| Contents | Previous | Next |
The gastrointestinal (GI) and genitourinary (GU) (elimination) health system is concerned with the elimination of waste products from the body. The nutrition health system is the complement to elimination and addresses the intake of nutrients and related metabolic processes. Patients presenting to the ED frequently are experiencing nausea, vomiting. diarrhea or constipation, abdominal pain, dysuria, and other problems in elimination. Although alterations in nutritional status are not seen as frequently in the ED, anorexia, impaired swallowing, and hypo- or hyperglycemia are encountered regularly.
|
Gastrointestinal-Genitourinary (Elimination) Health System |
||
| BOWEL | URINARY | RENAL |
| constipation | elimination | dialysis |
| diarrhea | incontinence | diuresis |
| elimination | hesitancy | electrolyte balance |
| incontinence | retention | fluid balance |
| ostomy | toileting | renal function |
| renal stones | ||
| INGESTION | DIGESTION | ABSORPTION AND ASSIMILATION | METABOLISM |
| Caloric intake | Abdominal distention | Electrolyte balance | Carbohydrate |
| Chewing | Food tolerance | Fluid volume | Fat |
| Diet | Gastric drainage | Hydration | Glucose |
| Nourishment route | Gastric enzymes | Nutritional status | Hormones (excluding sexual) |
| Oral mucous | Gastric motility | Weight | |
| Membranes | Nausea | Protein | |
| Self-care; feeding | Vomiting | ||
| Swallowing |
Gastrointestinal-Genitourinary (Elimination) Health System
dysreflexia
constipation
perceived constipation
colonic constipation
diarrhea
bowel incontinence
altered patterns of urinary elimination
stress incontinence
reflex incontinence
urge incontinence
total incontinence
urinary retention
toileting self-care deficit
Nutrition Health System
altered nutrition: more than body requirements
altered nutrition: less than body requirements
altered nutrition: potential for more than body requirements
fluid volume excess
fluid volume deficit
altered oral mucous membrane
feeding self-care deficit
impaired swallowing
ineffective breast-feeding
| Gastrointestinal-Genitourinary (Elimination) Health System | |||
| Level I | Level II | Level III | Level IV |
| Diarrhea; VS normal, not ortho-static | Abdominal pain with diarrhea; VSS, no orthostasis | Abdominal pain with diarrhea; orthostasis or other clinical signs of dehydration; fever > 101 °F | Severe abdominal pain with diarrhea and/or abnormal VS |
| Rectal itching, pain-mild; non-thrombosed hemorrhoids | small to moderate; VS wnl; thrombosed hemorrhoids | Tarry stools or bright red blood per rectum (BRBPR); VSS, no orthostasis | Tarry stools or BRBPR with orthostasis; hypotension, tachycardia, tachypnea; rectal prolapse |
| Constipation | Constipation; recent abdominal surgery | Constipation with severe abdominal pain | |
| Difficulty in starting stream of urine | Penile swelling; unable to void < 6 hr; no discomfort | Penile swelling; unable to void 6-12 hr; moderate discomfort | Inability to void for 12 or more hr |
| Dysuria; VS wnl; frequency, urgency | Dysuria; febrile < 102°F with or without hematuria | Dysuria; febrile > 102° F; nausea and/or vomiting with or without hematuria; no orthostasis | Gross hematuria; severe flank pain; positive orthostasis; pain suggestive of renal colic |
| Dialysis patient, in no acute distress, with unrelated complaint | Dialysis patient peritoneal dialysis (PD) with mild abdominal complaints; afebrile | Hemodialysis patient with abdominal complaints, febrile < 102°F; dialysis patient, problems with fistula | Hemo-or PD patient with altered mental status, seizure, syncope, cardiac chest pain, respiratory distress, bleeding, or fever > 102°F; PD patient with abdomi¬nal pain, febrile, nausea and/or vomiting |
| Vomiting, febrile, Dysuria, and/or urgency, with abdominal or flank pain; CVA tenderness | |||
| Abdomen distended, asymptomatic | Abdomen distended, peripheral edema, no respiratory distress; diminished bowel sounds; has not moved bowels or passed flatus | Abdomen diabetic; respiratory distress; unable to void > 12 hr | |
|
Nutrition Health System |
|||
| Requests glucose check; asymptomatic; Chemstrip wnl | Known diabetic, c/o fatigue and weakness; Chemstrip < 250 | Known diabetic, insulin-dependent; feels "funny"; VS stable, abnormal S and A, Chemstrip; history (hx) of vomiting and diarrhea | Known diabetic, insulin-dependent; change in mental status; respiratory rate decreased; Kussmaul respirations; VS abnormal |
| Vomiting; VS wnl; no orthostasis, no pain | Mild abdominal pain with vomiting; VSS; no orthostasis; streaks of blood in emesis | Moderate abdominal pain, guarding; vomiting and diarmea; VS wnl but orthostatic changes or other clinical signs of dehydration; temp>101°F | Severe abdominal pain; active hematemesis; VS abnormal, hy-potension; rigid, boardlike abdomen |
| Dysphasia; no suspicion of foreign body; no acute onset; no drooling or respiratory distress | Dysphasia; suspicion of foreign body; no respiratory distress | Dysphasia with respiratory distress | |
James Jay Hoelz, RN, MS, CEN
Robert is a 35-year-old white male who arrives in the ED on Sunday afternoon. Robert complains of right upper quadrant abdominal pain that he has had since awakening 4 hr ago. He slates he has vomited 6 times with no relief of his pain after vomiting. He describes the pain as sharp in nature and rates it 5 on a scale of 1 to 10. He describes no hematemesis or change in bowel or urinary habits. He denies chest pain but complains of mild shortness of breath. Robert has no previous medical problems but does admit to a 20-year history of alcohol use. He admits to drinking 2 to 3 six-packs of beer every day and 1/2 to 1 pint of whiskey on the weekends. He has had pain similar to this in the past but not as severe as the present episode.
Physical examination of the patient performed by the nurse at triage reveals a thin male, somewhat disheveled in appearance. He is sitting upright, leaning forward, clutching his right upper abdomen. His speech is normal and his manner is slightly anxious but cooperative. His vital signs are temperature 100.2° F. respirations 22, sitting pulse 110. sitting BP 146/75; standing pulse 130: standing BP !30' 60. His skin is warm and dry and his Glascow coma score is 15. His abdomen is diffusely tender with increased tenderness to palpation in the right upper quadrant. There is an odor of alcohol on Robert's breath.
Triage Assessment, Acuity Level 111: moderate to severe abdominal pain. vomiting, vital signs with 20 points orthostasis.
