2. Cardiovascular Health System
Overview
The cardiovascular health system is concerned with the supply of blood to the body tissues through the heart and blood vessels. Many patients present with either primary or secondary alterations in health status in this system. Alterations in cardiovascular status most frequently determine the patient's level of acuity and need for timely intervention regardless of presenting complaint.
Case studies selected for this section discuss patients with an alteration in red blood cells (sickle cell crisis) and with myocardial events (ischemia. infarction, and
tachydysrhythmia). Other patients who present with alterations in the cardiovascular health system but are not included in this grouping of studies include patients with peripheral vascular occlusion or disease, bleeding disorders such as hemophilia, high blood pressure, dyspnea on exertion or activity intolerance, and acute hemorrhages such as GI bleeding.
CUE WORDS
| BLOOD |
CARDIOVASCULAR |
| bleeding |
activity tolerance |
| clotting |
cardiac output |
| erythrocytes |
heart |
| plasma |
peripheral circulation |
| platelets |
tissue perfusion |
| volume |
vessels |
RELATED NURSING DIAGNOSES
activity intolerance
fatigue
potential activity intolerance
decreased cardiac output
fluid volume excess
fluid volume deficit
potential fluid volume deficit
altered tissue perfusion (specify)
Department of Emergency Medicine Triage Protocols
| Cardiovascular Health System |
| Level 1 |
Level 11 |
Level III |
Level IV |
| Chest pain, nonradiating; less than
30 years old; no personal or family hx of cardiac disease; VSS; no
associated cardiac symptoms; diaphoresis, N/V, SOB, pallor |
Chest pain, continuous, sharp,
increases with movement; associated with cough; no SOB |
Chest pain, relieved by NTG; hx
angina or cardiac disease; no current chest pain |
Chest pain, sudden onset;
diaphoresis, nausea, pallor; may or may not have cardiac hx |
| |
|
hx heart disease; peripheral edema
noted; 60 < HR < 120; c/o SOB on exertion, not currently SOB |
Pacemaker dependent, c/o weakness,
syncope; |
| |
|
|
Pulse rate more than 150 or less
than 45; |
| |
|
|
Irregular pulse rate, rapid, new
onset; symptomatic patient: weak, dizzy, hypotensive, impaired mentation,
recent syncopal episode |
| Bleeding disorder; presents for
elective transfusion; asymptomatic |
|
Bleeding disorder, presents with
(a) joint pain and/or swelling, (b) hx trauma, (c) presents for cryo-therapy |
Bleeding disorder, overt bleeding,
signs of bleeding into joints, etc. |
| |
Sickle cell patients experiencing
mild to moderate pain; afebrile; no nausea or vomiting |
Sickle cell patients now having
moderate to severe pain; may be febrile; nausea and vomiting |
Sickle cell crisis, severe pain,
symptoms of ischemia, hypoxia, respiratory distress |
| Wants BP check, entrance into
clinic system |
Hypertensive, ran out of
medication, in no acute distress; systolic < 200, diastolic < 115 |
Hypertensive, headache, no
neurological deficit; diastolic 115-130 |
Hypertensive crisis, diastolic >
130; hypertensive with weakness, chest pain, neurological deficits |
| |
Hypotensive; not symptomatic |
Hypotensive secondary to volume
deficit; orthostatic changes |
Hypotensive, shocky, unstable |
| |
|
Leg pain with decreased
circulation, decreased sensation; calf pain; warm erythematous, edematous
leg; positive Homan's sign |
Pulseless, cold, cyanotic
extremity; or same as III plus SOB |
Case Studies
2.1 SICKLE CELL ANEMIA: WHEN IT'S A CRISIS
Kathleen A. Williams, RN, BSN
H. C. is a 28-year-old black male with sickle cell anemia. He is well known to the ED staff and has visited the emergency room 3 times over the past week. He has had six visits over the past month. The precipitating cause for H.C. 's previous six visit.< appears to have been an upper respiratory infection. He has been experiencing a productive cough and low-grade fever both of which have resolved with antibiotic therapy. On H.C.'s most recent visit. 2 days ago. he described his usual crisis pain involving both knees, right hip. and back. His pain resolved with two morphine injections over a 5-hr period. He was able to tolerate oral rehydration and was discharged with a prescription for oral pain medication. Attempts to manage his pain at home with oral analgesics and fluids have been unsuccessful. He presently rate'- his pain as a 9 on a scale of I to 10. He has been experiencing intermittent nausea and vomiting.
H. C. 's previous history includes a seizure disorder due to a cerebral infarction at age 21 and a splenectomy due to a sickle cell sequestration event at age 14. H C denies any drug or alcohol abuse.
Triage Assessment, Acuity Level III: Sickle cell patient with moderate to severe pain.
Within 15 min H. C. is brought to the treatment area for further examination and intervention. On exam, H. C. appears to be in moderate to severe distress, rocking in pain on the stretcher. He is alert and oriented, pupils equal and reactive, conjunctiva pale, sclerae mildly icteric. Lung fields are clear to auscultation. Cardiac assessment is within normal limits. H.C.'s abdomen is soft and nontender with active bowel sounds. His extremities are without edema, cyanosis, pallor, or clubbing. Hi' distal pulses are present in all limbs. No priapism is observed.
H.C. denies any recent trauma to his knees, hip, or back. No deformities can he palpated, although each area is tender to touch. His orthostatic vital signs are lying BP 170/98. pulse 88. respirations 22: and standing BP 140/104 and pulse 120. Rectal temperature is 99.4° F. The labs ordered by the physician have the following results: hematocrit 22.8, white-blood-cell (WBC) count 12.1. reticulocytes 12 1. sodium 140, potassium 4.5, chloride 111. CO: 20, urea nitrogen 9, creatinine 1.7 glucose 8 2, total bilirubin 4.0, amylase67, and magnesium 1.5. These results arc not significantly different from H.C. 's lab results obtained during his visit 2 days-prior.
After consultation with the doctor, H. C. is medicated by the nurse with 8 mg morphine sulfate intramuscularly. Heating pads and pillows are applied to his knee'-and back. Because of his inability to tolerate oral fluids, an intravenous solution 1.11 5% dextrose and 0.4 5% normal saline is started at J50mi/hr. After 6 hr. H.C. 's vital signs are normal with intravenous rehydration. However, he is still unable to tolerate oral fluids and he has received two additional intramuscular morphine
sulfate injections without significant management of his pain. A decision is made by the physician to admit H.C. to the hospital with a diagnosis of vaso-occlusive sickle ceil crisis.
QUESTIONS AND ANSWERS
- What is a sickle cell crisis?
Sickle cell anemia (SCA) is an autosomal recessive disease. This homozygotic state afflicts 1 in every 500 blacks in the United States (1). The heterozygotic state of carrying the sickle cell trait is benign under normal conditions but is important from a genetic standpoint. If two individuals carrying the sickle cell trait mate, their children would have a one in four chance of having SCA (1). Therefore, patients with sickle cell trait need appropriate genetic and family planning counseling.
SCA is caused by abnormalities in the hemoglobin structure of red blood cells. The abnormalities are caused by substitutions that occur in the two amino acid chains which form hemoglobin (Hb). This substitution causes the "sickling" of erythrocytes under certain conditions. Red blood cells from patients with SCA contain 80 to 100% of the abnormal Hb chain known as HbS. Patients with sickle cell trait contain 20 to 45% HbS (l).
Decreased oxygen tension will induce a sickling episode, also known as a sickle cell crisis (SCC). Events which may contribute to a decrease in 02 tension include anesthesia (by causing a transient episode of hypoxia), exercise, infection, acidosis, and dehydration (1). During periods of decreased oxygen tension the solubility of HbS is decreased due to the "sickling" or clumping of Hb. The sickled red blood cell becomes very rigid and can assume dramatic changes in shape. This change in shape will decrease the available surface area for oxygen transport. Small vessels can become partially or completely occluded by these rigid,
sickled, erythrocytes. This occlusion of the blood vessel will cause ischemia, pain, infarction, and dysfunction of the tissue distal to the occlusion (1).
Certain organ systems are particularly susceptible to dysfunction due to SCA. These include organs where blood flow is slow and highly vascular such as the spleen, liver, and renal medulla. The brain, muscle tissue, and maternal placenta are also at risk for infarction due to SCA because of high metabolic demand (1).
- What are the types of SCCs, and which type is H.C. experiencing?
Generally, there are four types of SCCs.
Sequestration is usually seen in children and is caused by blood pooling in the abdominal viscera as the result of a sickling episode. This pooling of blood will cause a dramatic drop in the patient's hematocrit and may be fatal. The organ usually involved in the sickling episode is the spleen. Often sickle cell patients have had a splenectomy as a child or adolescent due to a sequestration episode. The spleen may also infarct due to a sequestration episode leading to fibrosis and atrophy of the spleen (1-3).
