of diazepam, and 1.0 mg of lorazepam (Roy-Byrne et al. in press). If a short-acting ben-zodiazepine is used (alprazolam and lorazepam) and the patient breaks through with anxiety attacks despite QID dosing, then switching to a longer acting benzodiazepine such as clonazepam is often effective. Clonazepam, lorazepam, and alprazolam have been associated with breakthrough depression (depression that becomes apparent as anxiety is decreased) in some patients, so the clinician should monitor these symptoms (Charney et al. 1987; Tesar et al. 1987b; Lydiard et al. 1987).
Successful Treatment of Panic Disorder With a BenzodiazepineMrs. A was a 43-year-6ld married white management banker. She presented to her primary physician with long-term anxiety problems. Mrs. A described acute attacks of rapid heartbeat, dyspnea, sweating, tremulousness, and nausea for more than 10 years. She avoided most social situations because of these attacks and her activities were limited to driving to work and back and spending time with her husband. She had recently received a promotion that would require taking clients to lunch and taking training seminars, and she was quite afraid she could not cope with these increased responsibilities with her social phobias. Mrs. A had taken diazenpam intermittently for 10 years, but it had not stopped her acute anxiety attacks. Mrs. A was initially tried on three different tricyclic antidepressant, but reported all cause side effects such as sedation, a “spacey”feeling, and constipation. She was then started on alprazolam 0.25 mg PO TID, which was increased to 0.5 mg PO TID over 2 weeks with a rapid cessation in panic attacks. Her primary care physician worked with Mrs. A to add several new social activities a month, and she was delighted to find she no longer experienced anxiety attacks. She accepted her promotion, was successful in taking several seminars, and began to actually enjoy going to lunch with colleagues and clients. She has remained asymptomatic for the last 2 years on alprozolam 0.5 mg PO TID. |
The prototypic MAOI that has been studied in panic disorder is phenelzine. Phenelzine may actually be the most effective medication in the treatment of panic disorder, and it is probably the medication of choice in treatment-resistant panic disorder (Sheehan et al. 1980,1984; Sheehan 1984).
These medications fell into disfavor in the United States because of the so-called "cheese reaction" they can cause (Tollefson 1983). Patients must closely monitor their diet, because foods that contain high amounts of tyramine can interact with the MAOI and cause a sympathomimetic crisis characterized by headache, diaphoresis, mydriasis, hypertension, neuromuscular excitation, and cardiac dysrhythmia. It is generally accepted that this "crisis" is caused by tyramine that is derived from the digestion of food products and enters the bloodstream in high concentrations, presumably because the liver's monoamine oxidase enzymes are inhibited (Tollefson 1983; Blackwell et al. 1967). Tyramine presumably acts by causing the release of the mobile fraction of norepinephrine into the intersynaptic cleft. In recent years, the monoamine oxidase inhibitors have enjoyed a resurgence of use in the United States, and they are generally accepted (with appropriate dietary constraints and caution with the use of certain specific medications) to be safer than formerly thought (Raskin et al. 1974).
The currently used MAOIs (table 4) inhibit the monoamine oxidase enzymes in all body tissues. The nonhydrazine MAOI, tranylcypromine, is a reversible monoamine oxidase inhibitor (albeit slowly), whereas phenelzine and isocarboxizide are irreversible (Roy-Byrne et al. in press). The monoamine oxidase enzyme's function is oxidative deamination within the outer membrane of neuronal mitochondria. It is one of the two major routes for the inactivation of nonmethylated biogenic amines, such as serotonin, norepinephrine, and dopamine (Tollefson 1983). By inhibiting inactivation of these biogenic amines, MAOIs are thought to increase the concentration of vasoactive amines available for synaptic release. However, recent evidence suggests this enzyme inhibition occurs rapidly at subtherapeutic dosages, whereas clinically the MAOIs often take 2 to 4 weeks for their therapeutic action (Roy-Byrne et al. in press). Thus, the mechanism of action is unclear.
