2 A 10-cm baseline is recommended for VAS scales
Source: Acute Pain Management Guideline Panel, 1992
| Contents | Previous | Next |
Assessment of pain in the cancer patient is imperative for all health care professionals because failure to assess pain can lead to its undertreatment. The critical role of the assessment of cancer pain was highlighted in a 1993 study of 897 oncologists who, collectively in the previous 6 months, had managed more than 70,000 cancer patients. According to these physicians, poor pain assessment was the greatest barrier to effective cancer pain management in their own practices (Von Roenn, Cleeland, Gonin, et al., 1993). Because of the multiple possible causes of pain, careful evaluation of pain is required.
The initial assessment should occur with each new report of pain and should focus on identifying the cause of the pain and developing a pain management plan. Subsequent assessments should evaluate the effectiveness of the plan and, if pain is unrelieved, determine whether the cause is related to the progression of disease, a new cause of pain, or the cancer treatment.
The initial evaluation of pain should include:
Attention to detail is important: a delayed or incorrect diagnosis, particularly with a syndrome such as spinal cord compression, can result in increased morbidity, needless pain and suffering, or both. The initial assessment should provide a detailed description of each type of pain (Table 3).
Health professionals should ask about pain, and the patient self report should be the primary source of assessment. The self-report should include a description of the pain; its location, intensity/severity, and aggravating and relieving factors; and the patients cognitive response to pain. Neither behavior nor vital signs should be used in lieu of a self-report (Beyer, McGrath, and Berde, 1990). It is best to use brief, easy-to-use assessment tools that reliably document pain intensity and pain relief and to relate these to other dimensions of pain such as mood. (Examples of both brief and comprehensive pain inventories are included in Attachment B.) One routine clinical approach to pain assessment and management is summarized by the mnemonic "ABCDE":
A Ask about pain regularly.
Assess pain systematically.
B Believe the patient and family in their reports of pain and what relieves it.
C Choose pain control options appropriate for the patient, family, and setting.
D Deliver interventions in a timely, logical, and coordinated fashion.
E Empower patients and their families.
Enable them to control their course to the greatest extent possible.
| Table 3. Initial pain assessment | |||
| A. | Assessment of pain intensity and character | ||
| 1 | Onset and temporal pattern—When did your pain start? How often does it occur? Has its intensity changed? | ||
| 2 | Location—Where is your pain? Is there more than one site? | ||
| 3 | Description—What does your pain feel like? What words would you use to describe your pain? | ||
| 4 | Intensity—On a scale of 0 to 10, with 0 being no pain and 10 being the worst pain you can imagine, how much does it hurt right now? How much does it hurt at its worst? How much does it hurt at its best? | ||
| 5 | Aggravating and relieving factors—What makes your pain better? What makes your pain worse? | ||
| 6 | Previous treatment—What types of treatments have you tried to relieve your pain? Were they and are they effective? | ||
| 7 | Effect—How does the pain affect physical and social function? | ||
| B. | Psychosocial assessment | ||
| Psychosocial assessment should include the following: | |||
| 1 | Effect and understanding of the cancer diagnosis and cancer treatment on the patient and the caregiver. | ||
| 2 | The meaning of the pain to the patient and the family. | ||
| 3 | Significant past instances of pain and their effect on the patient. | ||
| 4 | The patient's typical coping responses to stress or pain. | ||
| 5 | The patient's knowledge of, curiosity about, preferences for, and expectations about pain management methods. | ||
| 6 | The patient's concerns about using controlled substances such as opioids, anxiolytics, or stimulants. | ||
| 7 | The economic effect of the pain and its treatment. | ||
| 8 | Changes in mood that have occurred as a result of the pain (e.g., depression, anxiety). | ||
| C. | Physical and neurologic examination | ||
| 1 | Examine site of pain and evaluate common referral patterns. | ||
| 2 | Perform pertinent neurologic evaluation. | ||
| · | Head and neck pain—cranial nerve and fundoscopic evaluation. | ||
| · | Back and neck pain—motor and sensory function in limbs; rectal and urinary sphincter function. | ||
| D. | Diagnostic evaluation | ||
|
1 |
Evaluate recurrence or progression of disease or tissue injury related to cancer treatment. | ||
| · | Tumor markers and other blood tests. | ||
| · | Radiologic studies. | ||
| · | Neurophysiologic (e.g., electromyography) testing. | ||
| 2 | Perform appropriate radiologic studies and correlate normal and abnormal findings with physical and neurologic examination. | ||
| 3 | Recognize limitations of diagnostic studies. | ||
| · | Bone scan—false, negatives in myeloma, lymphoma, previous radiotherapy sites. | ||
| · | CT scan—good definition of bone and soft tissue but difficult to image entire spine. | ||
| · | MRI scan—bone definition not as good as CT; better images of spine and brain. | ||
In the initial assessment, document the onset and temporal pattern of the pain. Ask patients to point to the exact location of the pain on themselves or the clinician (The Brief Pain Inventory and the Initial Pain Assessment Tool in Attachment B). Determine whether the pain radiates or spreads to other parts of the body.