Aplastic crisis is due to a temporary cessation of erythropoiesis. This usually follows an infection. The patient's blood count and reticulocyte count need to be followed closely to determine that the patient has regained the ability to produce red blood cells. An aplastic crisis is usually self-limiting (3, 4).
Hemolytic crisis is manifested by a drop in Hb due to a rapid increase in hemolysis. A patient will have an increased reticulocyte count as the body attempts to compensate by increasing erythropoiesis. These patients will have a decreased hematocrit and elevated bilirubin. This type of crisis is rare and may be associated with a concomitant deficiency of the enzyme glucose-6-phosphate dehydrogenase (G6PD). This enzyme deficiency is also prevalent in the black population in general (1-3).
Occlusive crisis is caused by ischemia or infarction from vascular occlusion. A vaso-occlusive crisis may occur anywhere in the body but is common in long bones, joints, and the spine. Abdominal pain from an occlusive crisis must be differentiated from an acute surgical abdomen. An occlusive crisis may lead to a cerebral vascular accident. kidney infarction, lung infarction, bone necrosis, and priapism (1,2).
H.C.'s crisis would be described as a vaso-occlusive crisis in his knees, hip, and back, supported by his physical exam, labs, and history. His abdominal exam is benign, so he is not having a sequestration crisis. H.C.'s hematocrit, hemoglobin, and bilirubin are at baseline which rules out a hemolytic or aplastic crisis.
TIP: SCA is associated with a partially compensated hemolytic anemia. Red blood cells in SCA patients have a shorter life span. Red blood cells which have sickled will hemolyze due to structural damage to the cell and stasis in areas of diminished circulation. This is the pathophysiological reason for H.C. to have chronic anemia and hyperbilirubinemia (l).
- What are the nursing diagnoses appropriate for H.C.?
Diagnosis: Pain related to ischemia of joints and bones caused by SCA
Desired patient outcome: The patient will state that his pain has been relieved to a level he can manage at home on oral analgesics.
Diagnosis: Fluid volume deficit related to inadequate fluid intake, nausea, and vomiting
Desired patient outcome: The patient will receive adequate intravenous hydration demonstrated by nonorthostatic vital signs and will tolerate oral liquids.
Diagnosis: Altered peripheral tissue perfusion related to decreased oxygen tension and transport to bones and joints caused by SCA
Desired patient outcome: The patient will have adequate tissue perfusion demonstrated by fewer complaints of pain. adequate urinary output (> 30 ml/hr), and capillary refill less than 2 sec.
Diagnosis: Anxiety related to chronicity of Illness, pain, and frequency of ED visits
Desired patient outcome: H.C. will verbalize his feelings regarding his ED visit and remain actively involved in his care and management. He will state two coping strategies to manage his pain at home.
- What are the nursing interventions related to H.C.'s nursing diagnoses?
All patients presenting to the ED need a baseline set of vital signs, a physical assessment, and a pain assessment. The physical exam was presented earlier. It is important to look for other potential medical and surgical causes besides SCC. For example a patient complaining of abdominal pain may have an acute surgical abdomen. The nurse needs to keep a high index of suspicion for other potential problems with this patient population (5).
An initial and ongoing assessment of the patient's pain level will assist in the evaluation of nursing and medical interventions. The acronym PQRST is useful in pain assessment (5).
P: Provocation—what brought about this episode?
Q: Quality—is there anything different about this episode?
R: Region—is this the usual area of your SSC?
S: Severity—what rating would you give your pain on a scale from 1 to 10?
T: Time—how long have you had this pain?
To manage the patient's pain the nurse should provide increased fluid intake, orally if possible. The patient should be encouraged to drink juices, soda, or an electrolyte solution such as Pedialyte. Intravenous fluids are recommended to be started only if the patient is unable to take fluids orally. This preserves venous access (5).
TIP: Because of the renal sequelae of SCC, many SCA patients are unable to concentrate their urine. Therefore, water will not adequately rehydrate them. This inability to concentrate their urine can precipitate a vaso-occlusive crisis. The usual fluid requirement of a SCA patient is 3 to 5 liters per day (6). That's 250 to 400 ml per waking hour.
The nurse should monitor and record intake and output, ortho-static vital signs, and skin turgor to reassess hydration. The nurse should assess for fluid overload in older patients through frequent auscultation of the patient's lung fluids (6). Comfort measures should be provided such as pillows under joints, and heating pads. Distraction or diversional activities may also help break the pain cycle. Analgesics should be administered as ordered.
TIP: Meperidine is contraindicated with SCA patients who have known seizure disorders because it lowers the seizure threshold even with therapeutic anticonvulsant medication levels (6).
A physical assessment and pain assessment should be performed within 1 hr after each dose of medication to evaluate the patient's response. The patient should be allowed to control his pain management as much as possible including dose and frequency of medication based on his known baseline, and preferred site of administration. Persistent pain, unrelieved by medication and other measures described may indicate a more serious vaso-occlusive event. Although pain is always difficult to evaluate, it should not be assumed that the patient's presentation is related to drug-seeking behavior. A sickle cell crisis is real. The patient may have reached some tolerance level for the medications being prescribed necessitating more than the usual dosage and frequency. The nurse should work with the patient and the doctor to identify alternate medications or medication combinations to help relieve the pain. As with the pain associated with an acute myocardial infarction, the pain of SSC should be relieved as quickly as possible.
TIP: The patient should be encouraged to rest or lie quietly on the stretcher. "Rocking" in pain can increase the metabolic and oxygen demands of the affected tissue by 75% and exacerbate the crisis situation (4).
The nurse's role in managing H.C.'s altered tissue perfusion includes monitoring the patient for signs of increasing pain, decreased urinary output, and clinical presentation of shock like symptoms.
- How would the nurse evaluate H.C.'s care?
Because H.C. was not achieving the desired patient outcomes after 6 hr of definitive care, the physician, in collaboration with the nurse. decided that more aggressive therapy was needed including admission to the hospital. H.C. was scheduled for a CT scan of his hip to rule out infarction and plans were made for continuous intravenous morphine infusion to provide for more adequate pain control. In preparing the patient for admission to the hospital the nurse gave a report to the inpatient unit nurse describing the nursing problems identified, H.C.'s usual coping strategies, and the status of the current desired outcomes.
REFERENCE:
- Management and treatment of sickle cell disease. U.S. Department of Health and Human Services NIH
publication, Sept.1984.
- Charache S: Treatment of sickle cell anemia. Annu Rev Med 32:195-206,1981
- Barnhart M, Henry R, Lusher J: Sickle Cell. Kalamazoo, Michigan: Upjohn Co., 1976.
- Charache S: Management of patients with sickle cell anemia in the E.R. Balti-more: Johns Hopkins Hospital
Department of Medicine. Sickle Cell Protocol. 1986.
- Bourg P, sherer C , Rosen O: Standardized nursing care plans for the emergency department. St. Louis C . V. Mosby, 166-169,1986
- Nurses' Reference Library. Emergencies. Springhouse, Pennsylvania: Springhouse Corp., 480,1985.
2.2 ACUTE MYOCARDIAL INFARCTION: THROMBOLYTIC THERAPY IN THE EMERGENCY DEPARTMENT
Valerie A. Barren, RN, BSN, CCRN, CEN
Jason R. is a 38-year-old white male who called the ED about 30 min prior so arrival to ask if he should come to the hospital. Jason said over the phone. "\fy wife's bugging me to come over there. I've got a heavy pressure in my chest, sort of like indigestion. I've had it before, but it always went away. This time I can't gel rid of it. "Jason was advised by the ED nurse to come to the hospital, preferably by ambulance.
Jason arrived by car and is noted to look pale and uncomfortable. He complains of pain in the center of his chest that feels like a heavy pressure that is now going down both arms. The pain has increased in severity since he left home. Jason rates his pain as 8 on a scale of 1 to 10. Jason is assisted to a stretcher and while lying in semi-Fowler's position has no dyspnea. His vital signs are temperature 99° F. pulse 60 and regular, respiratory rate 22, and BP 108/60. Lung sounds are clear and heart sounds are regular with normal s1 and S2. He has an extra heart sound. S4
When questioned about recent health, Jason tells the nurse that he has had
chest pain off and on for about 1 week. Jason describes the pain as a tightness or heaviness in the center of his chest under the breastbone. Jason also mentions that the pain occurs with physical exertion and goes away with rest.
