Source: Royce, 1997
| Contents | Previous | Next |
The successful introduction and spread of the human immunodeficiency virus (HIV) into the global human population has occurred for many reasons. The discovery and widespread use of penicillin and other antibiotics meant that there was treatment and cure for most sexually transmitted diseases. The existence of these new drugs changed how people perceived risks associated with sexual activity. Soldiers in World War II increasingly used prophylactics and the subsequent development of hormonal contraceptives hastened the pace of change in sexual practices, as prevention of pregnancy became a real possibility. Lifestyles also were changing: people were moving into regions that were previously uninhabited by man and long distance travel became easier and was much more common, allowing for more social migration and sexual mixing. Although the virus may have first been introduced to humans earlier in the 20th century (most likely contracted from infected animals), it was in the 1970s that wider dissemination occurred.
For industrialized countries, the first evidence of the AIDS epidemic was among groups of individuals who shared a common exposure risk. In the United States, sexually active homesexual men were among the first to present with manifestations of HIV disease, followed by recipients of blood or blood products, then injection drug users, and ultimately children of mothers at risk.
Women have represented an increasing proportion of reported AIDS cases in the United States, accounting for 23% of adult cases from July 1998 to June 1999. (CDC, 1999) Eighty percent of AIDS cases in women are in African Americans and Hispanics, as compared to 61% of cases in men.
In developing countries, the AIDS epidemic manifested itself quite differently, both because the signs and symptoms were harder to identify due to other competing causes of morbidity and mortality and because the epidemic did not seem to be limited to high-risk groups and, instead, was more generalized. Worldwide, women now represent 43% of all adults living with HIV and AIDS (Table 2-1) and this proportion has been steadily increasing over time. (UNAIDS, 1998)
This chapter reviews the Epidemiology of HIV/AIDS beginning with how HIV is transmitted and the variables involved; the natural history of HIV infection, both without treatment and in the era of highly active Antiretroviral therapy (HAART); and concludes with future issues regarding the HIV/AIDS epidemic.
|
Table 2.1: Regional HIV/AIDS Statistics and Features, December 1998 |
||||||
| Region | Epidemic Started | Number of Persons with HIV Infection | Number of Persons with New HIV Infection | Prevalence Among Adults | Percent of Infected Adults Who Are Women | Main Modes of Transmission For Adults |
| Sub-Saharan Africa | Late 1970s nearly 1980s | 22.5 million | 4 million | 8.0% | 50% contact | Heterosexual |
| North Africa and Mid-East | Late 1980s | 210,000 | 19,000 | 0.13% | 20% | Injection drug use, heterosexual contact |
| South, South-East Asia | Late 1980s | 6.7 million | 1.2 million | 0.69% | 25% | Heterosexual contact |
| East Asia, Pacific | Late 1980s | 560,000 | 200,000 | 0.068% | 15% | Injection drug use, heterosexual contact, male/male sex |
| Latin America | Late 1970s nearly 1980s | 1.4 million | 160,000 | 0.57% | 20% | Male/male sex, injection drug use, heterosexual contact |
| Caribbean | Late 1970s nearly 1980s | 330,000 | 45,000 | 1.96% sex | 35% | Heterosexual contact, male/male sex |
| Eastern Europe, Central Asia | Early 1990s | 270,000 | 80,000 | 0.14% | 20% | Injection drug use, male/male sex |
| Western Europe | Late 1970s nearly 1980s | 500,000 | 30,000 | 0.25% | 20% | Male/male sex, injection drug use |
| North America | Late 1970s nearly 1980s | 890,00 | 44,000 | 0.56% | 20% | Male/male sex, injection drug use, heterosexual contact |
| Australia, New Zealand | Late 1970s nearly 1980s | 12,000 | 600 | 0.1% | 5% | Male/male sex, injection drug use |
| Total | 33.4 million | 5.8 million | 1.1% | 43% | ||
| Source: Modified from UNAIDS, 1998 | ||||||
Epidemiological studies have demonstrated that HIV is transmitted by three primary routes: sexual, parenteral (blood-borne), and perinatal. Virtually all cases of HIV transmission can be attributed to these exposure categories.
Transmission rates from the infected host to the uninfected recipient vary by both mode of transmission and the specific circumstances. Since HIV is a relatively large virus, has a short half-life in vitro, and can only live in primates, HIV cannot be transmitted from causal (i.e., hugging or shaking hands) or surface (i.e., toilet seats) contact or from insect bites.