Ask patients to describe their pain: the descriptive words they use can provide valuable clues as to the cause. For example, patients who describe back pain that radiates like a tight band around their chest and worsens with coughing or defecation should be evaluated for potential spinal cord compression, a complication of vertebral body metastasis. Patients who describe their pain as "burning" or "tingling" are likely to have a neuropathic cause of pain—particularly when it is associated with subjective numbness, loss of sensation, and weakness (Elliott and Foley, 1989).
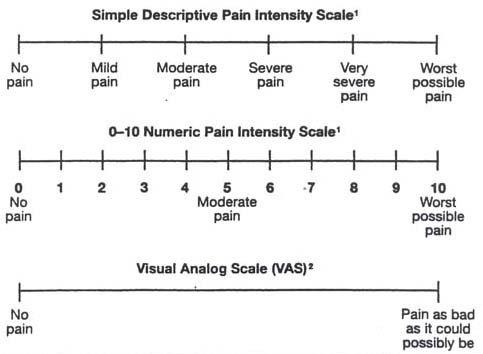
Three commonly used self-report assessment tools (Figure 4) are:
| Figure 4. Pain Intensity scales |
|
|
| 1 If used as a graphic rating scale, a 10cm baseline is recommended. 2 A 10-cm baseline is recommended for VAS scales Source: Acute Pain Management Guideline Panel, 1992 |
If the patient understands the scale and is capable of answering and if end points and adjective descriptors are carefully selected, each of these instruments can be valid and reliable (Gracely and Wolskee, 1983; Houde, 1982; Sriwatanakul, Kelvie, and Lasagna, 1982).
An assessment of pain intensity should include an evaluation of not only the present pain intensity but also pain at its least and worst. Knowing factors that aggravate or relieve pain helps clinicians to design a pain treatment plan. The initial pain assessment should elicit information about changes in activities of daily living, including work and recreational activities, sleep patterns, mobility, appetite, sexual functioning, and mood.
A psychosocial assessment should emphasize the effect of pain on patients and their families, as well as patients' preferences among pain management methods. Patients who are able to answer should be asked about the effectiveness of past and present pain treatments, such as antineoplastic therapy or specific pharmacologic and nonpharmacologic therapies.
The clinician should perform a physical and neurologic examination related to the pain report (see below, Assessment of Common Cancer Pain Syndromes). The painful area should be carefully examined to determine if palpation or manipulation of the site exacerbates the pain. Common sites of pain referral should be evaluated (e.g., shoulder pain may emanate from subdiaphragmatic abdominal sources; knee and hip pain may be referred from lumbar spine lesions). In addition, the patient should be observed for cues that indicate pain, e.g., distorted posture, impaired mobility, guarding the painful area, restricted movement of a limb, anxiety, attention seeking, or depression. However, absence of these behaviors should not be interpreted to mean that the patient has no pain.
Neurologic examination should be focused. For example, pain in the head and neck region requires careful cranial nerve examination to exclude intracranial pathology and lesions at the base of the skull, that may require definition by specialized magnetic resonance imaging (MRI) or computed tomography (CT). Neck or back pain require careful motor, sensory, and reflex examination of the arms and legs, as well as evaluation of rectal and urinary sphincter function to exclude plexopathy and spinal cord lesions.
Appropriate diagnostic tests should be performed to determine the cause of the pain and the extent of disease, and patients should be offered analgesia to facilitate these evaluations (e.g., to allow the patient to lie flat for CT or MRI scans). It is important to correlate the results of these studies with physical and neurologic findings to assure that appropriate areas of the body have been imaged and that identified abnormalities do in fact explain the patient's pain. Pain may be the first sign of tumor recurrence or progression and may appear or increase before changes are evident in imaging studies; therefore, imaging studies may have to be repeated.