Jason is married and has two children. He is a sales executive and received a promotion 1 month ago. Jason smokes about one pack of cigarettes per day. He has no previous medical history and does not take any drugs. Jason says that his father died of a heart attack and his mother has hypertension.
Triage Assessment, Acuity Level IV: Chest pain, unrelieved: pain continues at rest.
Jason is taken immediately to the treatment area to rule out myocardial ischemia or injury. A 12-lead ECG is immediately done and reveals ST segment elevation in leads I, II, III, A VF, V4, V5, and V6. T waves are inverted in VI, V2. and 1 '3. and an abnormal R wave is present in VI. The initial creatine phosphokinase
(CPK) is reported as 153 (0 to 225 is normal). The ED physician makes a diagnosis of acute inferior lateral myocardial infarction (MI). True posterior MI is also considered.
Jason is given oxygen via nasal cannula at 5 liters/min and sublingual nitroglycerin with significant reduction in his pain. After consultation with a
cardiologist. Jason is deemed a candidate for thrombolytic therapy. A lidocaine bolus is administered per protocol and a continuous infusion of lidocaine is started at 2 mg/min. Tissue plasminogen activator (t-PA) is selected as the thrombolytic agent for Jason. An intravenous bolus dose of 10 mg of t-PA is given by the physician, and an infusion of t-PA is initiated at a rate of 50 mg/hr. Jason is then transferred to the coronary care unit (CCU) for further definitive therapy and monitoring
QUESTIONS AND ANSWERS
- What is the significance of the risk factors for coronary heart disease that can be identified for Jason?
Although the mortality rate for cardiovascular disease has decreased from 39% in 1964 to 19% in 1984, 60% of the deaths related to myocardial infarction occur outside the hospital (1). Prevention of sudden death due to acute myocardial infarction (AMI) can be enhanced through efforts directed at early recognition of symptoms and risk factors. Risk factors identified for Jason include sex (incidence of AMI occurs more often in men than in premenopausal women), cigarette smoking (associated with an AMI risk 2.5 times greater than nonsmokers), and family history positive for coronary heart disease (1).
In addition, Jason seems to have experienced a recent increase in stress, related to his job promotion. Stress, hypertension, diabetes mellitus, and elevated cholesterol are all risk factors for CHD and occurrence of AMI.
Chest pain should also be recognized as a risk factor. The importance of the patient acknowledging chest pain as a potential precursor of sudden death is essential in order to reduce prehospital mortality from CHD. Educational efforts by the American Heart Association (AHA) emphasize the importance of not only recognizing risk factors. but also the importance of seeking medical intervention at the onset of symptoms. In particular, the role of the spouse or significant other is stressed; this person might encourage their loved one to seek medical attention when he or she has chest pain. Jason had chest pain for about a week before his AMI. He was reluctant to come to the hospital and called for advice only at the urging of his wife. Educational efforts need to continue to alert people like Jason to request medical help as soon as they experience chest pain.
- How does the progression of coronary artery disease relate to current theories about the pathophysiology of AMI?
The pathogenesis of coronary artery disease involves a progressive narrowing of the lumen of the coronary artery. The coronary arteries become stenotic due to an increase in the formation of plaque in the vessels. These narrowed coronary vessels give rise to the formation of thrombi.
A recent investigation of the pathophysiology of AMI demonstrated that in 80 to 90% of cases of transmural MI (through the entire thickness of the myocardium), coronary artery thrombosis was identified as the causative factor (2). Thrombus formation results in complete occlusion of the coronary artery. As blood flow and oxygen are drastically reduced to the myocardium, necrosis occurs. Research findings indicate that the process of myocardial necrosis is complete after about 6 hr (2).
- How is the diagnosis of AMI determined?
Three criteria are important for the diagnosis of AMI: clinical presentation, ECG findings, and cardiac enzyme studies. The most significant component is the clinical picture. If a person presents with symptoms of AMI, appropriate medical evaluation and treatment should be initiated even if the ECG. and/or cardiac enzymes are normal.
TIP: Careful analysis of a patient's complaint of chest pain is critical in order to develop the plan of nursing care. Use the following mnemonic to assist with collection of assessment data: PQRST (Fig. 2.2.1).
If the chest pain is associated with dyspnea. dizziness, weakness, or diaphoresis, the patient should be observed with cardiac monitoring and have further diagnostic evaluation. If ECG changes of ischemia. injury, or infarction (necrosis) are present and the patient has chest pain, the diagnosis of CHD is fairly certain.
Figure 2.2.1. Assessment of chest pain. (Illustration by Marsha A. Draa,
RN.)
|
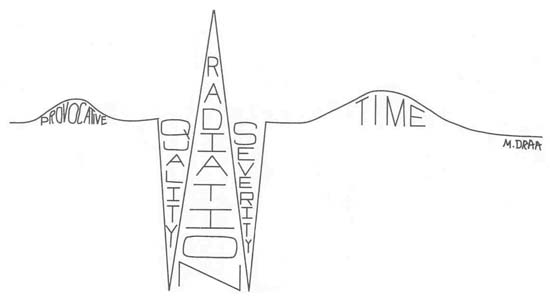 |
| P |
Provocative/Palliative (What makes pain worse/better?) Pain related to coronary heart disease is usually aggravated by exercise or
increase in physical activity. Ask patients if they noticed a pattern
associated with the chest pain. Pain associated with coronary heart-disease
typically is relived by rest or stopping physical activity. Pain of AMI is
not relived by anything.
|
| Q |
Quality (What does the pain feel like?) Cardiac chest pain is
often described as a heaviness, tightness, dullness, aching feeling,
pressure sensation, or indigation-like. The pain of myocardial infarction is
typically crushing, severe, like an elephant or truck sitting on the chest.
Patients might use a clenched fist (Levine's sign) to describe their pain. |
| R |
Reglon/Radiation (Where is the pain and where does it move to?)
The most common location of chest pain is the center of the chest, under the
breastbone (substernal) or behind the breastbone (retrosternal). Other areas
include right or left of the chest, arm (s), neck or jaw. Pain might radiate
down either arm or both arms or up into the neck and jaw. |
| S |
Severlty (How bad is the pain?) While the initial pain of
coronary heart disease might be mild, pain of myocardial infarction is
usually quite severe. Ask the patient to rate the pain on a scale such as
1-5 or 1-10. The scale is useful for the patient to quantify their pain and
the scale provides a measure for the nurse to evalute the effectiveness of
interventions to manage and relieve pain. |
| T |
Timing (What factors related to time are involved with the pain
and what other symptoms are there?) Ask the patient when the pain started,
how long does it last, how often does it occur (if intermittent) and is
there a change in the timing? Does the patient have nausea, vomitig,
diaphorsis, dizziness, and or pyspnea with the chest pain? These symptoms
are often associated with myocardial infarction. |
TIP: A normal ECG does not rule out the diagnosis of CHD or even myocardial in-farction. Initial ECG tracings may be normal in patients with AMI or angina. The 12-lead ECG should be repeated at periodic intervals, when a change in patient status occurs, and when the pattern of chest pain changes.
The 12-lead ECG can show changes associated with myocardial ischemia, injury, or infarction (Fig. 2.2.2), and these changes can occur without symptoms. For example, the patient with diabetes mellitus and related neuropathy might have a "silent" MI. In this situation the patient reports little or no chest pain, but the ECG shows AMI. This patient might present to the ED with new onset congestive heart failure (CHF). Another example involves the patient who experiences "silent" ischemia. ECG changes of myocardial ischemia are recorded during routine monitoring of an asymptomatic patient, via 24-hr Holter or ambulatory monitoring (3). This type of monitoring might be done for people who are at high risk for developing CHD but do not yet have symptoms.
ECG changes may be present only when the patient complains of chest pain, indicative of variant or Prinzmetal's angina (4). This atypical form of angina is not precipitated by factors of physical or emotional stress usually associated with angina. The symptoms occur without warning and are often short-lived. The etiology of variant angina is coronary artery spasm. When a spasm occurs in a coronary vessel, the coronary artery is temporarily occluded. The flow of blood and oxygen to a portion of the myocardium is severely restricted. At this time ECG changes of ischemia and injury are seen. When the vessel relaxes, blood flow is reestablished, and the symptoms and ECG change disappear. Serial recordings of the ECG are useful under these circumstances.
If an ECG shows any change initially, subsequent tracings can be used to evaluate patient progress. This is particularly important for the patient who receives thrombolytic therapy. If the occluded vessel is opened by the thrombolytic drug, the ECG should show resolution of the initial ischemia or injury pattern.