When the patient is brought to the treatment area, blood studies show an elevated serum amylase, an elevated WBC count, and prolonged clotting times. Robert's ABG shows a Pa02 of 76, a PaCO2 of 38, and a pH of 7.36. A diagnosis of acme pancreatitis is made by the physician.
Pancreatitis is an autodigestive disease resulting from premature activation of pancreatic enzymes. Damage that occurs within the acinar cells of the pancreas leads to an increased release and activation of pancreatic enzymes. This release of enzymes leads to local inflammation and necrosis (1). The inflammation and necrosis cause the sharp, unrelenting pain of pancreatitis. The irritation causes vomiting that does not relieve the pain (2). The pain associated with pancreatitis reaches a high intensity over a period of several hours and can persist for hours to days (3).
Pancreatic enzymes, once released, can circulate in the system at large. These enzymes can cause damage to the pleura of the lungs. Coagulopathies of the pulmonary microcirculation can occur and lead to respiratory compromise. Atelectasis, pleural effusion, and hypoxia are often complications of acute pancreatitis (1.4).
Pancreatitis can also cause electrolyte imbalances with related patient symptomatology. As the pancreatic enzymes cause necrosis, calcium soaps are formed from fats and can lead to hypocalcaemia. Circulating enzymes and necrosis can also cause damage to the (3 cells of the pancreas and lead to hypoglycemia from increased insulin production (5). Conversely, the serum glucose could be elevated.
In acute pancreatitis, pain is usually present and severe. It is often midepigastric, but may be difficult to localize. The pain often radiates to the back and is usually described as constant, not colicky. The patient can gain some relief of pain by leaning forward or squatting on all fours, as gravity will pull the abdominal viscera away from the inflamed pancreas. Nausea and vomiting are common symptoms. Constipation could occur, but is rare.
The patient's history review should include questions that explore the many etiologies of pancreatitis. Particularly important are a history of gallstones; use of steroids, thiazides, or oral contraceptives: endocrine disorders such as diabetes mellitus or hyperparathyroidism; or alcohol abuse (6).
Lab values that are useful in diagnosis include an elevated serum amylase, WBC count, glucose, calcium, liver function tests, and ABGs.
The serum amylase rises for the first 24 to 48 hr after onset of acute symptoms and then will begin to fall. This pattern is a useful diagnostic tool in patients who seek early medical attention (1). In some instances the serum amylase may actually be normal either due to chronic calcific pancreatitis (the pancreas can no longer produce amylase) or because of rapid clearance of the amylase by the kidney (6).
TIP: An elevated serum amylase is not exclusively diagnostic of pancreatitis. Other intra-abdominal events can also elevate the serum amylase such as perforated ulcer. perforated gallbladder, ruptured ectopic pregnancy, or diabetic ketoacidosis.
Alcohol seems to increase the likelihood of damage to the acinar cells of the pancreas, thereby causing an increase in the release of destructive enzymes. Alcohol has a direct effect on inflammation and. as such. can cause necrosis. Alcohol can also directly increase pancreatic enzyme secretion (3). Together these can trigger acute pancreatitis. Robert has presented with a 20-year history of alcohol abuse.
Studies show that patients who have used alcohol to excess for 5 to 10 years or more are prone to chronic pancreatitis. It is likely that this attack of acute pancreatitis is the first manifestation of a chronic process for Robert. He could stop drinking, but he might continue to experience episodes of pancreatic pain. However, these attacks may occur less frequently than if he were to continue his present drinking pattern (7).
Robert's problems during the ED visit center around his pain. fluid loss, and lack of knowledge about his condition. In addition, he has psychosocial problems related to his drinking that will require further analysis. For this ED visit the nurse will address the physical problems and knowledge deficit.
Diagnosis: Fluid volume deficit related to fluid loss from vomiting
Desired patient outcome: The patient will have no orthostasis. systolic BP > 90 mm Hg, HR < 100 beats/min, urinary output > 30 ml/hr, good skin turgor, and moist mucous membranes: the patient will have relief of vomiting.
Diagnosis: Potential for impaired gas exchange related to coagulopathies in pulmonary microcirculation that could lead to hypoxemia
Desired patient outcome: The patient will have ventilation and gas exchange maintained; the patient will have clear breath sounds, respiratory rate 12 to 24, and pink mucous membranes.
Diagnosis: Pain related to inflammation and necrosis of pancreas
Desired patient outcome: The patient will state that there is relief or reduction of pain; the patient will not exhibit nonverbal cues of discomfort.
Diagnosis: Knowledge deficit related to the diagnosis of pancreatitis and the implications of this diagnosis to life-style changes
Desired patient outcome: The patient will describe the symptoms of pancreatitis and how to treat himself; the patient will describe the relationship between alcohol intake and its effect on the pancreas
After consultation with the physician, the nurse should provide for vigorous intravenous hydration and management of pain for the patient with acute pancreatitis. A nasogastric tube should be inserted to keep the stomach free of irritants and reduce vomiting and distension. An intravenous line should be established and fluid replacement begun. Strict intake and output measures should be taken to assess for adequate urinary output. The nurse should examine the patient's skin turgor and mucous membranes for clinical signs of fluid deficit. Hourly monitoring of the patient's vital signs should occur, and hypotension, tension tachycardia, and orthostatic changes should alert the nurse to inadequate hydration.
Robert's ABG results and his shortness of breath indicate a need for supplemental oxygen. The nurse should check with the physician for an order for oxygen therapy. The nurse should assess the patient's lip color and nail beds for cyanosis and any changes in mental status for early signs of hypoxemia. Breath sounds should be assessed even 2 hr. Rate and character of respirations, chest movement, presence or absence of adventitious sounds, and use of accessory muscles should be noted. Repeat ABGs should be obtained as ordered by the physician to assess the outcome of the interventions.
The insertion of the nasogastric tube may also help with pain management by reducing pancreatic stimulation and episodes of vomiting. Pain medication will also be required.
TIP: Demerol is recommended over morphine since morphine may cause spasms of the pancreatic biliary duct (8) and spasms of the sphincter of Oddi. increasing pain (1).
Robert's teaching plan should include the disease process and answers to any questions he might have. If Robert is to be discharged from the ED, he should have an understanding of the effect alcohol has on his pancreas and his increased risk if he continues to drink. He needs to be aware of the signs and symptoms of pancreatitis and how to manage his own care at home, but he also needs to know when to return to the ED. He should be instructed in pain management techniques such as massage, relaxation, and distraction. He should also be given information on the use and side effects of any analgesics that may be prescribed. Robert should receive information on the importance of diet and nutrition versus the "empty" calories of alcohol. Finally, he should be assisted to find a primary care provider for regular follow-up.