The assessment of the patient's pain and the efficacy of the treatment plan should be ongoing, and the pain reports should be documented. Simply to record a patient's responses to the question "How is your pain?" invites misunderstanding and hinders quantification.
Pain should be assessed and documented:
Occasionally, discrepancies between behaviors and a patient's self report of pain may occur. For example, patients may describe pain as an 8, on a scale of 0 to 10, while smiling and walking freely, or conversely, as a 2 while experiencing tachycardia, splinting, and sweating, although this is less usual. These discrepancies may result from several factors, including the effectiveness of the patient's coping skills (see Chapter 4). The patient who uses distraction and relaxation techniques may engage in diversionary activities while still experiencing severe pain; in fact, this is a goal of many behavioral pain therapies. Patients may deny severe pain for a variety of reasons, including a perception that stoicism is expected or rewarded or a fear that the pain symbolizes disease progression. Similarly, patients managed with as-needed analgesia may perceive that medication will be given only if the pain score is very high. When discussing pain assessment and control with patients, members of the health care team should emphasize the importance of a factual report, thereby avoiding both stoicism and exaggeration. If anxiety or other concerns are significant, patients should be asked to rate their emotional distress separately from their pain, using similar scales (see Pain Distress Scales in Attachment B). They also may be asked to rate their mood or the effectiveness of analgesic therapies (see the Memorial Pain Assessment Card in Attachment B). When discrepancies between behaviors and self-reports of pain occur, these differences should be discussed with the patient, and the pain management plan should then be revised.
Most cancer patients are treated for pain in outpatient and home care settings. Plans should be made to ensure ongoing assessment of the pain and the effectiveness of treatments in these settings. Patients can keep a log of their pain intensity scores and report these scores during follow up visits or through telephone follow up. In addition, patients should be taught to report changes in their pain or any new pain so that appropriate reassessment and changes in the treatment plan can be initiated.
Patients unable to communicate effectively with staff require special consideration (see Chapter 7). Even patients previously able to communicate may be unable to do so as their disease progresses. Aggressive efforts should be made to find a translator for the non-English-speaking patient to determine a convenient way to assess pain. Many of the pain assessment tools have already been translated (Beyer and Wells, 1993; Cleeland and Syrjala, 1992).
When developing a treatment plan, members of the health care team should pay particular attention to the preferences and needs of patients whose education or cultural traditions may impede effective communication (see Chapter 7 for additional discussion). Certain cultures have strong beliefs about pain and its management, and members of these cultures may hesitate to report unrelieved pain or may have specific preferences for pain-relieving measures. When developing a treatment plan, clinicians should be aware of the unique needs and circumstances of patients from different age groups or various ethnic and cultural backgrounds.
Patients can experience acute or chronic pain from their cancer, diagnostic procedures, treatment, or preexisting conditions. Thus, patients should be carefully assessed to ensure that the cause of pain is established whenever possible and treated appropriately.
Some causes of cancer pain are relatively easy to diagnose and treat (e.g., pathologic fractures). However, clinicians treating patients with cancer should also be able to recognize readily the common pain syndromes that may cause intractable pain and that may signal disease recurrence in order to optimize therapy and minimize the morbidity of unrelieved pain. Furthermore, because many intractable pain problems involve neurologic structures (e.g., epidural spinal cord compression; metastatic brachial and lumbosacral plexopathy), prompt recognition and treatment of these syndromes may also minimize neurologic impairment (Elliott and Foley, 1989).
Multiple myeloma and cancers of the breast, prostate, and lung account for a large majority of bone metastases. The most common sites of bone metastasis include the vertebrae, pelvis, femur, and skull. Distal extremity metastases are uncommon (Malawer and Delaney, 1989). The most frequent symptom is pain, although 25 percent of patients with bone metastases have no symptoms (Wagner, 1984). Pain may result from direct tumor involvement of bone with activation of local nociceptors, or compression of adjacent nerves, vascular structures, and soft tissue. Because patients often have multiple sites of bone metastases, multiple areas of pain are common. Pain is usually described as dull and aching, is usually localized to the area of metastasis, and is increased by movement. However, spine metastases may impinge upon nerve roots and result in radicular pain. Patients with metastases to the base of the skull may complain of headache; pain on head movement; and face, neck, or shoulder pain (Greenberg, Deck, Vikram, et al., 1981). Besides pain and immobility, complications of bone metastases include fractures, hypercalcemia, and spinal cord compression. Pathologic fractures occur most commonly in cancers of the breast, lung, kidney, and thyroid and in multiple myeloma, usually in the proximal femur or humerus (Oda and Schurman, 1983). Hypercalcemia is most often observed in cancers of the breast, lung, and kidney and in multiple myeloma.