Various ECG leads record different surfaces of the myocardium. Leads II, III, and AVF record the inferior portion; leads I. AVL.V5, and V6 record the lateral surface; and VI through V4 reflect the anterior septal area. There is no direct lead to record the posterior surface. but changes in VI will show an abnormally tall R wave (sometimes referred to as a mirror image). ECG changes indicating right ventricular infarction can be seen with right ventricular leads. Right ventricular leads are recorded from the right side of the chest using the same landmarks as the standard precordial leads.
Figure 2.2.2. Lead ECG changes. (Illustration by Marsha A. Draa, RN.)
| Normal- |
Pt. who presents with chest pain may have normal ECG and
still have coronary heart disease. Pt. who had chest pain and is now
pain-free may have normal ECG and might have variant angina.
Obtain ECG at frequent intervals and with pain. |
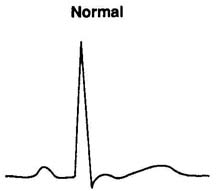 |
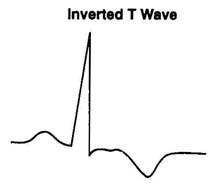
| Ischemia- |
Usually reversible Endocardial tall peaked T
Epicardial inverted T
AMI- localized to involed area, inferior, anterior, lateral
CVA, other conditions- global
Conduction alterations result in T wave changes |
 |
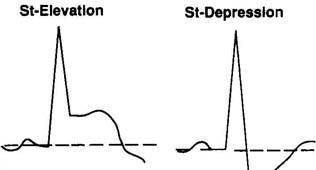
| Injury paterns |
Endocardial-depression
Epicardial-elevationDepression diagnostic of CAD during GETT
Elevation during episodes of pain only variant angina
Changes localized to area
Digoxin effect global |
 |
| Necrosis |
irreversibly dead tissue electrically silent Changes localized to
areas involved; inferior, anterior, lateral
True posterior MI mirror image in V1, tall wave in V 1 |
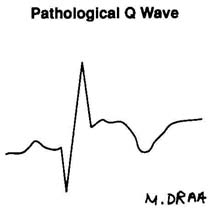 |
The third diagnostic tool of AMI is the cardiac enzyme. CPK and CPK MB isoenzymes will show changes within the first 4 to 6 hr after AMI. Cardiac enzymes are frequently not helpful for the patient presenting to the ED with chest pain of short duration since the values obtained from the initial sample drawn in the ED may be normal. However, the decision to use thrombolytic therapy cannot be delayed. Therefore, the diagnosis of AMI will be determined by the patient's clinical presentation and ECG, as demonstrated by Jason's case.
- Why was thrombolytic therapy chosen for Jason?
Thrombolytic therapy is the administration of pharmacological agents that dissolve blood clots. As mentioned above, coronary thrombosis is recognized as the etiology of AMI. Thrombolytic agents are administered to dissolve the thrombus in the coronary artery and thus reestablish blood flow through the vessel and to the myocardium. If the blood flow can be reestablished very soon after the occlusion, myocardial necrosis can be prevented. The onset of symptoms of chest pain is generally correlated with the onset of coronary artery occlusion. Since myocardial necrosis evolves during the first 4 to 6 hr after occlusion, it is critical that thrombolytic drugs are given in this time period.
The chest pain experienced by Jason began about 2.5 hr prior to his arrival in the ED. This meant that there was good potential for reversal or minirnalization of his myocardial damage.
- What are the effects, side effects, indications, and contraindications of the various thrombolytic agents currently on the market?
Currently there are three thrombolytic agents available for the treatment of AMI. The thrombolytic drugs are streptokinase. urokinase. and tissue type plasminogen activator (t-PA) or alteplase. In addition. there are two investigational drugs in clinical trials: anisoylated plasminogen-streptokinase activator complex (ASPAC, eminase) and single-chain urokinase plasminogen activator (scu-PA. prourokinase) (5).
Streptokinase is derived from the streptococcal bacteria. The thrombolytic effect produced by streptokinase is systemic since the drug combines with circulating plasminogen. Plasmin. the substance that actually dissolves fibrin clots, is then produced in the circulation. As the plasmin circulates through the body. a systemic "lytic" state develops. This means that all blood clots will be dissolved and the patient might develop bleeding problems.
Other problems associated with streptokinase are the prolonged effect on the coagulation system (18 to 24 hr after the drug is given), hypotension associated with administration of the drug. and the possibility of allergic reactions to this "foreign" substance and of resistance to the drug because of patient antibodies to streptococcus bacteria (6). Major contraindications to the use of streptokinase are recent strep infections, recent intracranial or intraspinal surgery or trauma, intra-cranial aneurysm. neoplasm or arteriovenous malformation, history of cerebrovascular accident (CVA), active internal bleeding, uncontrolled hypertension, or known bleeding tendency or disorder.
Urokinase is a naturally occurring human enzyme, found in small amounts in urine and produced by kidney cells. Urokinase converts plasminogen to plasmin in the circulation and produces a systemic lytic state much the same as the effects of streptokinase. Since uroki-nase is a natural substance, there is less chance of allergic reactions and hypotension.
Tissue plasminogen activator is a natural substance found in blood cells. It is referred to as a clot-specific or clot-selective drug since it binds with Fibrin at the site of the newly formed blood clot. This binding results in the conversion of plasminogen at the site of the clot to plasmin. The effects oft-PA then are limited to specific fresh blood clots. Side effects are minimal—bleeding from fresh blood clots might occur. Major contraindications to t-PA are similar to those for other thrombolytic agents. Other possible contraindications include surgery within the last 10 days, cerebrovascular disease, hypertension, pregnancy, and any other conditions in which the risk of bleeding is great. Patients must be evaluated individually on a risk/benefit basis.
The investigational drugs previously mentioned are modified versions of the originals. These drugs, like t-PA, are designed to be clot-specific and do not result in systemic lytic states.
- What are the specific nursing care concerns related to patients receiving t-PA?
The first concern of the nurse is to assist in the identification of patients who might be candidates for t-PA or any thrombolytic therapy. Patients presenting with chest pain must be carefully screened and evaluated according to the above criteria. If the nurse suspects that the patient might be a candidate for thrombolytic therapy, blood samples should be obtained for coagulation studies along with the other initial blood work. Blood samples should be obtained through an intravenous catheter with a heparin lock adapter to prevent open veni-puncture sites. If veni-puncture is attempted and missed, a pressure dressing should be applied and the site clearly identified. Generally three intravenous sites will be needed.
If the thrombolytic agent is not readily available in the ED. the pharmacy should be notified as soon as possible. It is imperative that the drug be administered within 6 hr after the onset of the patient's chest pain. It should be noted that thrombolytic agents are very expensive and should be handled carefully. If t-PA is the agent selected. the intravenous bolus dose should be prepared for the physician to administer and then the intravenous infusion immediately started. Intravenous infusion devices must be used to ensure the accuracy of the drug administration. Protocols may vary by hospital, but the usual dose of t-PA is 100 mg within 6 hr: an initial bolus of 6 to 10 mg followed by 50 mg administered by infusion over the first hour and 40 mg by continuous infusion over the next 2 to 5 hr.
While hypotension is usually not associated with t-PA. the patient requires close monitoring of vital signs during drug administration. Continuous cardiac monitoring will help to identify reperfusion when it occurs. During reperfusion of the myocardium (if t-PA has dissolved the clot and reestablished blood flow through the coronary vessel), a variety of dysrhythmias will be noted on the monitor. These dysrhythmias occur due to reestablishment of blood flow to a hypoxic myocardium, making cells irritable and capable of chaotic electrical impulse formation.
- What nursing diagnoses can be determined for Jason?
The following nursing diagnoses are appropriate for Jason.
Diagnosis: Potential/or decreased cardiac output related to development of lethal dysrhythmias as evidenced by premature ventricular contractions (PVCs), ventricular tachycardia, (VT), and ventricular fibrillation (VF)
Desired patient outcome: The patient maintains a normal sinus rhythm, systolic BP > 90 mm Hg, and urinary output > 30 ml/hr: the patient remains alert and oriented.
Diagnosis: Altered coronary tissue perfusion related to decreased coronary artery blood/low as evidenced by chest pain and by ischemia or injury pattern on 12-lead ECG
Desired patient outcome: The patient states that there is a decrease in and/or relief of chest pain, and 12-lead ECG shows return to baseline or normal.
Diagnosis: Anxiety related to hospitalization and potential for death as evidenced by facial tension, restlessness, and questions asked by patient
Desired patient outcome: The patient will demonstrate reduction of anxiety by relaxed facial muscles and ability to rest: the patient will describe his feelings and demonstrate coping strategies that reduce feelings of anxiety.