MAOIs are well absorbed after an oral dose and undergo acetylation by the liver (Tollefson 1983). The MAOIs do not produce the stimulatory effects that tricyclic antidepressants do in some patients with panic disorder (Lydiard 1988). They also have less sedative and anticholinergic effects. In an NIMH study, 1,110 patients on maintenance phenelzine demonstrated no adverse hypertensive reactions (with proper dietary discretion); one case of elevated liver function tests and a small number of anticholinergic side effects were reported (Raskin et al. 1974). This suggests that the prevalence of MAOI side effects has been overestimated.
Blackwell (1967) also documented that even patients ingesting high-risk food products rarely experience hypertensive effects. Insomnia is a common side effect, and encouraging patients to take the drug earlier in the day is often helpful. Other research suggests that low dosage amitriptyline 25 mg PO QHS can decrease the side effect of insomnia and may actually act to prevent the hypertensive reaction induced by tyramine (Pare et al. 1982). Patients with insomnia who will be prescribed amitriptyline need to be tapered off the MAOIs and kept off for 7 to 10 days; then they can have small dosages of amitriptyline and the MAOI started at the same time (see below).
| Table 4. Monoamine oxidase inhibitors | |||
| Generic | Trade name | Initial dosage | Maintenance dosage |
| Phenelzine sulfate | Nardil | 15 mg PO BID | 45 mg to 90 mg |
| Tranylcypromine | Parnate | 10 mg PO BID | 10 mg to 40 mg |
| Isocarboxizide | Marplan | 10 mg PO BID | 10 mg to 50 mg |
| Side effects: Hypertensive reaction, orthostatic hypotension, insomnia, tachycardia, dry mouth, constipation, blurred vision, hepatocellular damage, delayed ejaculation, impotency, edema. | |||
Another side effect of MAOIs is orthostatic hypotension, which often begins 3 to 4 weeks after starting the medication (Kopin et al. 1965). Potential mechanisms to decrease hypotension include lowering the dosage, salt tablets, abdominal binders, and the use of fluorodeoxy-corticosterone. Other less common side effects are listed in table 4.
The hypertensive reaction is the major potential toxicity of the MAOIs. The patient needs to remain on a tyramine-free diet (exhibit 11) to minimize the risk of this reaction (McCabe and Tsuang 1982). This diet should be continued for 2 weeks after treatment ends, owing to the irreversible inhibition of the MAOIs (Goldberg 1964). The patient must also avoid medications that can precipitate hypertensive crises when used in conjunction with MAOIs. These include sympathomimetic agents of any kind, such as over-the-counter stimulants, amphetamines, many cold tablets containing ephedrine-like compounds, meperidine, cocaine, L-dopa, methyldopa, and asthma inhalants (Pare 1977). Also, it is not safe to switch from one MAOI to another; the first MAOI must be stopped for 10 to 14 days before starting another, or an adrenergic crisis can ensue. Tricyclic antidepressants should not be added to MAOIs that have been already prescribed—they can cause fever, delirium, and seizures (Roy-Byrne et al. in press).
Hypertensive reactions are characterized by severe, crushing, throbbing headache, accompanied by sweating and flushing, nausea and vomiting or neck stiffness and photophobia. Blood pressure increases associated with headache have been about 55 mm systolic and 30 mm diastolic. One useful strategy is to prescribe one to two 10-mg tablets of nifedipine for patients taking MAOIs to carry around at all times for use if a sudden "throbbing" headache associated with these other symptoms occurs (Clary and Schweitzer 1987). Dosages of 5 mg IV of the alpha-adrenergic blocker phentolamine will also reduce the blood pressure.