The diagnosis of bone metastasis is established by radiographic confirmation and, rarely, biopsy. Radionuclide scintigraphy and magnetic resonance imaging are the most sensitive means of detecting bone metastases, often demonstrating abnormalities before those seen on plain radiographs. Plain.radiographs showing typical lytic, blastic, or mixed lesions are usually diagnostic and easily distinguished from lesions resulting from nonmetastatic causes (Wilner, 1982). However, plain radiographs and bone scintigraphy may be negative early in the course of myeloma, in some osseous metastases, and at sites of previously radiated bone (Kelly and Payne, 1991). Magnetic resonance imaging may be helpful in such cases when bone involvement is suspected.
Epidural metastasis is the most ominous complication of bone metastasis to the vertebral spine and is a medical emergency. Failure to diagnose and treat this condition will lead to permanent neurologic deficits due to spinal cord dysfunction. Early diagnosis, before overt neurologic deficits, should result in improved outcome (Byme, 1992). Epidural metastasis is a common complication in patients with breast, prostate, or lung cancer; multiple myeloma; renal cell carcinoma; or melanoma. The tumor enters the epidural space by contiguous spread from adjacent vertebral metastases in the vast majority of cases (Rodriguez and Dinapoli, 1980). The remaining cases arise from the direct invasion of retroperitoneal tumor or tumor located in the posterior thorax through adjacent intervertebral foramina or, rarely, from bloodborne seeding of the epidural space. The pain is usually midline, but patients whose tumor involves nerve roots have sharp or shooting pain in a radicular distribution. Untreated, the pain slowly intensifies with a mean duration of 7 weeks from the onset of pain to the onset of neurologic deficits due to spinal cord compression (Gilbert, Kim, and Posner, 1978). Signs of spinal cord compression include motor, sensory, and autonomic (e.g., bladder and bowel) dysfunction.
More than 70 percent of patients with spinal cord compression have an abnormal plain radiograph in the region of pain (compression fracture, blastic, or lytic metastases) (Portenoy, Lipton, and Foley, 1987). Because pain is such a reliable early sign, epidural metastases can often be diagnosed and treated before neurologic deficits develop. Patients with persistent back pain in the region of abnormality on plain spine radiograph, with or without neurologic deficits, should undergo evaluation with MRI. Patients with progressive back or neck pain whose plain radiograph is normal should also undergo an imaging study of the epidural space, even if their neurologic examination is normal. Administration of analgesics and corticosteroids constitutes the mainstay of pharmacologic therapy. Radiation therapy or surgical excision followed by radiation therapy are the two standard treatments.
Table 4 lists common metastases to the skull, which often cause pain in patients with cancer.
| Table 4. Metastases to the skull | |
| Type of metastases | Signs and symptoms |
| Middle fossa syndrome | Similar to trigeminal neuralgia, i.e., numbness, paresthesia, and pain referred to the second or third divisions of the fifth nerve, except that objective signs of neuropathy, e.g., corresponding sensory deficits and masseter weakness, may be present. Diplopia, dysarthria, headache, and dysphagia may develop as well. |
| Jugular foramen syndrome | Occipital pain often radiating to the vertex and ipsilateral shoulder or neck; may be accompanied by local tenderness and. exacerbation with movement of the head. Neurologic signs consistent with dysfunction of cranial nerves IX through XII and Homer's syndrome may be present. Lancinating throat pain (glossopharyngeal neuralgia) has been observed in association with the above symptoms or as the sole complaint. |
| Clivus metastases | Vertex headache exacerbated by neck flexion; may be accompanied by either unilateral or bilateral cranial nerve dysfunction (IV through XXII). |
| Orbital metastases | Retro-orbital or frontal headache often with diplopia, visual loss, proptpsis, and extraocular nerve palsies. |
| Parasellar metastases | Symptoms similar to those of orbital metastases. |
| Sphenoid sinus metastases | Bifrontal headache radiating to both temples with intermittent retro-orbital pain. Nasal stuffiness, diplopia, and a unilateral or bilateral VI cranial nerve palsy may be present. |
| Occipital condyle invasion | Severe occipital pain that is exacerbated by movement and that may be accompanied by XII cranial nerve dysfunction. |
| Odontoid fractures | Usually caused by tumor or metastasis to atlas bone. Risk of spinal cord compression due to vertebral instability. |
| Source: from Elliot and Fotey, 1989; Greenberg, Deck, Vikram, et al., 1981. | |
Cervical, brachial, and lumbosacral plexi can be sources of intractable pain in cancer patients (Elliott and Foley, 1989). Pain is produced when these structures are infiltrated by tumor or compressed by fibrosis after radiation therapy to adjacent structures. Pain tends to be less prominent in radiation induced plexopathies than in tumor-related ones. Traction injury related to the positioning of a patient during a prolonged operation may also produce brachial plexopathy.