Diagnosis: Potential for injury related to complications of thrombolytic therapy such as bruising and bleeding
Desired patient outcome: The patient will have no evidence of bleeding and minimal bruising.
Diagnosis: Potential ineffective family coping related to onset of critical illness as evidenced by wife's questions and interaction with patient
Desired patient outcome: Wife of patient will verbalize understanding of husband's condition and support system needs. The patient and his wife will state strategies for assisting each other through this illness; the patient and his wife will state other resources available such as other supportive family members.
- What are the nursing interventions necessary to manage the care of Jason?
Prompt attention to the first two nursing diagnoses takes priority since these diagnoses involve potentially life-threatening conditions. The nurse should obtain a history of the patient's chest pain and risk factors for CHD. The patient should be assisted with lying down on a stretcher, in a comfortable position. Rest and comfortable body position can help to decrease myocardial oxygen demands. The 12-lead ECG should be recorded quickly and cardiac monitoring initiated at once. This will allow for quick detection of potentially life-threatening dysrhythmias. Myocardial ischemia, injury, or necrosis should also be quickly identified.
Since all patients with cardiac chest pain (or chest pain with undetermined etiology) are possible candidates for thrombolytic therapy. care must be taken with venipuncture as previously described. Blood samples should be sent for cardiac enzymes (including MB isoenzymes). coagulation studies, CBC, blood urea nitrogen (BUN), glucose. and electrolytes.
Oxygen is administered via nasal cannula at 3 to 5 liters/min. If the patient is known to have chronic obstructive pulmonary disease and has a tendency to retain CO;, oxygen should be given at 2 to 3 liters/ min. It is important to increase the amount of circulating oxygen to the myocardium. Reassurance and explanations about the hospital routines help to decrease anxiety and therefore myocardial oxygen demands.
Chest pain management is directed at efforts to enhance coronary blood flow. Medications of choice include nitroglycerin (sublingual. oral, topical, or intravenous); calcium channel blockers such as nifedipine: and analgesics such as morphine and meperidine. Beta-blockers might also be administered to decrease myocardial oxygen demands. Patient response to these cardiovascular medications should be monitored closely by the nurse. Frequent evaluation of chest pain and vital signs is essential. Medications are usually titrated to control chest pain as long as vital signs are stable. Communication with family and/or significant others is important in terms of aiding the family as well as patient coping. The family should be allowed to see the patient as soon as reasonably possible. Patients may benefit from familiar support systems as they make crucial decisions about their care (for example. thrombolytic therapy or choosing a physician). In addition, family members should be encouraged to call upon their own support systems. AMI is a life-threatening illness. Spiritual support should be offered and provided as requested by the patient or family member.
Emergency equipment, defibrillator, medications, airway, and ambu bag should be kept at the bedside and transported with the patient. The staff should be prepared to treat sudden cardiac arrest at any time.
The patient who comes to the ED with chest pain is usually very anxious. The notion of a heart attack is feared by the patient. Fears associated with a heart attack include fear of death and disability. Many patients fear that if they do survive, their lives will never be the same. The nurse must respond to the patient's verbal and nonverbal concerns. Honest, clear explanations should be provided for the patient and family. Concrete terminology should be used to explain what will happen to the patient. Explanations provided by the ED nurse will include a description of the CCU and the admission procedures. When the patient is transferred to the CCU. the ED nurse should accompany him and introduce him to the CCU nursing staff (7), provide a complete report, and update the nursing care plan based on the patient's response to treatment.
REFERENCES
- American Heart Association. Textbook of advanced cardiac life support. Dallas, Texas, 1987.
- Misinski M: Pathophysiology of acute myocardial infarction: A rationale for thrombolytic
therapy [Part 2 supplement].
Heart Lung 1 7(6):750-755, 1988
- Assey ME: Ischemia without symptoms. Emerg Med 20 (7):26-36, 1988.
- Schakenback LH: Prinzmetal's angina: Current perceptions and treatments. Crit Care Nurse (2):90-99, 1987.
- Kleven MR: Comparison of thrombolytic agents: Mechanism of action, efficacy, and safety. [Part 2 supplement] .
Heart Lung 17(6) : 750-755, 1988.
- Henderson E: Clinical experience with thrombolytic agents [Part 2]. J Emerg Nur
15(2):174-181,1989.
- Throwe AN. Fought SG: Psychosocial nursing care of the emergency patient. New York: John Wiley & Sons; 1984.
SUGGESTED READINGS
Belle-Isle C: Patient selection and administration of thrombolytic therapy; Part 2.
J Emerg Nurs 15(2):155-162, 1989
Conover M: Electrocardiography update:1988. J Cardiovasc Nurs 2(4): 45-52, 1988
Dracup KA. Weinberg SL, eds: Symposium proceedings: Nursing interventions in limiting infarct size in acute MI patient.
Heart Lung 16(6) (suppi): 739-800. 1988.
Erickson DE, Kleven M: Patient care guidelines, the acute myocardial infarction patient receiving tissue-type plasminogen
activator. .Emerg Nurs 14(4):253-259. 1988.
Giebl RA, Pavey SS, Bryant PP: t-PA therapy in acute myocardial infarction. J Emerg
Nurs 14(4);253-259, 1988.
Gillis CL, Sparancino PSA, Gortner SR, Kenneth HY: Events leading to the treatment of coronary artery disease: Implications for nursing care.
Heart Lung 14(4):350-356. 1985.
Funk M: Diagnosis of right ventricular infarction with right precordial leads.
Heart Lung 15(6):562-570, 1986.
Henderson E: Thrombolytic therapy in acute mvocardial infarction: An overview [Part 2].
J Emerg Nurs 1 5(2): 145-149. 1989.
Lee TH. Rouan GW. Weisberg ME. el al.: Clinical characteristics and natural history of patients with acute Ml sent home from the ED.
Am J Cardinl 60:219-2:4. 1987.
Nurses' reference library: Emergencies. Springhouse, PA: Springhouse Corporation, 1985.
Perchalski DL. Pepine CJ: Patient with coronary artery spasm & the role of the critical care nurse.
Heart Lung 16(4): 392-402,1987.
Rea RE. Bourg PW. Parker JG. Rushing D. et al., eds: Emergency nursing core curriculum. Philadelphia: W.B. Saunders, 1987.
Riegel BJ: The role of nursing in limiting myocardial infarcl size. Heart
Lung 14(3):247-254. 1985.
Schiro AG, Curtis DG: Asymptomatic coronary disease. Heart Lung 17(2): 144-14'-). 1988.
2.3 MYOCARDIAL ISCHEMIA: THE COCAINE CONNECTION
Barbara Mlynczak-Callahan, RN, MS, CCRN
Mark is a 33-year-old black male who was brought to the ED by ambulance, fie is experiencing significant chest pain, 7 on a scale of 1 to 10. that began a few minutes after he injected cocaine intravenously. He states he had used a similar amount from the same supply the previous day with no unusual effects. Mark's pain is crushing in nature and radiates down his left arm and up into his left jaw. His BP is 202/110. and his pulse is 120 and irregular. PVCs were seen by the paramedic on his cardiac monitor at the rate of 6 per minute. His respirations are 26 and heaving. His skin is warm and dry, and his Glascow coma score is 15. He states he is nauseated, and he is observed to intermittently begin to retch. The paramedics have initiated an Intravenous solution of
D5 in water (keeping the vein open) and oxygen by nasal prongs at 2 liters/mi n. Mark has been given one dose of sublingual nitroglycerin by the paramedics with no relief'
of symptoms. He states he has been told he has high blood pressure but does not take hi.' medication. He drinks '/: pint of whiskey per day, smokes two packs of cigarettes per day, and is a daily user of intravenous cocaine. He states this is his first admission to the ED related to
his cocaine habit. Mark's social history reveals that he is a very successful real estate agent with his own company. His wife has been contacted at her place of employment and is driving to the ED.
Triage Assessment. Acuity Level IV: chest pain sudden onset, unrelieved by nitro-glycerin, continues at rest. The patient is escorted immediately to the treatment area. A subsequent 12-iead ECG done in the treatment area shows S-T depression in leads II III and AVF indicating inferior wall myocardial ischemia.
QUESTIONS AND ANSWERS
- What is the physiological basis for the systemic responses being presented by Mark?
Cocaine is classified as a local anesthetic and sympathomimetic drug. It is an alkaloid derived from the leaves of the coca shrub which grows in Peru and other parts of South America. When a moderate amount is absorbed systemically, cocaine stimulates the CNS resulting in a fight-or-flight reaction. The pulse is stronger and more rapid. BP is elevated, respirations are faster and deeper, and the activities of the brain are increased. The patient is more talkative, is more alert mentally, and feels exhilarated. The patient's pupils are dilated, blood sugar increases, and there is peripheral vasoconstriction (1).