Sexual transmission of HIV from an infected partner to an uninfected partner can occur through male-to-female, female-to-male, male-to-male, and female-to-female sexual contact. Worldwide, sexual transmission of HIV is the predominant mode of transmission. (Quinn, 1996) Among U.S. women with AIDS, sexual transmission constitutes 40% of reported cases as of June 1999. (CDC, 1999) This 40% is probably an underestimate when you take into consideration that a large proportion of the women with AIDS who report no identifiable risk (an additional 15% of AIDS cases in women) are actually also infected via sexual transmission. While receptive rectal and vaginal intercourse appear to present the greatest risk of infection (approximately 0.1-3% and 0.1-0.2%, respectively, per episode), insertive intercourse (both rectal and vaginal) have also been associated with HIV infection (approximately 0.06% and 0.1%, respectively, per episode). (Vittinghoff, 1999; Mastro, 1996) In addition, there have been a few case reports of male-to-male transmission from receptive oral intercourse with an HIV-infected male partner (approximately 0.04% per contact) and female-to-female transmission from oral-vaginal, oral-anal, and digital intercourse. (Marmor, 1986; Monini, 1996; Monzon, 1987; Perry, 1989; Rich, 1993; Sabatini, 1983)
Parenteral transmission of HIV has occurred in recipients of blood and blood products, either through transfusion (estimated 95% risk of infection from transfusion of a single unit of HIV-infected whole blood (CDC, 1998)) or clotting factors, in intravenous or injection drug users through the sharing of needles (approximately 0.67% risk per exposure (Kaplan, 1992)), and in healthcare workers through needle sticks (approximately 0.4% risk per exposure, depending on the size and location of the inoculum (Tokar, 1993)) and less commonly mucous membrane exposure. (Hessol, 1989) Among cumulatively reported AIDS cases in U.S. women through June 1999, 42% had injection drug use as their exposure risk and 3% receipt of blood, blood products, or tissue. (CDC, 1999) Parenteral transmission patterns vary by geographic region due to social and economic factors. For instance, in regions where the prevalence of HIV infection is higher, the risk of occupational or nosocomial transmission of HIV is increased over regions where there is lower prevalence. (Consten, 1995) The transmission risk is therefore related to the prevalence of HIV in the population as well as the frequency of exposure to infected body fluids and organs and the method of exposure. (Fraser, 1995) In addition, many developing countries that have a high prevalence of HIV infection also lack the resources to implement universal precautions adequately (Gilks, 1998) and may experience a greater amount of transfusion-associated HIV transmission due to a lack of HIV antibody screening in some areas, a higher residual risk of contamination in blood supplies despite antibody screening (McFarland, 1997), and high rates of transfusion in some groups of patients.
Perinatal transmission can occur in utero, during labor and delivery, or post-partum through breast-feeding. (Gwinn, 1996) Perinatal transmission rates average 25-30% (Blanche, 1989), but vary by maternal stage of disease, use of antiviral therapy, duration of ruptured membranes, practice of breast-feeding, as well as other factors. In the U.S. as of June 1999, 91% of cumulative pediatric AIDS cases were attributed to perinatal transmission.
Transmission of HIV infection can be influenced by several factors, including characteristics of the HIV-infected host, the recipient, and the quantity and infectivity of the virus. A summary of factors affecting sexual transmission of HIV is presented in Table 2-2.