Pain originating in the cervical plexus often occurs as an aching discomfort that may radiate into the neck and occiput. It is most commonly caused by metastases to the cervical lymph nodes or the local extension of primary head and neck tumors.
Brachial plexopathy is a common complication of breast and lung cancer and lymphoma, but it can also be caused by metastasis to the brachial plexus from a remote primary tumor (Kori, Foley, and Posner, 1981). Pain occurs in up to 85 percent of patients with brachial plexus involvement and may precede weakness or sensory loss by months (Foley, 1987). When the upper plexus is damaged by tumor, pain usually begins in the shoulder and is associated with shooting or electrical sensations in the thumb and index finger. When the lower plexus is involved, as is more common, pain begins in the shoulder and radiates into the elbow, arm, and medial forearm, and into the fourth and fifth digits. In about 25 percent of patients, both upper and lower divisions are involved. Compared with tumor-related plexopathy . radiation damage to the brachial plexus causes less severe pain, distributed initially in the upper division.
Epidural extension may occur in up to 50 percent of patients with superior pulmonary sulcus ("Pancoast") tumors (Kanner and Foley, 1981). Epidural disease is more likely to occur when the entire plexus is involved and Homer's syndrome is present, which indicates medial and paraspinal spread of tumor. Lymphoma may produce brachial plexopathy and spinal cord compression in the absence of vertebral body erosion. CT and MRI of the brachial plexus and epidural spaces are the diagnostic procedures of choice, and are essential to define the extent of disease and to determine the appropriate radiation ports.
The lumbosacral plexus, embedded in the psoas muscle, may be invaded by tumors of the abdomen and pelvis. Colorectal, endome-trial, and renal cancers, as well as sarcomas and lymphomas, may invade this plexus by direct spread. However, 25 percent of lumbosacral plexopathies are metastatic (Jaeckle, Young, and Foley, 1985). Pain is usually felt in the lower abdomen, buttock, and leg. Infiltration of the sacral plexus may produce perineal and perirectal pain, which is exacerbated by sitting and lying prone. Pain typically precedes, by weeks or even months, the neurologic signs of weakness, sensory loss, or urinary incontinence. Abdominal and pelvic CT or MRI may provide the diagnosis and allow definition of radiation portals. Similar to patients with brachial plexopathy, patients with diffuse or bilateral lumbosacral plexus involvement may have an epidural extension of tumor, in which case, MRI of the epidural space is also required. Epidural disease of the cauda equina or leptomeningeal tumor may produce a clinical syndrome similar to lumbosacral plexopathy (Elliott and Foley, 1989).
Pain may precede overt neurologic signs in spinal cord compression, plexopathies, and spinal metastasis. Prompt recognition of these syndromes and institution of appropriate treatment can avoid paralysis and incontinence.
Peripheral nerves can be compressed or infiltrated by tumor or constricted by fibrosis, which in rare instances is a complication of radiation treatment. They may also be damaged by neurotoxic chemotherapy or by cutaneous incisions and the retraction of tissues during surgery (Table 5).