In early cocaine stimulation, with moderate dosages, the effects occur from the head downward. The initial response is euphoria and restlessness. Motor activity increases with an increase in repetitive motor actions such as picking and stroking. The cardiovascular effects occur causing increased heart rate (sometimes 30 to 50% above normal), increased contractility, and vasoconstriction. An overall BP rise of 15 to 20% above normal can occur. Cocaine also stimulates the respiratory vasomotor and vomiting centers in the medulla which results in increased respiration, cold sweats, and nausea, vomiting, and vertigo. Mark's symptoms of tachycardia, PVCs. elevated BP. sweating. and vomiting may have two causes: cocaine and acute myocardial ischemia.
- Is there a relationship between the sympathetic nervous system effects of cocaine and the onset of Mark's myocardial ischemia?
Death caused by cocaine toxicity usually occurs largely because of the progressive effects on the CNS. Acute toxic effects are likely to occur if the drug is absorbed rapidly. Excessive absorption may cause headache, excitement, palpitation, fainting, and convulsions. Death usually occurs from failure of the patient to breathe or from progressive hyperthermia. The patient may progress very rapidly from advanced stimulation to significant depression of the CNS within a very brief period of time (1-4).
Idiosyncratic cardiovascular effects of cocaine have been reported and may be related to myocardial oxygen imbalances from adrenergic stimulation of heart rate and coronary artery vasoconstriction, electro-cardiographic conduction defects, and hypertension (5-7). Mark's preexisting history of hypertension may be aggravated by the use of cocaine. However, there have been other case reports of significant dysrhythmias and myocardial ischemia and infarction in young patients who have had few risk factors (8-10). Of the cases reported, when arteriograms were performed, coronary arteries were found to be normal (7-9). The mechanism of action for the primary ischemic event therefore is not clear. Effects may be related to the release of catechol-amines and stimulation of platelet aggregation, sympathetic stimulation of the myocardium and increased oxygen consumption, or isolated occurrences of coronary artery thrombosis or vasospasm (10).
- Are the cardiovascular effects experienced by Mark related to his intravenous use? In other words, if he was taught to change how much cocaine he used and stopped injecting the drug intravenously, would he still be at great risk for another ischemic event?
Certainly cocaine absorption is related to the dose administered and the route of administration. Cocaine can be made into a paste. liquid, powder, or crystal and taken orally, intranasal. or parenterally. or it may be smoked in cigarette form as a freebase (a purified form) (11). Street cocaine is usually combined with other substances such as amphetamines, caffeine, or lidocaine which can potentates the effects of the drug (1,2, 11, 12). "Crack." a very pure crystallizes form of cocaine, is a new hazard to be addressed.
The effect of intravenous cocaine occurs in a matter of minutes. and its effect is dose dependent (1,2, 11). The effect of vasoconstriction caused by cocaine can prolong its absorption through mucous membranes (such as the nares), subcutaneous tissue, or muscle. Vaso-constriction can slow the peak effect to 30 to 60 min after use but can prolong duration of action up to 3 hr (1, 2, 11). Cocaine is rarely taken orally because minimal absorption occurs in the GI tract due to the acidity in the stomach. The cocaine user frequently progresses from intranasal to intravenous use for its rapid and intense effect.
Cocaine is primarily metabolized in the liver where it is hydrolyzed by liver enzymes and plasma pseudocholinesterases. The effects of cocaine can be more prolonged or more toxic in a patient who has liver disease (1). It is not unusual for persons like Mark to have an underlying problem with significant alcohol consumption and poor nutritional habits that may alter his liver functions. The myocardial ischemia experienced by Mark may be related to the sudden sympathetic stimulation that occurred with the injection of the cocaine. However, it is also likely that the same event would have occurred had he chosen another route of administration.
Although there are dose-related changes in peak plasma concentrations of cocaine, the timing of these levels usually remains consistent for each individual (11). In addition, as the person reaches tolerance for increasing dosages of the drug, the individual may crave more drug even though plasma concentrations remain elevated (11). hence reaching toxic levels.
- What nursing diagnoses are applicable to this situation?
The patient who is experiencing acute myocardial ischemia or infarction related to cocaine use should be recognized and managed as any other patient with myocardial ischemia precipitated by other factors such as arteriosclerosis and vasospasm. However, there are additional challenges due to the direct effect of the drug on the CNS. The appropriate nursing diagnoses (13) for this patient's care are:
Diagnosis: Acute pain related to myocardial ischemia or decreased oxygen supply to the myocardium
Desired patient outcome: The patient will state that there is relief or reduction in the chest pain and does not exhibit cues of discomfort.
Diagnosis: Potential/or decreased cardiac output related to life-threatening arrhythmias secondary to injury or to enhanced automaticity of the myocardium, or potential for impaired contractility secondary to myocardial muscle injury
Desired patient outcome: Patient remains in normal sinusrhythm; patient maintains a normal cardiac output as evidenced by systolic BP > 90 mm Hg and HR < 100 beats/min: patient remains oriented to person, place, and time: skin remains warm and dry: and urinary output > 30 ml/hr.
Diagnosis: Knowledge deficit related to use of cocaine, myocardial ischemia, and high blood pressure implications for life-style changes
Desired patient outcome: Patient verbalizes an understanding of the relationship between his cocaine habit and the effect on his heart: patient verbalizes an understanding of his risk factors related to his heart including diet. smoking, drug use. and management of his high blood pressure.
- What interventions should the nurse initiate in this situation?
Nursing interventions for Mark are similar to those for any patient with acute myocardial ischemia. The goal is to relieve the patient's pain and anxiety and reduce cardiac work load. The nurse should assess the patient's chest pain for location and radiation, duration, intensity. quality, and chronology (precipitating factors), and other related symptoms. The patient's HR and BP should be monitored with each episode of chest pain as the sympathetic stimulation caused by the pain and by the cocaine may significantly increase both of these. However, if the patient has experienced significant myocardial muscle damage, there may be reduced cardiac output and a low BP. The sympathetic stimulation of the pain and cocaine action are also probable causes for Mark's PVCs. Mark's history of high blood pressure should be taken into serious consideration in monitoring these parameters. To relieve Mark's pain careful titration of intravenous morphine sulfate was ordered by the physician and administered by the nurse with constant reassessment of Mark's overall pain condition. Morphine is also a useful adjunct for reducing preload and the workload of the heart, thus improving cardiac output.
TIP: A low BP is not necessarily a contraindication for administering intravenous morphine to a patient. BP may be reduced related to the ongoing ischemia that is reducing myocardial contractility or the increased preload that is reducing myocardial pump efficiency.
Oxygen was ordered by the physician at 3 liters/min (usual range is 2 to 6 liters/min) for the myocardial oxygen demands. Serial 12-lead ECGs and blood samples to measure cardiac enzymes along with other routine labs are also indicated and were performed. Monitoring of cardiac output in the ED is usually through indirect measures of systolic BP and HR, skin temperature, urinary output, and the evaluation of the patient's degree of orientation and restlessness. Significant changes in these are recorded and reported to the physician for further orders.
In some cases of myocardial infarction or ischemia, interdependent nursing actions around the administration of nitroglycerin for the reduction of preload may be indicated. In the case of cocaine-induced ischemia. the therapeutic goals are to minimize nervous system stimulation and support ventilation and circulation. Intravenous propranolol (1 me administered slowly and repeated every minute to a maximum of 6 mg) has been used to block the (3-adrenergic stimulation of HR and BP (14). Because the a-adrenergic effects of vasoconstriction are left unopposed when propranolol is used, some authors recommend the use of a-adrenergic blocking agents such as phentolamine, or afterload reducing agents such as sodium nitroprusside (15). Antiarrhythmic drugs such as lidocaine may also be indicated if other measures fail to relieve or reduce the cardiac instability that may exist.
Other physiological effects of myocardial ischemia should also be considered such as fluid balance overload related to poor cardiac contractility. Although Mark did not experience this problem while in the ED, his lungs were assessed regularly by the nurse for the presence of rales and his heart sounds for S3 gallop.
Although the relationship between Mark's cocaine use and his heart attack are an important educational concern for Mark and his family. the immediate educational need in the ED should be to assist the patient and his family to acknowledge the relationship of his drug use to his current illness. Orienting Mark and his wife to important significant events of the upcoming hospitalization are indicated. His discharge teaching plan should include assisting him to recognize his risk factors for heart attack and assisting with a plan for risk factor modification including BP control. Mark will be admitted to a special telemetry unit for cardiac monitoring. He should be informed of the need for more ECGs and blood drawing to ensure his safe recovery. He should be instructed to inform his nurse immediately if his chest pain returns.