Exhibit 11. Dietary restrictions for patients taking monoamine oxidase inhibitors |
|
| Foods that must be avoided | |
| Beer and wine, particularly Chianti Cheese, except cottage and cream cheese Smoked or pickled fish, especially herring Beef or chicken liver Summer (dry) sausage Fava or broad bean pods (Italian green beans) Yeast vitamin supplements (brewer's yeast) |
|
| Foods that are unlikely to cause problems unless large quantities are consumed | |
| Other alcoholic beverages Ripe avocado Ripe fresh banana Sour cream Soy sauce Yogurt |
|
| Insufficient evidence of adverse interaction Chocolate Figs | |
| Meat tenderizers Raisins Yeast breads Coffee, tea, and other caffeine-containing beverages |
|
| Source: McCabe, B., and Tsuang, M.T. Journal of Clinical Psychiatry 43(5):178-181,1982. Copyright 1982 Physicians Post-Graduate Press, Inc. Reprinted with permission. | |
For the patient with panic disorder, a dosage of 15 mg PO each morning of phenelzine for the first 2 days should be followed by 15 mg PO BID for 1 week. An increase of 15 mg per day should continue each week thereafter until all panic attacks have ceased. The highest daily dosage that should be used is 90 to 105 mg, but most patients improve at 45 to 90 mg.
Successful Treatment of Panic Disorder With an MAOIMr.M was a 27-year-bld businessman who presented with a 6-month history of acute attacks of dyspnea, sweating, tachycardia, tremulousness, and dizziness. He had to make frequent oral presentatlions at work to groups of colleagues, and the attacks were making these more and more difficult. In addition, he had been avoiding social situations such as parties because the attacks had embarrassed him during several previous social interactions. He was one of seven children, with at least two other siblings suffering from panic disorder. His mother had recurrent severe depressions. Mr. M was diagnosed as having panic disorder and started on imipramine 25 mg, which was increased to 100 mg over 2 to 3 weeks. He had increased jitteriness and a sense of being “speeded up” on the imipramine, which did not improve over a 1-month period. The imipramine was topped, and after 10 days, phenelzined was started at 15 mg. The dosage was increase to 60mg over 2 to 3 weeks with a rapid amelioration of all panic attacks. His anticipatory anxiety with business speeches and presentations and parties gradually decreased over 2 months as he reexperience these situations and found that he no longer developed anxiety attacks. He has been seen in weekly psychotherapy that has addressed chronic self-esteem problems and his tendency to pressure himself to always be “perfect” to compensate for his low self-concept. His phenelzine was tapered to a maintenance dose of 15 mg during this time. |
Once an effective dosage of a tricyclic antidepressant, MAOI, or benzodiazepine is found and the patient's panic attacks have entirely ceased, the patient should be kept on that medication for 6 to 12 months. The dosage of medication should then be tapered. Tricyclic antidepressants should be tapered by 25 to 50 mg every 1 to 2 weeks. Monoamine oxidase inhibitors should be tapered by 10 to 15 mg (one tablet) per week.
Benzodiazepines appear to be more difficult to taper in patients with panic disorder. A recent placebo-controlled study of alprazolam tapering in patients with panic disorder demonstrated that 27 percent of the alprazolam group reported a rebound of panic attacks and 13 percent had rebound anxiety (Pecknold et al. 1988). No serious or life-threatening withdrawal symptoms were reported, but a distinct, transient mild to moderate withdrawal syndrome occurred in 35 percent of the alprazolam-treated group and in none of the placebo-treated group. The recommendation from this study was to treat panic disorder for at least 6 months and to taper benzodiazepines quite slowly over at least 8 weeks. If the patients receiving benzodiazepines experience recurrence of symptoms, stopping the taper is advised. If the symptoms subside over the next several days, the likely cause was rebound anxiety or withdrawal. The patient can then be gradually withdrawn at a slower rate (Lydiard 1988).
Several additional strategies appear to be helpful in patients with panic disorder when tapering a benzodiazepine has been difficult. Preliminary evidence suggests that the addition of carbamazepine at 400-800 mg daily will decrease benzodiazepine withdrawal symptoms, even if the benzodiazepine is tapered rapidly over 4 to 7 days (Roy-Byrne et al. in press). Alternatively, the addition of a tricyclic antidepressant to therapeutic levels (100 mg to 300 mg) will often decrease the likelihood of rebound panic attacks with tapering of benzodiazepines.
Should the patient relapse during withdrawal or soon after withdrawal of one of the three classes of antipanic medications (and the symptoms do not subside in several days), then another 6- to 12-month course of medication is prescribed before a second attempt to taper medication is instituted.