Kathleen Keenan, RN, MS, CCRN
Mrs. A. is a 60-year-old white female whose family called an ambulance because she was very weak and was having trouble talking. Field interventions by the emergency medical technicians (EMT) included oxygen, consultation, and transport. Intravenous access was unobtainable. The impression of the EMTs was that Mrs. A. was experiencing a cerebral vascular accident (CVA).
On arrival in the ED, Mrs. A. is a wake but unable to respond verbally. She weakly attempts to follow commands. Her pupils are equal and reactive to light, and her upper and lower extremity strengths are 1/4 bilaterally.
Mrs. A. 's past medical history, obtained from her daughter, includes type II diabetes mellitus, two MIs, and peripheral vascular disease. She was admitted to the hospital in 1986 for a femoral-popliteal bypass graft without complications. Mrs. A.'s daily medications include 40 units of NPH insulin every morning, digoxin. Lasix, and Persantine. She has no known allergies. Mrs. A. 's daughter says the signs and symptoms of weakness and slurred speech developed over several days and became most noticeable this morning. Mrs. A. 's vital signs at triage are BP 146/84. HR 122, and respiratory rate 22. Her skin is pale, diaphoretic, and cool. A finger stick blood glucose level obtained at triage is 24 mg/dl. The patient is immediately brought back to the treatment area for definitive therapy.
TIP: Any patient presenting with an altered level of consciousness should immediately have a finger stick blood glucose level checked. A patient presenting with primary psychiatric complaints may be at high risk for missed hypoglycemia. A seizure patient should also have an immediate blood glucose level performed since the seizure may be the presenting sign for hypoglycemia.
Triage Assessment, Acuity Level IV: Known diabetic, insulin-dependent with change in menial status, vita/signs abnormal, blood glucose below normal.
Mrs. A. is a type II, insulin-dependent (exogenous) diabetic. Type I insulin-dependent diabetics require insulin injections to prevent diabetic ketoacidosis (DKA). A type I diabetic, with no endogenous insulin, cannot metabolize glucose without the aid of exogenous insulin via injection. In the absence of insulin, a type I diabetic metabolizes fats which produces ketosis. A type II insulin-dependent diabetic requires insulin for control of hyperglycemia. This type of diabetic has enough endogenous insulin to be able to use glucose instead of fats as a fuel source but not enough insulin to sufficiently metabolize glucose and prevent hyperglycemia. Both type I and type II diabetic patients are at risk for hypoglycemia from their use of insulin and variations in life-style, diet, and health status.
Fasting hypoglycemia is the most common type of hypoglycemia and can result from use of such drugs as insulin, sulfonylureas, propranolol, excessive use of acetylsalicylic acid (ASA), and alcohol. Fasting hypoglycemia is also associated with hepatic failure, sepsis. and severe malnutrition, among other pathologies. Hormones involved in glucose homeostasis include growth hormone, cortisol, and adrenaline.
The signs and symptoms that occur with hypoglycemia may be divided into two categories: adrenergic and neuroglycopenic. The signs and symptoms that develop are related to the speed of the serum glucose drop, the severity of the decrease, and the duration of the event. Because cerebral cells are highly dependent on glucose metabolism, symptoms generally cluster around the neurological-cerebral effect.
Adrenergic signs and symptoms are related directly to the sympathetic nervous system's (SNS) response to an acute, rapid drop in blood glucose level. These symptoms include weakness, faintness, tremors, palpitations, sweating, hypertension, flushing, hunger, and nervousness. Neuroglycopenic symptoms occur when the fall in blood sugar is gradual or less profound. Symptoms include lethargy, personality changes, motor weakness, confusion, inability to concentrate, slurred speech, seizures, paralysis, and headache. These symptoms result from decreased cerebral oxygen uptake as the prolonged fall in blood glucose progresses.
Besides the etiologies already described, such as some medications. alcohol use, and some pathologies, other factors to consider when evaluating the cause of hypoglycemia for a particular patient include the type, amount, and method of administration of insulin; diet; and the eating and exercise habits of the patient. Knowing the type of insulin a patient is using helps the nurse to understand the time of peak action and duration of action.
In addition, the nurse should explore with the patient if the type of insulin being used has changed from beef or pork insulin to the newer form of insulin preparation known as Humulin. Since insulin preparations contain a number of protein impurities, a patient frequently develops antibodies to the insulin. Beef and pork insulin are known to be immunogenic, and each patient's dose of insulin is adjusted according to the individual's resistent response. The newer Humulin insulin is a more purified substance generating a reduced immunogenic response and therefore a more potent response at lower dosages. A patient whose type of insulin has been changed from a beef or pork preparation to Humulin may require an overall reduction in dose and frequency of insulin use for maximal effect and prevention of hypoglycemia.
Another concern in evaluating a patient about insulin use is to determine if the patient has changed the site of administration. If the patient switches from an area with poor absorption (poor perfusion from repeated injections) to an area of good absorption (an area with good perfusion and more rapid delivery of the drug into the circulation), the dose of insulin may actually be too high.
Patients who miss or delay meals, or eat meals lower in calories than what their insulin is intended to cover, are also at risk for hypoglycemia. The most common cause of increased insulin requirement is obesity. For the obese patient who has had insulin adjustments upward to manage this glucose load, diet adjustments should only be made with the supervision of their physician so that insulin adjustments can be made as well.
Patients who consume large amounts of alcohol have impaired glyconeogenesis in the liver which is associated with depleted glycogen stores. Compounding this effect, patients who consume a large amount of alcohol usually do not eat correctly. This combination also affects the requirement for insulin and could result in hypoglycemia.
TIP: When caring for alcoholic patients, stress the importance of eating while they are drinking to prevent hypoglycemia.
When questioning Mrs. A. about her insulin administration and her activities over the last several days, the nurse discovered that Mrs. A. had recently begun exercising in an attempt to lose weight. Mrs. A. also admitted that she had not been following her 1800-calorie-per-day diet very well. She had been eating beyond her calorie restriction for several months, causing her to put on weight. This prompted her decision to resume her 1800-calorie diet and begin exercising this past week. She had not sought medical assistance. This change in diet and activity both contributed to Mrs. A.'s hypoglycemia. which would be considered a fasting hypoglycemia.
TIP: When patients present to the ED with complaints unrelated to their diabetes. take the opportunity in your nursing assessment to include the areas of diet. exercise, insulin administration, and glucose monitoring. Reinforce the need for medical supervision if the patient wishes to change any of these.