REFERENCES
- Gay GR: Clinical management of acute and chronic cocaine poisoning.
Ann Emery, Med 1 1:562. 1982.
- Jonsson S. O'Meara M. Young JB: Acute cocaine poisoning. Am J Med 75:1061. 1983.
- Nanji AA. Filipenko JD: Asystole and ventricular fibrillation
associated with cocaine intoxication. Chest 85:132. 1984.
- Bozart MA. Wise RA: Toxicity associated with long term intravenous heroin and cocaine self-administration in the rat.
JAMA 254:81. 1985.
- Coleman DL. Ross TF. Naughton JL: Myocardial ischemia and infarction related to recreational cocaine use.
East J Med 136:444, 1982.
- Kossowsky WA. Lyn AF: Cocaine and acute myocardial infarction: a probable connection.
Chest 86:729. 1984.
- Cregler LL. Mark H: Relation of acute myocardial infarction to cocaine abuse.
Am J Cardini 56:794, 1985.
- Pasternak PF. Colvin SB. Baumann FG: Cocaine induced angina pectoris and acute myocardial infarction in patients
younger than 40 years. Am J Cardiol 55:847. 1985.
- Howard RE, Huetcr DC. Dans GJ: Acute myocardial infarction following cocaine abuse in a young woman with normal coronarv arteries.
JAMA 256:95.1985.
- Cregler LL, Mark H: Cardiovascular dangers of cocaine abuse. Am Heart J 111:793. 1986.
- Javaid Jl. Fischman MW. Schu-.ter CR. et al.: Cocaine plasma concentration in relation to physiologic and subjective effects in humans.
Science 202:227. 1978.
- LoveysBJ: Physiologic effects of cocaine with particular reference to the cardiovascular system.
Heart Lung 16(2):175, 1987.
- Swearingcn PL. Sommers MS, Miller K Manual of critical care. applying nursing diagnosis to adult critical illness. St Louis: C. V. Mosby. 78. 1988.
- Rappolt RT. Gay GR. and Inaba DS: Propranolol: A specific antagonist to cocaine.
Clin Toxicol 10:265. 1977.
- Benowitz NL. Rosenburg J, Becker CE: Cardiopulmonary catastrophes in drug overdosed patients.
Med Clin North Am 63:267, 1979.
2.4 PRIMARY TACHYDYSRHYTHMIAS IN THE EMERGENCY DEPARTMENT
Valerie A. Barron, RN, BSN, CCRN, CEN
Rick W. is a twenty-year-old white male who walks into the triage area complaining of palpitations and dizziness. Rick tells the triage nurse, ' 'My heart's pounding so fast. I can hear it in my ear! I feel dizzy." Rick looks anxious and pale. Hi' breathing is slightly labored and his skin is cool and moist.
Rick is assisted with lying supine on a stretcher. His vital signs are temperature 98.2° F, pulse 180 and regular, respirations 24, and BP 90/60. Lung sounds arc clear and heart sounds are normal.
Rick states he is unmarried, graduated from college 2 months ago. and is
living with his parents and two siblings. He has been unable to obtain a job since graduation. Rick uses alcohol occasionally and smokes "grass" once in a while. When asked about previous health problems, Rick replies. "They told me that I had some kind of heart condition.'' Rick says that he has been to the hospital twice in the past for rapid heartbeats. The last episode was about 2 years ago. Rick mentions that fie was instructed to return to the clinic for follow-up but never went.
Triage Assessment, Acuity Level IV: HR > 150 beats/min. BP < 90 systolic; dizzy .
Rick is taken immediately to the treatment area where a 12-lead ECG shows tachycardia with slightly wide ventricular complexes and no discernible P waves. A diagnosis of supraventricular tachycardia (SVT) is made. An intravenous line is established and blood samples are obtained for CBC, BUN, glucose, electrolytes, calcium, and cardiac enzymes. With physician direction and nurse support. Rick is directed in Valsalva maneuvers. These measures are unsuccessful, so the physician performs carotid sinus massage. As there is still no change in the cardiac rate or rhythm. Rick is given verapamil 5 mg intravenous push. The dose is repeated until a total dose of I5 mg of verapamil is given and there is conversion of Ricks heart rhythm to normal sinus at a rate of 76 beats/min. The 12-lead ECG taken after the change in cardiac rhythm reveals
sinus rhythm with a PR interval of O.08 sec and a QRS duration of 0.14 sec. Delta waves arc noted on the upstroke of the QRS complexes. A diagnosis of
Wolff-Parkinson- White syndrome is made by the ED physician.
QUESTIONS AND ANSWERS
- What is the etiology of SVT?
SVT is a rapid heart rhythm that is initiated by an impulse above the ventricles. The term SVT is used when the precise mechanism causing the tachycardia is unable to be determined. The umbrella term, SVT, then, might include atrial or junctional tachydysrhythmias. The initial impulse originates in the atrial or junctional area and travels down the conduction system to the ventricles (antegrade conduction). The same impulse can then travel back up the conduction system (retrograde conduction). As the impulse travels retrogradely, portions of the conduction system may no longer be refractory. These nonrefractory portions of the conduction system can, therefore, be electrically stimulated by the retrograde impulse. The result is recurrent rapid depolarization of the ventricles. As this process continues in a circular fashion (down the conduction system. up the conduction system, and back down again), tachydysrhythmias are generated. This phenomenon is often termed "reentry" since the initial impulse reenters the conduction system and produces tachycardia. The impulse may reenter the conduction system through a portion of the normal conduction pathway, such as the junctional area. The original impulse might also reenter the conduction system through an abnormal extra or accessory conduction pathway. Persons with accessory cardiac conduction pathways are said to have pre-excitation syndrome.
Preexcitation syndrome is a common etiology of SVT. The term preexcitation refers to early activation of the ventricles by an atrial impulse through an alternative conduction pathway. The two known types of preexcitation syndromes are Wolff-Parkinson-White (WPW) and Lown-Ganong-Levin (LGL). In both types of preexcitation, conduction bypasses the normal pathways and travels through the accessory tracts. Accessory tracts that have been identified include Kent's bundle, a direct connection between the atria and ventricles; Mahaim fibers, a connection between the junctional area and the ventricles; and James's fibers, a connection between an internodal tract and the Bundle of His (1). Conduction through these pathways is more rapid than normal, since the usual conduction system is bypassed and thus the delay at the atrioventricular (AV) junctional area does not occur. This rapid activation of the ventricles is demonstrated on the ECG by a shortened PR interval and slurring of the upstroke of the R wave or initial portion of the QRS complex. This abnormal slurring is referred to as a $ wave.
Persons with preexcitation syndrome are predisposed to developing SVT. The accessory pathways facilitate the development of reentrant tachycardias. Cardiac electrical impulses can reenter the conduction system via the accessory pathways. The result is rapid antegrade activation of the ventricles and retrograde reactivation. The cardiac impulse travels down the conduction system, back up. and then down the system again in a circular fashion. This circular movement of the cardiac stimulus is referred to as a circulating wave (2). This process is also known as "circus" movement (3).
- How is the diagnosis of SVT determined?
The diagnosis of SVT is generally determined from the ECG. ECG criteria include QRS duration of less than 0.15 sec and ventricular rate of 150 to 250 beats/min. Often P waves are not discernible. If P waves can be identified, there is a regular, consistent relationship between the P waves and the QRS complexes.
The patient's clinical presentation and history may help the diagnosis. Rick's reported medical history is vague. However, preexcitation syndrome should be suspected in a young man with a history of rapid heatbeats but no history of coronary heart disease. The ECG characteristics for WPW are duration of PR interval less than 0.12 sec, the presence of 5 waves, and prolongation of the QRS interval greater than 0.12 sec (4). Since the pattern of repolarization is also altered, secondary ST-T wave changes will be seen on the ECG.
TIP: Differentiating between ventricular tachycardia and supraventricular tachycardia with aberrant conduction is essential to ensure proper patient management
The following ECG criteria support the diagnosis of ventricular tachycardia: the presence of AV dissociation; QRS duration of > 0.14 sec; axis deviation (left of-30° or right of+120°); and abnormal morphology of the QRS complex such as initial taller peak ("rabbit ear") of the R wave in V 1, a broad R wave, slurred or notched downslope of the S wave in VI or V2, and a duration of more than 0.06 sec from the beginning of the QRS complex to the lowest point of the S wave (3. 5). Other criteria that favor ventricular tachycardia include presence of fusion beats, presence of compensatory pause, HR between 130 and 170 beats/min, and concordancy—ventricular complexes in the pre-cordial leads are all negative or are all positive (6).