Many patients find that a smaller dosage of medication is needed for maintenance than for acute treatment. With relapse, the dosage of medication should be increased to just above the level at which relapse occurred, which is often below the highest dosage needed in the past. A small subgroup of patients will need chronic lifelong treatment or will relapse frequently. For these patients, lifelong use of a medication (tricyclic antidepressant, MAOI, or benzodiazepine) is preferable to the frequent relapses or chronic symptoms of panic disorder and their adverse effects on the patient's personal, family, and vocational lives.
Panic disorder, like peptic ulcer disease, is a relapsing, remitting disorder that is often precipitated by stressful life events. Studies have reported that anywhere from 30 to 90 percent of patients relapse within 1 year (Roy-Byrne and Katon 1987). The combination of psychotherapy and medication may decrease this propensity toward relapse because psychotherapy frequently improves self-esteem and successful coping with stress and generally improves the patient's sense of self-efficacy (Bandura 1977).
Patients with panic disorder vary considerably in the life stresses that precipitate their disorders; the strength of their social support network; their use of healthy coping mechanisms, such as exercise, recreational hobbies (hiking, swimming, fishing), and relaxation techniques that can decrease the effects of stress; and their baseline self-esteem and coping mechanisms. Brown and colleagues' series of prospective studies have convincingly demonstrated that although patients may have genetic susceptibility to a disorder such as depression or panic disorder, life stresses invariably precipitate the acute episode (Finlay-Jones and Brown 1981). Moreover, patients with chronic poor self-esteem or chronic ongoing problems (such as an unhappy marriage) are at increased risk of developing an episode of panic disorder or major depression should a stressful life event or series of events occur (Brown et al. 1987). Panic disorder may also occur at key developmental transition phases such as leaving home for the first time, after the birth of a first child, or a promotion at work.
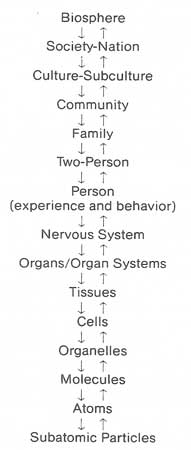
| Figure 9. The biopsychosocial model |
|
|
| Source: Adapted from Engel, G.L. The clinical application of the biopsychosocial model. American Journal of Psychiatry 137:537, 1980. Copyright 1980 by the American Psychiatric Association. Reproduced with permission. |
Along with social factors, it is useful to determine the patient's psychologic vulnerability to stressful life events. Panic disorder occurs in a wide variety of patients, from those who have many psychological strengths to those with severe personality disorders. Histories of patients' experiences in their family of origin (looking for key factors such as early loss of parents, rejecting parents who provided little nurturance, childhood physical or sexual abuse, parental substance abuse and other mental illness, a family history of panic disorder and/or agoraphobia) and the patients' experience with adult intimate relationships often are quite helpful.
Understanding the social context in which panic disorder developed as well as the psychologic strengths and vulnerabilities of the patient helps to evaluate whether any ongoing problems could be addressed by psychotherapy. If a marital crisis and potential separation provoked the panic attacks, marital therapy should be initiated. A long-term problem with poor self-esteem, a tendency to have problems with passivity, avoidance, and lack of assertiveness, or recurrent relationships that have led to feelings of rejection would indicate psychodynamic therapy. As with all clinical illness, it is useful for the primary care physician to assess patients with panic disorder utilizing the biopsychosocial model (figure 9).
Such a model dispenses with the dualism and reductionism of traditional biomedical teaching and replaces the simple cause-and-effect explanations of linear causality with reciprocal causal models (Engel 1977,1980). The biopsychosocial model expands clinicians' treatment options as well as their understanding and therapeutic alliance with their patients. Somatic symptoms often express patients' distress in many dimensions of their lives, and the job of the clinician is to determine whether the etiology of that distress is in the biologic, social, or psychologic sphere or a combination of the three.
In this model, the patient's symptoms could result from perturbations in their family and marriage and lead to changes at the subcellular level. Alternatively, changes in a tissue or organ (such as ischemia of myocardial tissue) could cause marital or family problems.