Nursing diagnoses appropriate to the hypoglycemic patient relate primarily to the emergent problem of decreased level of consciousness. Following successful treatment, the patient's knowledge deficit should be assessed and managed. The following nursing diagnoses apply to Mrs. A.
Diagnosis: Potential/or ineffective airway clearance related to decreased level of consciousness
Desired patient outcome: The patient maintains a patent airway and is free of the complications of aspiration, Atelectasis, and hypoventilation as evidenced by normal respiratory rate and depth, and absence of adventitious breath sounds.
Diagnosis: Potential for injury related to decreased level of consciousness and increased potential for seizure activity
Desired patient outcome: The patient will remain free of injury as evidenced by freedom from falls and complications of seizures, and by an intact musculoskeletal system.
Diagnosis: Potential for decreased cardiac output related to current stress effect and history of MI
Desired patient outcome: The patient will remain in sinus rhythm and have an adequate cardiac output as evidenced by warm, dry skin, systolic BP> 90mm Hg. HR 100 beats/min. urinary output >= 30 ml/hr; and the patient will be oriented to person, place, and time.
Diagnosis: Knowledge deficit related to signs and symptoms, etiologies, and treatment of hypoglycemia
Desired patient outcome: The patient and daughter will state the causes, signs, symptoms, and self-treatment for hypoglycemia, as well as the indications for calling 911 to bring the patient to the hospital. The patient will use appropriate resources (i.e., Visiting Nurses Association (VNA) and private physician) for continuing follow-up and assistance in dealing with a chronic illness (i.e., proper insulin use, diet, and exercise, allowing for tight glucose control).
As with any physiological crisis, the ABCs have top priority. In the hypoglycemic patient, the ABCs are followed by D for dextrose. Hy-poglycemia is an easily treatable problem that should be quickly diagnosed because of its potential for poor outcome. A patient with a decreased level of consciousness should be assessed for a patent airway. and cough and gag reflexes. The patient should be positioned on her side, with the head of the bed slightly elevated. Suction should be readily available. Side rails should be kept up, and the stretcher's wheels locked at all times. If the patient is restless or confused, a vest restraint is indicated to prevent injury.
Reversal of the lowered blood glucose is important to prevent further patient compromise such as seizures and cardiac arrest. Glucose may be ordered by the physician and administered intravenously or orally. The patient should be observed for the development of arrhythmias (SNS response), seizures, and coma. Because Mrs. A. has a cardiac history, she was placed on a cardiac monitor. An intravenous line was inserted. Mrs. A. was given oxygen at 4 liters/min via nasal cannula. She was administered two premixed-predosed syringes of glucose of 25 g each intravenous push. Within minutes. Mrs. A. had complete resolution of symptoms.
TIP: After treatment for hypoglycemia, the patient should be observed for signs and symptoms of recurrent hypoglycemia. If the patient is to be observed for an extended period of time, the appropriate meal tray should be ordered for the patient so that hypoglycemia will not recur.
The patient's level of consciousness and the severity of the SNS response need to be considered when determining which is the appropriate route for administration of glucose. If the patient's level of consciousness is depressed, as was Mrs. A.'s. or if the SNS response is extreme, the intravenous route is preferred.
Glucose is usually ordered in doses of 25 g. Twenty-five g of glucose equals 100 calories. The designation D5o indicates a 50% solution of glucose and water, which means that there are 50 g of glucose in 100 ml of solution or, equivalently, 25 g of glucose in 50 ml of solution. The usual dose of glucose for an average-sized adult is 25 to 125 g. If the initial dose of glucose has not reversed the symptoms, more glucose should be administered. The usual response to intravenous glucose occurs within 1 or 2 min.
Patients who are alert and able to protect their airways, and who are not exhibiting signs of marked SNS stimulation, can safely take glucose orally. Since glucose absorption occurs in the small intestine, the goal is to administer a glucose-containing drink in a form that will travel from the stomach to the small intestine as soon as possible. Sugar water or honey water is a palatable drink with a rapid GI transit time. The response to oral glucose occurs within several minutes. If the patient remains unresponsive to several administrations of glucose, consider another cause for the patient's signs and symptoms.
TIP: Prior to and during the administration of Dso, the nurse should aspirate on the plunger of the syringe to confirm proper line placement by blood return. If Dso infiltrates, it is very irritating to the tissues and could cause tissue sloughing.
Mrs. A. was discharged from the ED within 3 hr. The reason for her hypoglycemia (exercise and diet) had been discovered, and appropriate discharge instructions and follow-up appointments had been given to the patient and family. In addition, the data collected in the ongoing nursing assessment supported the decision for discharge. The patient was able to maintain a serum glucose level between 100 and 150 mg/dl (checked at hourly intervals); the patient was alert and oriented; the patient and her family were able to describe the signs and symptoms of hypoglycemia, and they described the appropriate actions to take if hypoglycemia reoccurs.
If the cause of Mrs. A.'s hypoglycemia had not been so readily discovered, an in-depth assessment of the cause would have been indicated. If insulin or other drugs were suspect, the patient would not have been stable for discharge and may have required hospital admission for further testing and evaluation.
Alspach J, Williams S: Core curriculum for critical care nurses. Philadelphia: Saunders 1985.
Carpenito L: Nursing diagnosis: application to clinical practice, 2nd ed. Philadelphia: Lippincott, 1987.
Dornbrand L, Hoole A, Fletcher R, Pickard C: Manual of clinical problems in adult ambulatory care. Boston: Little, Brown, 1985.
Hudak C, Lohr T, Gallo B: Critical care nursing, 4th ed. Philadelphia: Lippincott, 1985.
Kennedy P, Gerich J: Hypoglycemia: separating fact from fiction. Diagnosis 10(1): 61-70,1988.
Rosen P: Emergency medicine. St. Louis: Mosby, 1988.
Safrit H: Diagnosis: hypoglycemia. Hosp Med 17(2):37-44, 1981.
Kathleen Keenan, RN, MS, CCRN
Mr. B. is a 26-year-old male who presents to the triage desk appearing acutely ill and very lethargic. The triage nursing assessment reveals sitting BP 110/88. pulse 120, and respirations l4anddeep. Mr. B.'s standing vital signs are BP 80/60, pulse 155, and respiratory rate 16.