Most tachydysrhythmias with wide ventricular complexes represent ventricular tachycardia (2). If the ECG diagnosis of a tachydysrhythmia with wide ventricular complexes is not immediately evident, the dysrhythmia should be treated as ventricular tachycardia. Ventricular tachycardia is often incorrectly diagnosed as SVT. sometimes resulting in life-threatening conditions (7).
- What factors contribute to the development of SVT?
Factors such as high caffeine intake and smoking are associated with an increased incidence of tachydysrhythmias. Overexertion and emotional stress have also been identified as possible causative agents. In addition, toxic levels of certain medications or drugs can result in tachydysrhythmias.
TIP: Solicit a comprehensive medication and drug history from the patient. If the patient is taking theophylline or antiarrhythmic medications, obtain an order for serum drug levels. If indicated, toxicology screens should also be obtained.
- What nursing diagnoses can be determined for Rick?
The following nursing diagnoses are appropriate for Rick.
Diagnosis: Decreased cardiac output related to electrical alteration in cardiac rate and rhythm (tachycardia) as evidenced by palpitations and rapid heartbeat with hypotension
Desired patient outcome: The patient will maintain a normal sinus rhythm. BP systolic>90mm Hg; HR< 100 beats /min. urinary output > 30 ml/hr: skin warm and dry: the patient will remain oriented to person, place, and time.
Diagnosis: Anxiety related to tachycardia and uncertainty about its effect on health status
Desired patient outcome: The patient will verbalize an understanding of current health status and activities that will help reduce anxiety. The patient will relate an increase in psychological and physiological comfort.
Diagnosis: Knowledge deficit related to heart problem as evidenced by patient's 'statement, "... some kind of heart condition"
Desired patient outcome: The patient will be able to describe type and name of heart problem, possible complications of heart problem, patient's role in management of heart problem, and when to seek medical attention for heart problem.
Diagnosis: Noncompliance related to follow-up care with known cardiac condition
Desired patient outcome: The patient will identify factors that contribute to personal noncompliance; the patient will develop strategies to reduce impediments and comply with follow-up care.
- What nursing interventions are appropriate in Rick's care?
Rick's decreased cardiac output related to his dysrhythmia has the potential to become life-threatening and requires immediate intervention. The patient should be assisted to a supine position on a stretcher so that further assessment can be done and to improve venous return. If resuscitation measures become necessary, the patient will be easier to manage. Emergency equipment for resuscitation should be at the bedside. A full 12-lead ECG is recorded immediately, providing diagnostic information as well as documentation of the dysrhythmia. The patient should remain connected to the ECG machine to record various lead tracings during therapeutic interventions. Cardiac monitoring should also be initiated so that the nurse can observe the rhythm as other procedures are performed. An intravenous line should be established to provide access for medications and fluid support as needed. Since Valsalva and carotid massage maneuvers might produce severe slowing of the ventricular response, it is important to have the intravenous line in place before the maneuvers are initiated. Blood samples should be obtained, as ordered, for electrolytes, calcium, and CBC. If Rick had been on any antiarrhythmic medications or theophylline. serum drug levels would be sent to the lab. Oxygen via nasal cannula should be administered to increase oxygenation.
As the nurse performs each task. reassurance and explanations should be provided for the patient. The rationale for the treatment plan should be discussed to elicit maximal patient cooperation. The patient will need step-by-step instructions for the Valsalva maneuvers and support throughout the procedures. The patient should be told that he might feel as though his heart has "stopped" as the heart rate slows and converts from SVT to sinus rhythm.
A patient who comes to the ED with heart problems is usually very anxious. The patient needs reassurance that the health care team understands his problem and has the knowledge and skills to treat it. The nurse should give the patient a brief explanation of the nature of the problem and of the plan for treatment. In addition the nurse should explain the role of the patient in the treatment plan. As the treatment continues, the patient may feel some sense of control as he participates in his own care.
Health teaching needs include the type and correct name of the heart condition and education on the management and prevention of tachydysrhythmias. A brief description of the pathophysiology of WPW syndrome should be shared with Rick and written on paper for Rick to carry with him.
The importance of follow-up care with a cardiologist or cardiology clinic should be discussed. The nurse should explore reasons for Rick's noncompliant behavior and assist him to identify strategies to deal with these. Rick should also be instructed to avoid potential cardiac stimulants such as caffeine, smoking, alcohol, and emotional stress. Rick might be referred for job counseling if he determines that his unemployment is stressful.
What other medical therapies are appropriate for the treatment of SVT? What are the particular nursing considerations associated with these therapies?
If the patient is hemodynamically unstable, synchronized electrical cardioversion is indicated. Prior to countershock the patient should receive intravenous sedation such as with diazepam. Careful monitoring and ventilatory support should be instituted as needed.
TIP: Patients who take digoxin are very sensitive to electrical countershock and are more likely to develop ventricular tachydysrhythmias. Therefore, only small amounts of electrical energy should be used for digitalized patients. Digoxin should not be used as an antiarrhythmia medication for patients that might require counter-shock.
Verapamil is the usual drug of choice for the management of SVT. Verapamil is given intravenous push in increments of 5 mg doses or 0.075 mg/kg of body weight up to a total dose of 15 mg in 30 min (7). Hypotension is a common side effect experienced by patients receiving verapamil. The BP should be checked every 5 min during drug administration. Verapamil should be used very cautiously with patients who already have left ventricular failure.
Although verapamil is the usual drug of choice for SVT. several cautions should be noted. If there is any doubt about the dysrhythmia diagnosis, or if there is any suspicion that the rhythm might be ventricular tachycardia, verapamil should not be given (5). Fatalities have occurred in patients with ventricular tachycardia who were given verapamil (3).
Verapamil may shorten the refractoriness in the accessary pathways of patients with WPW (8). The shortened refractory periods can result in an acceleration of the ventricular response particularly in atrial flutter or fibrillation, and thus can produce ventricular fibrillation. Therefore, verapamilshould not be given to patients with WPW and atrial fibrillation or flutter (9). In addition patients with WPW and wide QRS complexes should not be given verapamil (10).
Because Rick could not tell the nurse and physician what heart condition he had. verapamil was chosen. Fortunately, he experienced no acute side effects. For future attacks, verapamil should not be selected as first line management and Rick should be informed of this precaution. Intravenous procainamide should be used for patients with WPW and atrial fibrillation. Procainamide is also indicated for patients with wide complex tachycardias in which the origin of tachycardia is unclear.
REFERENCES
- Layer JH: Electrical activity of the heart: dysrhythmias. In: Cardiovascular nursing: Body-mind tapestry. St. Louis: C.V. Mosby, 1984.
- Marriolt HJL: Practical electrocardiography. Baltimore: Williams & Wilkins. 1983.
- Conover M: Electrocardiography update. J Cardiovasc Nurs 2(4) :45-52,1988.
- Zipes D: Arrhythmias. In: Comprehensive cardiac care. St. Louis: C.V. MosbY,1983
- Wellens HJ: The wide QRS tachvcardia. . Ann Intern Med 104:79. 1988.
- Karncs N: Differentiation of aberrant ventricular conduction from ventricular ectopic beats.
Crit Care Nurse 7(4) :56-67,1987.
- Lowenstein SR. Harken AH: A wide complex look at cardiac dysrhythmias. J Emerg Med 5(5): 519-531,1987.
- American Heart Association textbook of advanced cardiac life support, Dallas, Texas, 1987.
- Zimmerman JH, Nieminski KE: Atrial fibrillation in Wolff-Parkinson- White syndrome.
Crit Care Nurse 7(3):84-88, 1987.
- Erickson SL: Wolff-Parkinson-White syndrome: a review and an update. Crit Care Nurse 9(5):28-35,1989.
SUGGESTED READINGS
Cook JR, Nieminski KE: Ventricular tachycardia. Crit Care Nurse 8(7):15-19, 1988.
Lanros NE: Assessment and intervention in emergency nursing. Norwalk, Connecticut: Appleton & Lange, 1988.
Petrie JR: Distinguishing supraventricular aberrancies from ventricular ectopy.
Focus Crit Care 1 5(4): 15-21. 1988.
Rea RE, Bourg PW, Parken JG. Rushing D, eds.: Emergency nursing core curriculum. Philadelphia: W.B. Saunders. 1987.
Valladares BK, Lemberg L: Wide complex tachycardia: diagnosis and treatment.
Heart Lung 15(6):644-647, 1986.
Weller DM, Noone J: Mechanisms of arrhythmias: enhanced automaticity and reentry.
Cit Care Nurse 9(5):42-66,1989.