Panic Disorder and the Biopsychosocial Model 1Mr.W was a 28-year-old Cambodian refugee who had several emergency room and primary care visits over a 2-month period due to chest pain. He also had a 3-day admission in the coronary care unit where EKG, echocardiogram, and exercise treadmill test were negative. Because of his anxiety, psychiatric consultation. He described his chest pain as associated with dyspnea, paresthesia, sweatiness, dizziness and a fear of heart attack. Mr. W had migrated to Seattle after spending 3 years in a resettlement refugee camp in Thailand. He had been brought up in a middle-class family in Phnon Phen prior to the Communist takeover. After the takeover, he and his four siblings and parents were forcibly marched out of their home in the capital of Cambodia to a rural area and had to work as farmers. There was little food, and his father and one sister died from starvation. Mr. W and his brother escaped by walking more than 200 miles to Thailand, where they spent 3 years in poverty before emigrating to America. Mr. W had tried to contact his mother and twa surviving sisters by mail, but had not been able to reach them in 3 years. He had frequent nightmares of their starvation experience and had been bothered by the autonomic episodes described above for over a year. Mr W was diagnosed as meeting DSM-III criteria fro panic disorder stress disorder. He was started on imipramine, and his panic episodes rapidly were ameliorated on 100 mg a day. He was also treated with ssupportive psychotherapy once a week during which he described in depth much of this and his family’s suffering over the the 3-year period. Eventually, he wrote a 50-page account of this period of his life, and the ventilation and abreaction (reliving the frief and terror of these years) seemed to be quite therapeutic. Mr. W was able to taper and discontinue imipramine after 6 months of treatment, and he has remained asymptomatic for 3 years. |
Case 1 demonstrates how major perturbations in one's society and nation can dramatically effect one's culture, community, and family, causing overwhelming stress to the individual. Mr. W had both posttraumatic stress disorder and panic disorder. The medication ameliorated his panic attacks, and supportive therapy aimed at ventilation and abreaction of his suffering in the Cam-bodian revolution helped decrease his psychologic pain as well as cementing the therapeutic alliance.
Case 2 demonstrates how a current life stress may bring up overwhelming, painful childhood memories associated with the onset of panic attacks.
Panic Disorder and the Biopsychosocial Model 2Mrs. M was a 40-year-old white female who presented to her primary care physician with a 1-month history of insomnia, nightmares, and acute attacks of rapid heartbeat, dizziness, paresthesia, hot flashes, and depersonalization. She wondered if she were "going through the change." Asked about life stresses, Mrs. M reported the onset of her disorder disorder after her husband (she had been married for 15 years and had two children) returned home from work 2 hours late. He had gone out drinking (which was not typical) with his friends after work and had lost track of time. She and her husband got into a verbal argument and he lost his temper and, for the first time in the marriage, slapped her. Mrs. M stated she had gradually forgiven him but, subsequently, wherever her husband attempted to hug or touch her, it would make her skin "crawl" and she would yell at him. Because of her vivid description, she was asked about any past history of physical or sexual abuse. Mrs. M tearfully recounted that she had been both physically and sexually abused as a child by an alcoholic father, and that she had been having nightmares and flashbacks of his trauma since her husband had struck her. Mrs. M was diagnosed as having panic disorder secondary to both marital distress arid psychologic vulnerability due to problems in her family of origin, Imipramine, 150 mg a day, stabilized her panic disorder, and Mrs. M was referred for psychodynamic therapy. Over a year’s time, she was able to deal with her childhood trauma, which helped stabilize and strengthen her relationship with her husband. |
In this case, problems with her relationship with her husband had brought up repressed traumatic memories from her family experience and had resulted in overwhelming stress that led to dysregulation of her autonomic nervous system.