Mr. B.'s chief complaints are nausea, vomiting, thirst, and abdominal pain. Mr. B. relates a history of type I insulin-dependent diabetes mellitus. Because the patient is lethargic, the triage nurse asks the patient about drug and alcohol use, which he denies. The triage nurse also notes a dressing on the patient's arm covering an extremely infected abrasion that the patient relates to an injury which occurred 10 days before. Additional physical assessment findings include a weak, rapid pulse; slow, deep respirations; and a dry, furrowed tongue. A finger stick blood glucose level obtained at triage is greater than 500 mg/dl. Mr. B. also states he has been having polyuria and polyphagia prior to the onset of nausea and vomiting.
Triage Assessment. Acuity Level IV: Known diabetic, insulin-dependent, with orthostatic hypotension, pulse rate > 155, blood sugar > 500 mg/dl.
Mr. B. is brought immediately to the treatment area. Initial lab results are sodium 150 mEq/liter, potassium 6.2 mEq/liter, phosphorous 0.9 mg/dl, bicarbonate 10 mEq/liter, and ABG values of PaO2 =92, PaCO2 = 26, pH = 7.32.
Medical and nursing treatment focuses on the reversal of the hyperglycemia, dehydration, and electrolyte imbalance, and the treatment of infection. Mr. B. is given 2 liters of NS over the first hour of his ED stay, and then NS with 40 mEq of potassium chloride per liter at 500 ml/hr. After an initial bolus of 10 units of regular insulin intravenous push, Mr. B. is started on an insulin drip at 8 units per hr. Three hours later, Mr. B. has the following: 20 points orthostasis by pulse and BP, urine output of 20 ml/hr, lungs clear, improved alertness, serum glucose of 300 mg/dl, and positive serum ketones. Because Mr. B. remains acutely ill he is transferred to the intensive care unit.
Diabetic ketoacidosis is a complex pathophysiological state in which the diagnosis as well as the treatment center around reversing the hyperglycemia, ketoacidosis, and dehydration. The presence of hyperglycemia alone does not constitute DKA. Hyperglycemia combined with serum ketones signifies that fat, instead of glucose, is being metabolized resulting in metabolic acidosis. This combination of factors is essential to the diagnosis.
DKA is usually associated with a transient glucose intolerance that is the result of some stressful event. The precipitating stressor may be an infection, occasionally an MI, or noncompliance with the treatment plan. The event increases the body's production of glucagon, cortisol, and growth hormone as well as catecholamines, resulting in an overproduction and underutilization of glucose. The production of these hormones and catecholamines results in an increase of free fatty acids which produce ketones and ultimately metabolic acidosis. The glucose utilization and production, and the altered release of insulin produce severe hyperglycemia causing osmotic diuresis and dehydration to occur. Osmotic diuresis occurs through the renal tubules.
Normally the renal tubules do not "see" glucose because it is reabsorbed before it reaches the tubules. With hyperglycemia, the excess glucose exceeds the renal threshold. Some glucose is reabsorbed, but the remainder is circulated to the tubules. Because the presence of glucose makes the tubules hypertonic, large amounts of water enter the tubules to help dilute the hyperosmolar state. The glucose and water combination is then excreted in the urine. Large amounts of water and potassium are lost in this manner causing dehydration. Dehydration combined with acidosis can lead to a decreased level of consciousness for the patient, hypotension, altered respiratory pattern (Kussmaul's respirations), nausea, and vomiting.
TIP: When in doubt about the reason for a patient's decreased level of consciousness, treat as if it were caused by hypoglycemia. Administer 25 g of glucose. If the diagnosis is incorrect, the added glucose can be easily treated with regular insulin.
Since the diagnosis of DKA is based on the presence of hyperglycemia, ketoacidosis, and dehydration, the patient's presenting signs and symptoms relate to these three states. The patient will often describe signs of the presence of the three Ps: polyuria, polydipsia, and polyphagia. Polyuria (osmotic diuresis) leads to dehydration. Mental status changes occur from brain cell dehydration and may vary from minimal changes to coma. The slow, deep character of Kussmaul's respirations are part of the body's compensatory buffer mechanism for metabolic acidosis. These respirations can be associated with a fruity odor but should not be used as a diagnostic tool since it can be confused with the odor of alcohol and may not be present at all. The complaints of abdominal pain, nausea, and vomiting are not unusual. and are usually related to the electrolyte imbalance and acidosis. Unless a patient has an additional process going on, abdominal findings will be negative.
TIP: Documentation of baseline and ongoing abdominal assessments is important so as not to overlook acute abdominal pathology. Keeping the patient from eating or drinking will help to limit the amount of nausea, vomiting, and discomfort for the patient.
Treatment of DKA is managed through rehydration, electrolyte replacement, and insulin therapy. Dehydration and hyperglycemia can be reversed with fluid administration. Fluid administration will decrease the blood glucose level even without the administration of insulin. Fluids accomplish this by promoting glucose excretion in the urine.
NS is suggested as the initial fluid to be given. The amount and speed of the fluid replacement must be determined by the patient's status and past medical history. Most patients with severe dehydration will receive initial fluid replacement with 1 to 2 liters of NS over the first hour.
Potassium replacement is determined by the patient's initial serum potassium level. If the patient is hypokalemic, replacement should begin immediately. With normal or elevated potassium levels, replacement is usually required within the first few hours after improvement in the patient's perfusion status. Potassium phosphate may be an alternate replacement to potassium chloride thus increasing both potassium and phosphate levels simultaneously.
TIP: Potassium replacement of 10 to 15 mEq/hr or as much as 20 to 40 mEq hr may be needed depending on the degree of deficit. The patient should be on a cardiac monitor and observed for untoward cardiac arrhythmias that occur with hypo-and hyperkalemia.
Only fast-acting regular insulin should be used to treat patients with DK.A. Insulin may be administered intramuscularly, subcutaneously. by intravenous bolus, or by intravenous continuous drip. Regular insulin administered intramuscularly has a half-life of about 2 hr. This half-life generally results in a decline in blood glucose of approximately 80 to 100 mg/dl/hr. The intramuscular dose is usually 10 to 20 units per hour. If peripheral perfusion is poor in a hypotensive or dehydrated patient, the potential for poor tissue absorption should preclude the use of intramuscular insulin. There is also the risk of delayed hypoglycemia if perfusion improves and a large amount of insulin is absorbed later.
Regular insulin administered intravenously has a half-life of 20 min. Repeated intravenous bolus doses of insulin should range from 10 to 25 units per hour. Because the half-life of intravenous insulin is short. intravenous continuous drip administration is recommended. Intravenous insulin was chosen for Mr. B. because of his dehydrated state.