Myeloma may cause a progressive painful neuropathy in about 15 percent of patients. In as many as 8 of 10 such patients, neuropathy precedes the onset of other symptoms (Davis and Drachman, 1972). This sensorimotor neuropathy is characterized by distal paraesthesias, sensory loss, weakness, and muscle wasting, and it may occasionally ascend upward in a manner similar to Guillain-Barre syndrome.
| Table 5. Common cancer pain syndromes due to peripheral nerve injury | ||
| Pain syndrome | Associated signs and symptoms | Affected nerves |
| Tumor infiltration of a peripheral nerve | Constant, burning pain with
dysesthesia in an area of sensory loss. Pain is radicular and often unilateral |
Peripheral |
| Postradlcal neck dissection | Tight, burning sensation in
the area of sensory loss. Dysesthesias and shocklike pain may be present. Second type of pain may occur mimicking a drooped shoulder syndrome. |
Cervical plexus |
| Postmastectomy pain | Tight, constricting,
burning pain In the posterior ami, axilla, and anterior chest wall Pain exacerbated by arm movement |
Intercostobrachlal |
| Postthoracotomy pain | Aching sensation In the
distribution of the incision with sensory loss with or without autonomic
changes Often exquisite point tenderness at the most medial and apical points of the scar with a specific trigger point Secondary reflex sympathetic dystrophy may develop |
Intercostal |
| Postnephrectomy | Numbness, fullness, or
heaviness in the Dysesthesias are common |
Superficial flank |
| Postlimb amputation | Phantom limb pain usually
occurs after pain in the same site before amputation Stump pain occurs at the site of the surgical scar, several months to years after surgery. It is characterized by a burning dysesthetic sensation that is exacerbated by movement |
Peripheral endings and their central protections |
| Chemotherapy-induced peripheral neuropathy | Painful paresthesias and
dysesthesias Hyporeflexia Less frequently: motor and sensory loss; rarely, autonomic dysfunction Commonly associated with the vinca alkaloids, cisplatin, and Taxol |
Distal areas of peripheral (e.g., polyneuropathy) |
| Radiation-induced peripheral nerve tumors | May promote malignant
fibrosarcoma Painful, enlarging mass hi a previously Irradiated area Patients with neuroflbromatosis more susceptible |
Superficial and deep |
| Cranial neuropathies | Severe head pain with
cranial nerve dysfunction Leptomeningeal disease Base of skull metastasis |
Cranial V. VII, IX, X, XI, XII are most common |
| Acute and postherpetic neuropathy | Painful paresthesia and
dysesthesia Constant burning and aching pain Shocklike paroxysmal pain Immunosuppression from disease or treatment is a risk factor; postherpetic neuropathy Incidence Increases with age |
Thoracic and cranial (VI) are most common |
|
Note: See Chapters 3,4, and 5 for treatment of cancer-related neuropathies. Source: Adapted from Fotey, 1985b; Kanner. 1985; Payne 1985. |
||
Vincristine, cisplatin, and taxol produce dose-related peripheral neuropnthies. usually manifested as dysesthesia in the feet and later (as the neuropathy progresses) in the hands; continuous burning pain is rarely a problem. Vincristine neuropathy may also give rise to cranial neuralgias, including jaw claudication. Treatment of chemotherapy-related neuropathy involves decreasing or stopping the offending agent (when possible) and the use of analgesics.
In the absence of recurrent tumor, persistent pain following surgery may result from intraoperative injury to cutaneous or deeper nerves. Postsurgical pain syndromes are characterized by either persistent pain after the surgical procedure or recurrent pain after the initial surgical pain has resolved. The clinical characteristics relate to the location and extent of nerve injury (Kelly and Payne, 1991). Treatment of these syndromes involves the use of analgesics and, occasionally, regional nerve blocks.
| Table 6. Common causes of abdominal pain |
| Obstruction of small or large bowel. |
| Occlusion of blood flow to visceral organs (e.g., liver, kidney, large and small bowel). |
| Thrombosis and engorgement of splenic or renal veins. |
| Omental metastasis. |
| Volvulus of the small intestine. |
| Infectious or chemical peritonitis. |
| Metastasis or lymphomatous liver distention. |
Varicella-zoster virus infection or reactivation ("shingles") is more likely to occur in patients with cancer than in the general population because of the higher incidence of immunosuppression in the former. Zoster neuralgia may cause acute and chronic pain (Rusthoven, Ahlgren, Elhakim, et al. 1988). Disseminated zoster is twice as likely to occur in patients with progressive tumor than those in remission (Rusthoven, Ahlgren, Elhakim, et al., 1988). Thoracic and cranial dermatomes are most commonly affected, and the incidence of psotherpetic neuralgia (pain after healing of rash) increases with age (Watson, Evans, Reed, et al., 1982).