To date, no studies have examined the specific efficacy of psychodynamic, marital, or family therapy in patients with panic disorder, either alone or in combination with psychopharmacology (Roy-Byrne and Katon 1987). However, these therapies often decrease social and psychologic distress and improve self-esteem. They also provide models for the use of more appropriate problem-focused coping mechanisms instead of maladaptive mechanisms such as avoidance, wishful thinking, and passivity, which are commonly used by patients with panic disorder. As such, these therapies may decrease the tendency to relapse, as well as decrease the morbidity should panic attacks recur. In major depression, new evidence has suggested that the combination of a psychotherapeutic and psychopharmacologic approach is usually superior to reliance on any single modality (Dimascio et al. 1979), and the same approach is recommended in panic disorder.
Another specific type of therapy for panic disorder and its sequelae of multiple phobias deserves mention. Pharmacotherapy, by reducing or ameliorating spontaneous anxiety attacks, can quickly relieve suffering and facilitate movement toward reentering phobic situations. A behavioral technique termed in vivo exposure does not directly affect the occurrence of panic, but instead, it gradually reduces the anticipatory anxiety felt toward stimuli and situations that have been associated with panic attacks (Marks 1987; Ghosh et al. 1984; Rapee and Barlow 1988; Roberts 1984). As the anticipatory anxiety diminishes and the individual develops a sense of confidence and mastery, the episodes of panic may diminish.
In vivo exposure involves a simple principle—persuading patients to reenter those frightening situations that they have long avoided, and to stay in them long enough (often an hour or more) for the fear to diminish (Marks and Horder 1988). The exposure does not need to occur with a friend, family member, or with the physician.
The primary care physician can carry out this treatment in patients with multiple phobias and/or agoraphobia and panic disorder. The physician must first develop a list with the patient of the specific fears and places that are avoided. He then explains that avoiding situations perpetuates phobias. The rapid avoidance or escape from a feared situation prevents patients from learning that if they just stayed in the situation, the anxiety would subside anyway (Marks and Horder in press). In fact, avoidance and escape make anxiety worse the next time the patient must face a similar situation. The old proverb and folk wisdom about "When you fall off a horse, you should get right back on" is familiar to most people and often helpful. Exposure works better if the patient is able to stay in the situation long enough for the anxiety to lessen.
The primary care physician and the patient should rank the list of fears from the most difficult to the least difficult and then, starting with the least difficult situations, negotiate and agree each week about which specific fears or situations to face. Thus, an agoraphobic might walk one block away from home and stay there for 1 hour the first week; this would be followed by a longer walk to a local shop, and then to more distant locales; next might be riding public transportation, such as buses, and exposure to crowds; finally, the patient would schedule a job interview. Patients are encouraged to keep diaries of their exposure "homework" to bring each week to the physician. It is important to reinforce the patient for successful exposure and to encourage the patient to try again when avoidance has occurred.
Studies have described excellent results for in vivo exposure in both psychiatric and primary care patients as far as decreasing phobic behavior (Marks 1987). It is not entirely clear whether these patients' panic attacks go away with effective exposure treatment; however, in some patients, this treatment is probably effective for both spontaneous panic attacks and for agoraphobia (Marks and Horder in press; Roberts 1984). Its ease and low cost make this procedure a useful alternative for patients who refuse to take medications or as an adjunct to pharmacotherapy.
Several studies have suggested the possibility that the behavioral treatment and tricyclic antidepressant treatment may both be effective because they act on similar receptors (Marks and Tobena 1986). Exposure to repeated stress in rodents leads to a gradual decrease in physiologic and behavioral responses to that stress and correlates with widespread down regulation or decrease in central beta-adrenergic postsynaptic receptor sensitivity (Stone 1983). Tricyclic antidepressants also cause down-regulation of beta-adrenergic postsynaptic receptors and decrease panic attacks and phobic behavior (Charney et al. 1981b).
Several groups are experimenting with new methods of behavioral therapy to add to in vivo exposure. Many authors have reported that patients with panic disorder seem to have a "fear of fear" (Clark 1986a, b; Rapee and Barlow 1988; Hibbert 1984). That is, their panic attacks are so frightening that any somatic symptom that reminds them of a panic attack (such as rapid heartbeat or sweating) may lead to the sudden increase of anxiety and a subsequent panic attack.