The insulin drip should be prepared according to hospital and pharmacy policies and procedures. In this case a drip of 100 units of regular insulin was added to 100 ml of NS to deliver 1 unit per ml. making the dosage calculation quite simple.
In administering insulin, it is important to remember that the goal of insulin therapy is not to reverse hyperglycemia. but to correct ketosis. The progressive fall in the serum glucose level should be monitored closely, along with interval monitoring of serum ketones. Insulin is still indicated to continue control of blood glucose. but it may be administered in a smaller dose or, if given by the intravenous bolus method, at longer intervals. Because Mr. B. still had ketones in his blood after 3 hr, insulin therapy was continued at 2 units per hour.
TIP: The impairment in glucose metabolism is reversed much quicker than the ketoacidosis. Even though the blood glucose may be only slightly elevated (250 mg/dl), the patient still needs insulin for the reversal of acidosis. At this glucose level the patient will require the administration of glucose in order to prevent hypoglycemia.
Mr. B.'s hand wound was suspected as the precipitating cause for his DKA. His wound was fully cultured to determine the presence of any occult infection. Wound dressings and antibiotics were started immediately. Mr. B. was given a thorough physical exam to determine if there were other causative factors such as other areas of infection. A review of his diet and use of insulin was also indicated.
Nursing care for the patient with DKA is a multifaceted problem. Attention must be paid not only to treating the presenting problem, but also to preventing complications of therapy. The following nursing diagnoses apply to Mr. B.
Diagnosis: Fluid volume deficit related to osmotic diuresis from hyperglycemia
Desired patient outcome: The patient will maintain BP and HR within normal limits, both on resting and on orthostatic assessment as evidenced by systolic BP > 90 mm Hg; HR < 100 beats/min; and urinary output > 30 ml/hr. Lab results, if available, will indicate a normal serum glucose (60 to 110 mg/dl), a normal serum osmolality (275 to 295 mOsm/liter), and a normal serum sodium (135 to 145 mEq/liter); the patient will have good skin turgor and moist mucous membranes.
Diagnosis: Altered cerebral, renal, and peripheral tissue perfusion related to dehydration
Desired patient outcome: The patient will demonstrate improved cerebral, renal, and peripheral perfusion as evidenced by an appropriate response to stimuli (awake and oriented); warm, dry skin; pink mucous membranes; urine output at least 30 ml/ hr; and strong peripheral pulses.
Diagnosis: Infection related to dirty arm abrasion
Desired patient outcome: The patient will be free of infection as demonstrated by normothermia, intact nondraining skin, and clear lungs.
Diagnosis: Potential for injury related to decreased level of consciousness
Desired patient outcome: The patient will remain free of injury as evidenced by freedom from falls and an intact musculoskeletal system.
Diagnosis: Potential/or ineffective airway clearance related to a decreased level of consciousness
Desired patient outcome: The patient will maintain a patent airway and be free of the complications of atelectasis, aspiration, and hypoventilation as evidenced by normal respiratory rate and depth, and absence of adventitious breath sounds.
Diagnosis: Knowledge deficit related to the signs and symptoms of DKA and its causes
Desired patient outcome: The patient and family will state the causes and prevention of DKA, including the early signs and symptoms of an elevated blood glucose level.
Ongoing nursing assessment is critical in the care of these highly complex patients. Particular attention must be paid to identifying precipitating factors, response to fluid therapy, and the development of the potential complications of hypokalemia and hyperkalemia. Elderly diabetics are particularly prone to having an MI that may be masked by the other presenting symptomatology. A 12-lead ECG should be performed early in the treatment course and analyzed for tall, peaked T waves, and widened QRS's. The patient should be placed on a cardiac monitor and observed for arrhythmias such as PVCs indicative of hypokalemia. The patient's vital signs should be recorded at least every 15 min for the first hour and, if stable, once every hour thereafter. Urinary output should be monitored and recorded to determine that hydration and renal perfusion are adequate. The nurse should be alert to the presence of an infection, particularly of the lungs, feet, toes, and urine. The patient should be assessed for compliance with the treatment plan, and a patient teaching plan conducted, or community referrals made- For Mr. B., the nurse in the intensive care unit will continue the treatment plan and reinforce the education that was started in the ED related to infection control and signs and symptoms of DKA.
Carpenito L: Nursing diagnosis, application to clinical practice. 2nd ed. Philadelphia: Lippincott, 1987.
Foster D, McGarry J: The metabolic derangements and treatment of diabetic ketoacidosis. N Engl J Med 309(3) : 159-169, 1983.
Guyton A: Textbook of medical physiology 7th ed. Philadelphia: Saunders, 1986.
Kappy M: Avoiding the pitfalls in managing diabetic ketoacidosis. Emerg Med Rep 4(15):89-94, 1983.
Lipson A: Diabetic ketoacidosis: why man- agement is never routine. Drug Therapy (11):65-75, 1981.
Lueg M: Hyperglycemic dehydration: a swift but thorough approach to this subtle disorder. Consultant (11 ):91 -100. 1981.
Sauve G: A primer on insulin use. Postgrad Med 82(3): 176-179. 1987.
Swearingen P, Sommers M, Miller K: Manual of critical care. applying nursing diagnoses to adult critical illness. St. Louis: Mosby. 1988.
Tressler K: Clinical laboratory results. Englewood Cliffs: Prentice-Hall. 1982.
Whitehouse F: Diabetes mellitus: current noses concepts of proper management. Hosp Med 22(5):231-247. 1986.
Polly Thornton, RN
Mr. Thode, a 68-year-old Caucasian male, is brought to the ED in a wheelchair: he is pale, diaphoretic, and restless. He is nauseated and vomits approximately 100 ml of clear liquid immediately upon arrival. He is complaining of severe, diffuse abdominal pain.
With his family's cooperation, a triage history and assessment is completed. Mr. Thode stales that he is allergic to penicillin and iodine. His BP has been normal on past routine physical examinations. At this time his BP is 154/90. pulse 96 and regular, respirations 22. and temperature 98.6° F. Mr. Thode stales that he has been in considerably good health, although he had prostate surgery 8 years ago. Two days ago he experienced a sudden, sharp pain in the right flank area which resolved itself almost immediately. The pain was less severe and more localized than [he pain he is experiencing this evening.
Triage Assessment. Acuity Level IV: Severe abdominal pain: pain suggestive of renal colic and/or renal stone.