Varicella-zoster virus infection is characterized by a burning, aching pain. Lancinating or shocklike pain may be superimposed in the area of the crusted (or healed) herpetic skin lesions, in which there is usually sensory loss. Hyperpathia may be profound. For acute zoster, antiviral therapies in combination with analgesics are recommended. For postherpetic neuralgia, antiviral therapies are of limited use, and therapies for neuropathic pain are used (see Chapter 3). Empiric observations suggest that nerve blocks during acute herpes zoster infection reduce pain, shorten the acute episode, and prevent the emergence of postherpetic neuralgia (Bonica, 1990). Treatment approaches for neuropathic pain are discussed later (see also Figure 1).
Abdominal tumors are frequently characterized by pain that is colicky, worse after eating, and associated with nausea. Pain may be referred widely throughout the abdomen to distant cutaneous sites (e.g., shoulder, neck, and back). Patients with tumors of the small or large intestine occasionally have a combination of obstruction, pain,and hematemesis or rectal bleeding. Common causes of abdominal pain for these patients are listed in Table 6.
| Table 7. Assessment of mucositis |
| Examine lips and all mucosal surfaces for number, size, and location of lesions. Pain intensity is usually related to the degree of tissue damage. |
| Include assessment of local edema and erythema as well as preexisting periodontal disease that may also be painful. |
| Ask patient to identify painful or burning areas; even if there is no apparent tissue damage, these may become involved later. |
| Culture suspicious lesions to rule out concomitant infection (bacterial, viral, fungal) that may intensify pain and delay healing. |
| Evaluate patient's ability to swallow (including oral analgesics), and restrict oral intake if necessary. |
| Repeat the assessment frequently because clinical signs and symptoms may change. |
Patients with cancer are vulnerable to developing the same nonmalignant medical and surgical causes of abdominal pain, such as appendicitis, cholecystitis, and pancreatitis, as are individuals without cancer. Opioid analgesic therapy is often constrained in this group of patients by nausea, constipation, and ileus related to tumor-related bowel obstruction. Treatment of abdominal pain with nerve blocks is discussed in Chapter 5.
Mucositis can occur in any patient receiving cytotoxic chemotherapy or radiation to the head and neck. In patients receiving chemotherapy, the incidence and severity of mucosal toxicity is influenced by the individual drugs, their dosages, and the schedule of their administration. Preexisting poor oral hygiene may also contribute to mucositis. Pain is often intense and interferes with oral intake. Chemotherapy-induced mucositis usually begins 3 to 5 days after therapy is started, reaches its peak at 7 to 10 days, and slowly resolves over the next 5 to 7 days unless complicated by infection or hemorrhage (Dreizen, 1990). Clinical signs of mucositis include diminished mucosal thickness and keratinization, superficial sloughing, and ulceration.
Radiation of the oropharyngeal and esophageal mucosa results in predictable inflammatory effects, usually appearing at the end of the second week of treatment, plateauing during the fourth week of radiation, and sometimes persisting for 2 to 3 weeks after the completion of treatment (Baker, 1982). Initially, Ihe mucosa in the path of radiation appears reddened and swollen; as treatment continues, the mucosa can be covered with a fibrous exudate.
In both chemotherapy- and radiation-associated mucositis pain, intensity is related to the extent of tissue damage and the degree of local inflammation. Typically, the patient describes a burning sensation, often accompanied by erythema. Because clinical signs and symptoms may change, patients with mucositis should be assessed frequently (Table 7). Management involves the aggressive use of analgesics (such as systemic patient-controlled analgesia) and agent-specific antimicrobial agents (Epstein, 1990; Janjan, Weissman, and Pahule, 1992).
Pain assessment is an ongoing process requiring constant attention to new pain (see Figure 1). Changes in pain patterns or the development of new pain should not be attributed to preexisting causes but should instead trigger diagnostic evaluation. New pain may signal treatable problems such as infection or fracture. A change in pain often signals advancing disease, and because pain management relies on the treatment of the underlying disease, establishing a medical diagnosis with the criteria discussed earlier is critical. A 1992 report showed that a comprehensive pain assessment revealed new causes of pain in 64 percent of 270 oncology patients with new pain complaints; most of the new diagnoses were neurologic (Gonzales, Payne, Foley, et al., 1992). Thus, the need to reassess persistent pain to identify new causes cannot be overemphasized.