One researcher demonstrated that in a patient with panic disorder attached to a biofeedback heart monitor, the bogus report that his heart rate was increasing led to panic attacks (Margraf et al. 1987). Weekes (1976) has described the phenomenon of sensitization in patients with panic attacks whereby the autonomic and somatic sensory nerves react to internal organ signals (missed or ectopic heartbeat, heartburn, giddy stomach, epigastric distress) with heightened nervous sensations and often excessively strong and precipitant emotional responses (anxiety attacks).
Several groups have reported good results with therapy in which the patient exercises or hyperventilates to provoke internal physiologic cues associated with panic attacks (Rapee and Barlow 1988; Salkovskis et al. 1986). The patient is gradually exposed and habituated to these cues in the same way agoraphobic patients are gradually exposed and desensitized to external cues associated with panic. Patients are also taught to slow down their breathing as a mechanism to decrease symptoms of hyperventilation. Barlow and Craske (1989) have recently developed a self-programmed manual called Mastery of Your Anxiety and Panic that helps patients understand their anxiety and provides exercises (such as the hyperventilation exercise) that provoke panic symptoms and teach the patient to develop mastery over these physiologic sensations.
A useful behavioral strategy in primary care in both diagnosis and treatment may be to ask the patient to breathe in and out rapidly at 40 to 60 breaths per minute to see if hyperventilation simulates the presenting symptoms. Patients are then taught to slow down their breathing to a normal 15 to 20 respirations per minute by timed breathing (i.e., one breath every 5 seconds) rather than their internal breathing cues. Repeated trials of having the patient hyperventilate to provoke symptoms, then slowing down the breathing to ameliorate symptoms seems to allow some patients to gain control of panic attacks. Uncontrolled studies have found this behavioral method quite successful (Rapee and Barlow 1988; Lum 1971).
Panic disorder Treated With In Vivo ExposureMr. L was a first-year medical student who presented to his primary care physician with episodes of shortness of breath, tachycardia, anxiety, and epigastria pain. Further history revealed these episodes had started while watching a move of open heart surgery and had quickly and had quickly generalized to watching movies in theaters and going to large parties. Also, several episodes had occurred during class when patients with particular illnesses were presented. Mr. L’s physician explained that avoidance of these situations would lead to more and more anxiety in the future and explained the technique of in vivo exposure. Mr. L felt if he could just have more exposure to patients in a controlled situation in which his instructor knew of his anxiety, he could become desensitized. The physician arranged a once – a – week 2 hour experience with an emergency room physician in which the student would follow this physician’s rounds and watch him in his patient care. After 3 weeks, the student reported that he had seen 14 or 15 patients with the emergency room physician and a range of cases from a patient with a severe skull injury to patients with influenza. His anxiety had decreased each week, his confidence had improved, and he no longer reacted with anxiety to classroom movies of medical procedures or patient presentations. Also, in vivo exposure to movies and parties the following few weeks worked quite well to decrease these fears. |
Panic Disorder Treated With Hyperventilation Exposure and Breathing TrainingMrs. B was a 35-year-old married black female who presented to the emergency room four times in 2 weeks with shortness of breath, chest pain, tachycardia, sweatiness, and fear that she was having a heart attack. These attacks started anger her best friend, a 40-year-old female, died suddenly from a myocardial infarction. Mrs. B was told she had panic disorder and was hyperventilating. She doubted this diagnosis, stating she breathed fast because she felt she couldn’t catch her breath. She was instructed to breathe at 40 to 60 breaths per minute in the office. This precipitated her exact symptoms and convinced her she was hyperventilating,. Mrs. B was then taught to breath “by the watch” at a rate of one breath every 5 seconds to stop a panic attack or hyperventilation episode. Several trials of rapid breathing followed by breathing once every 5 seconds were tried in the office, teaching her to ameliorate her own attacks. The patient had a rapid recovery and no medication was required. |
These simple behavioral techniques are helpful to convince the patient of the diagnosis. In some patients with less severe disorders, they seem to be curative. Controlled trials of behavioral treatments that desensitize patients to their own somatic symptoms have not been reported, but many case reports in the literature suggest that these techniques are quite helpful (Roy-Byrne and Katon 1987).