The patient is taken immediately to the treatment area and prepared for a complete physical examination. The nursing assessment confirms a normal rectal temperature of 99.6° F. The patient's BP did rise to 180/90 with an increase in pain. The physical assessment of the patient confirms that he has normal bowel sounds. no abdominal distention or rigidity, and no palpable masses. Pulses (femoral, popliteal. posttibial. and dorsalis pedes) are palpable and of equal intensity bilateral!)'. Mr. Thode is still experiencing severe abdominal pajn. The rectal examination indicates that blood is not present in the stool. The patient is put on a cardiac manner. and intake and output measures are recorded.
Blood work is obtained and sent to the lab for CBC. electrolytes, BUN, creatinine, amylase, bilirubin. PT, and PTT. The results are reported as normal. An intravenous line of Ringer's lactate is instituted promptly with a no. 16-gauge catheter In addition, a large bore catheter with a heparin lock attached is inserted in anticipation of a possible transfusion and as an access port for intravenous medications
A 12-lead ECG shows an RBBB. However, there are no previous tracings available for comparison. Chest and abdominal x-rays are negative. A clean-caught urine sample is tested with a chemstrip in the ED: the test indicates there is blood in the urine. A urine specimen is sent to the lab which confirms mild RBCs. occasional WBCs. negative protein, 3+ glucose, and few bacteria. The assessment data supports the conclusion that the patient's pain is originating in the urinary system.
Approximately 1 hr after vigorous hydration, the patient, although exhausted, is suddenly almost pain-free. His color has improved and his BP has dropped to 134 90. Because of Mr. Thode's allergy to iodine, an intravenous pyelogram is not considered. Rather, an emergency CT scan of the abdomen is done. This procedure rules out an abdominal aortic aneurysm and confirms no gross evidence of hydronephrosis.
Mr. Thode's urologist is contacted, and arrangements are made for the pattern !o see him in the morning. Explicit instructions are given to the patient concerning adequate hydration, the necessity of straining all urine, the saving of any questionable stones, and the possible reoccurrence of pain. Ten 50-mg Demerol tablets arc given to the patient to lake home. Additional blood studies are drawn for calcium. phosphorous, and uric acid. These reports will be forwarded to his doctor.
The ED staff made a follow-up call to the patient the next afternoon. That morning, when Mr. Thode voided through the strainer provided by the ED nurses, he passed a 1.5-cm stone. He took the specimen to the urologist. who forwarded it 10 a laboratory for analysis. The patient was put on a low-calcium diet for several days while further testing was done. One week later the urologist also ordered a 24-hr urine collection from the patient on an outpatient basis to determine calcium and uric acid content.
Urinary calculi occur most often in sedentary individuals, in males rather than females (3:1), between the ages of 30 to 60. and in whites. Calculi are more likely to occur in persons living in the Southeast or the Southwest, followed by the midwest, and more so in persons living in hot. dry climates, and during the summer. Other predisposing factors include diet. recent immobilization (e.g.. traction), abdominal surgery, metabolic disease, hypertension, gout. and strong family history. Patient history may include use of the following medications that may precipitate stone formation: antibiotics, allopurinol, anti high blood pressure medications, sodium bicarbonate, phosphates. and thiazides (1,2).
The stones themselves are called calculi: the actual stone formation is lithiasis. Calcium oxalate is the most common stone, occurring more frequently in men than in women. Although it is usually small, it can be trapped in the ureter. Idiopathic in nature, the stone formation is not dependent upon the urine pH. Therapeutic measures include increased hydration, reduced dietary oxalate, use of thiazide diuretics, and phosphate and calcium lactate therapy.
Struvite (magnesium ammonium phosphate) is 3 to 4 times more prevalent in women. It is usually of staghorn formation, crumbles easily, and is always associated with urinary tract infections. Struvite is the second most common type of stone. Surgical intervention is often needed to remove this stone. Antimicrobial agents and urine acidification are also considered as therapeutic measures.
Calcium phosphate stones are usually mixed, with proportions of struvite and calcium oxalate. Predisposing factors would include persons with alkaline urine and hyperparathyroidism. Treatment is the same as for calcium oxalate and struvite stones.
Uric acid stones are commonly found in men, especially Jewish males. Gout is a predisposing factor. These stones form only in acidic urine. Uric acid stones cannot be seen on x-ray without dye enhancement. Therapeutic measures include reducing the uric acid in the urine and the administration ofallopurinol and sodium citrate. A diet low in caffeine, theobromine, and other purines is ordered.
Cystine stones are formed by a genetic recessive defect which causes malabsorption and excessive concentration of cystine in the GI tract and kidneys. They are formed only in acid urine, sometimes joining to form staghorn stones. Medications considered are Dpenicillamine and sodium bicarbonate. A decrease in intake of milk products and a high fluid intake are advised (2, 3).
Stones, depending upon their composition, may be either radiopaque (cystine) or radiolucent (pure uric acid calculi). Also. a small object (in this case. a 1.5-cm stone) could be concealed easily if it were lined up with a bony prominence such as the iliac crest (4).
Many calculi present no symptoms for years. But when a calculus obstructs one or more calyces, the renal pelvis, or the ureter, severe back pain and/or renal colic may occur. The movement of the calculus will stimulate increased flank or costovertebral angle tenderness. The pain. which can be excruciating, can radiate across the abdomen along the ureter, down to the genitalia, and to the inner thigh. Nerve plexus and vascular density in the area increase the likelihood of diffuse, exaggerated pain. Mr. Thode was experiencing the passage of a stone through the right ureter at the time of admission, manifested as right flank pain. Complications, in the event of obstruction, include hydronephrosis and infection.
TIP: Drug addicts have mimicked these symptoms well in their quest for narcotics A nursing assessment that includes detailed history taking and knowledge of risk factors for stone formation is very important.
For the patient with a renal stone, the following six nursing diagnoses should be considered.
Diagnosis: Pain related to the obstruction and movement of the renal calculus
Desired patient outcome: The patient describes relief or a significant decrease in the pain experienced.
Diagnosis: Altered pattern of urinary elimination (frequency), related to the presence of renal calculi in the ureter and the bladder
Desired patient outcome: The patient describes relief of or a reduction in urinary frequency.
Diagnosis: Potential for altered renal tissue perfusion, related to the obstruction of urine flow from the kidney, causing pressure in the kidney
Desired patient outcome: The patient maintains normal renal tissue perfusion as evidenced by a systolic BP > 90 mm Hg and urinary output > 30 ml/hr; the skin remains warm and dry.
Diagnosis: Fluid volume deficit related to nausea, vomiting, and di-arrhea
Desired patient outcome: The patient will have adequate intake (at least 3000 ml/day) and proportionate output: the patient will describe relief of nausea and vomiting.
TIP: The patient should not be excessively hydrated when having an intravenous pyelogram.
Diagnosis: Anxiety related to concern and apprehension about pain and its cause
Diagnosis: Knowledge deficit concerning the pathophysiological process involved concerning renal calculi
Desired patient outcome: The patient verbalizes a working knowledge of the etiology, signs, symptoms, treatment, and prevention of his disease.
Nursing interventions include assessing and documenting the patient's pain—its intensity, frequency, duration, and location. Findings are reported to the attending physician. Medication is evaluated and documented. Ureteral colic is relieved by narcotics, and antispasmodics alleviate ureteral spasms.
TIP: If an intravenous pyelogram is to be done, the patient should sign the consent form prior to the administration of narcotics.
To maintain adequate hydration accurate intake and output documentation should occur. Urinary frequency is common because the bladder is stimulated by the calculus. Renal or ureteral irritation. caused by the movement of the stone, may induce hematuria. A clean-caught or catheterized urine specimen for routine urinalysis and for culture and sensitivity to rule out (or to confirm) a urinary tract infection should be obtained.
Any obstruction in the kidney or the ureter can cause a backup of urine. The patient will experience back pressure, decreased blood flow, and decreased urinary output, which will stimulate renin production. Monitor the patient's BP, since a decrease in blood flow can result in acute tubular necrosis. Renin production will raise the BP in an attempt to increase renal blood flow. Monitor lab values for elevated BUN and creatinine, and for a decreased hemoglobin and hematocrit due to over hydration.
The nurse should observe the patient's level of consciousness. Decreased movement, incoherence, and slow response to commands or to painful stimuli can indicate an accumulation of wastes and electrolytes due to inadequate filtration. These waste products and electrolytes could prove toxic to the CNS.
Renal colic causes a generalized abdominal and pelvic irritation. Nausea, vomiting, and diarrhea are frequent complaints of persons with urinary calculi. Emesis can be controlled with hygiene (mouth care) and antiemetic medications. The patient may have clear liquids and a bland diet if his tests are completed and his nausea has subsided.
Physical and emotional support must be provided for the patient. Explain the likely cause of the pain, that the stone is moving, and that. hopefully, he will be relieved of it soon. Medicate the patient on schedule or as indicated. Delaying relief of pain only intensifies it. and the patient becomes more apprehensive. When the pain has subsided, the patient can cope more easily with this illness. Assure the patient that all tests ordered are completed as rapidly as possible and that the physician is notified of the results.
The patient should be instructed in the mechanism for retrieval of the calculus. He is instructed how to use the urinary strainer and is provided with one. The nurse should emphasize the importance of retrieving any stone which might be passed, no matter how small. The nurse should advise the patient to place such findings in a dry container (if passed at home), not in alcohol, oil, or other base. Laboratory analysis will help to confirm the probable cause of the stone formation (1,2, 4).
The nurse should discuss with the patient and his family the importance of adequate daily fluid intake to help prevent stone formation. Six to eight liters of water may be needed. Cranberry juice will help to acidify the urine and prevent the formation of stones. If the patient is sedentary, more exercise is encouraged.
Strict adherence to a prescribed diet—usually reduced in calcium and phosphorous and low in purines—will help to reduce the reoccurrence of urinary calculi (Table 4.4.1). A high fluid intake dilutes the urine. An acid and ash diet forms slightly acid urine and helps to prevent phosphate stones. The ingestion of cranberry juice during an infection helps to form acid urine (2, 5, 6).
The patient must be urged to take appropriately prescribed drugs conscientiously.
By speaking with the patient in lay terms and with sincere concern. the nurse can help him to better understand the pathophysiology, treatment, and prevention of further stones.
There are two new procedures for removal of kidney stones that do not require major surgical intervention. The nurse should know what these are to help educate the patient and to alleviate some fear and anxiety. These are percutaneous lithotripsy (PUL) and extracorporal shock wave lithotripsy (ESWL).
Although lithotripsy would not be routine in an ED setting, it provides an innovative alternative course of treatment for the person suffering from hydronephrosis and/or severe infection due to the presence of renal calculi. In the past, the patient frequently had to withstand the trauma of major surgery and spend several weeks recuperating at home.
Table 4.4.1 Foods Containing High Amounts of Purine, Calcium, and Oxalate
| Purine | Calcium | Oxalate |
| Bacon | Beans (except green beans) | Asparagus |
| Chicken | Cheese | Beets |
| Crab | Chocolate | Cabbage |
| Goose | Cocoa | Celery |
| Herring | Dried fruits | Chocolate |
| Kidney | Fish | Cocoa |
| Liver | Foods containing flour | Instant coffee |
| Meat soups | Ice cream | Nuts |
| Mussels | Lentils | Ovaltine |
| Salmon | Milk | Parsley |
| Sardines | Nuts | Runner beans |
| Sweetbreads | Ovaltine | Rhubarb |
| Veal | Yogurt | Spinach |
| Venison | Tea | |
| Tomatoes |
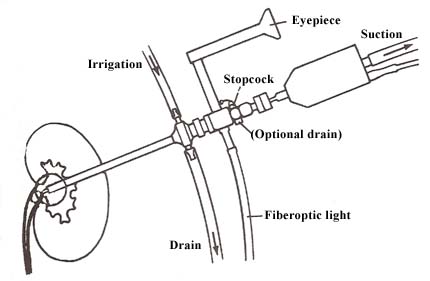
As opposed to surgery, PUL offers an easier, less traumatic means of removal by pulverizing the stone with ultrasound.
The PUL procedure (Fig. 4.4.1) involves the insertion of a nephroscope into the portion of the kidney where the stone is lodged. Initial attempts are made to grasp and break the stone without ultrasound. If these are unsuccessful, a hollow probe is used which sends out high-frequency sound waves that shatter the stone. With constant irrigation and suctioning the stone is removed.
 |
| Figure 4.4.1 Basic mechanisms of lithotriptor. (From Harwood C: Pulverizing kidney stones: What you should know about lithotripsy. RN July 1985: 35. Copyright 1985, Medical Economics Company Inc., Oradell, NJ. Reprinted by permission. |
PUL is not a painless procedure. The patient experiences more colicky, severe pain than that experienced after conventional surge?..
ESWL is entirely noninvasive. There is no surgery involved. The patient is immersed in water and the kidney stones are shattered by shock waves which are produced by an underwater electrode. The equipment for this procedure costs more than a million dollars. whereas the equipment for PUL therapy costs about $15.000. Both procedures, now being done in major university settings, are being brought into local communities. However, it is quite obvious that PUL therapy will be more popular because of its lower cost (3. 7).