| Table 2-2: Biologic and Host-Related Factors Affecting Sexual Transmission of HIV | |||
| Biologic Factor | Host-Related Infectivity Factors | ||
| HIV Concentration in Genital Secretions | Infectiousness (Transmission) | Susceptibility (Acquisition) | |
| Mutation of chemokine receptor gene | ? | ? | ▼▼▼ |
| Late stage of HIV infection | ▲▲ | ▲▲▲ | Not applicable |
| Primary HIV Infection | ▲▲ | ▲▲ | Not applicable |
| Antiretroviral therapy | ▼ | ▼▼ | ▼? |
| Local infection | ▲▲ | ▲▲ | ▲▲ |
| Presence of cervical ectopy? | ▲▲ | ▲? | ▲▲ |
| Presence of foreskin? | ? | ▲▲ | ▲▲ |
| Method of contraceptives | |||
| Barrier | Not applicable | ▼▼▼ | ▼▼▼ |
| Hormonal contraceptives | ▲▲ |
|
|
| Spermicidal devices | ? | ▼? |
|
| Intrauterine devices | ? | ? | ▲▲ |
| Menstruation | ? | ▲▲ | ▲ |
| Factors that lower cervicovaginal pH | ▲? | ▼? | ▼? |
| Immune activation | ▲? | ▲ | ▲ |
| Genital tract trauma | ▲? | ▲▲ | ▲▲ |
| Pregnancy | ▲▲ | ▲? | ▲? |
| The degrees of positivity (▲to ▲▲▲) and
negativity (▼ to ▼▼▼) of the associations are
indicated with arrows, with three arrows indicating a very strong
association. The symbol
Source: Royce, 1997 |
|||
There is an association between the quantity of virus transmitted and the risk of HIV infection. (Roques, 1993) Several studies have found that HIV-infected persons may be more likely to transmit the infection when viral replication is high, both during the initial stage of infection (Palasanthiran, 1993) and at more advanced stages of HIV disease. (Laga, 1989) People with high blood viral load are more likely to transmit HIV to recipients of blood, their sexual partners, and their offspring. (Vernazza, 1999; Quinn, 2000) HIV has been quantified in semen (Coombs, 1998; Speck, 1999; Vernazza, 1997) and detected in female genital secretions (Ghys, 1997; Mostad, 1998), and virus in these locations may facilitate transmission. However, the association between infectivity and disease stage is not absolute; HIV-infected women may transmit virus to a first-born child while not to a second-born child (deNartubim 1991), and temporal studies of semen from HIV-infected men demonstrate waxing and waning viral titers over time. (Krieger, 1991; Tindall, 1992)
Factors that decrease viral titers, including anti-retroviral therapy, may decrease but not eliminate the risk of HIV transmission. (Hamed, 1993) Zidovudine has been shown to reduce vertical transmission from mothers to their fetus even when administered late in pregnancy or during labor. (CDC, 1998) Individuals receiving Antiretroviral therapy have also shown reduced transmission rates of HIV to their sex partners. (Musicco, 1994) Several studies have suggested that anti-retroviral treatment reduces detection of HIV in female genital secretions (Cu Uvin,1998) and the concentration of HIV in semen. (Gilliam, 1997; Gupta, 1997) Providers counseling patients on treatment should be clear that precautions to prevent transmission of the virus should be maintained since not all treatments reduce infectiousness and transmissions have been reported among individuals with undetectable HIV RNA levels. (The European Collaborative Study Group, 1999)
Factors which increase the risk of exposure to blood, such as genital ulcer disease (Cameron, 1989; Plummer, 1991), trauma during sexual contact (Marmor, 1986), and menstruation of an HIV-infected woman during sexual contact (The European Collaborative Study Group, 1992; Nair, 1993; St. Louis, 1993) may all increase the risk of transmission.
Method of contraception also affects the likelihood of HIV transmission. (Daly, 1994) There is overwhelming evidence that the correct and consistent use of latex condoms protect both men and women against HIV. However, because of methodologic difficulties in studies of contraceptive use and HIV transmission, it remains unclear whether the use of hormonal contraceptives, IUDs and spermicides alter the risk of HIV transmission.
Similarly, characteristics of the uninfected individual may increase the likelihood of infection for a given exposure to HIV. Specifically, inflammation or disruption of the genital or rectal mucosa (which can occur with sexually transmitted diseases and trauma), and lack of circumcision in heterosexual men may increase the risk of infection. (Cameron, 1989; Moses, 1994; Quinn, 2000) Sex during menstruation may increase women’s risk of acquiring HIV infection (Lazzarin, 1991) as may bleeding during sexual intercourse. (Seidlin, 1993) In women, both uclerative and non-uclerative sexually transmitted diseases have been shown to be risk factors for getting infected with HIV. (Laga, 1993; Plummer, 1991) Cervical ectopy has been identified as a risk factor for acquisition of HIV infection in some (Nicolosi, 1994; Plourde, 1994) but not all (Mati, 1994) studies that have evaluated this condition. There is also some evidence that changes in the vaginal flora, as characterized by bacterial vaginosis, may facilitate acquisition of HIV. (Sewankambo, 1997)
Non-barrier contraceptive methods have also been investigated in association with risk of HIV transmission but the results are inconclusive. The most frequently studied methods of contraception have been oral contraceptives, injectable hormones, intrauterine devices, and nonoxynol-9. (Daly, 1994; Plummer, 1998) Traditional vaginal agents, used in African women for sexual enhancement and self-treatment of vaginal symptoms, has also been investigated as a potential co-factor for HIV transmission. (Dallabetta, 1995) For many of these studies, limitations of the study design preclude any definitive conclusions.
There is increasing evidence that host genetic or immunologic factors may protect against HIV infection. This has been investigated in cohort studies of Nairobi sex workers (Willerford, 1993) and in United States homesexual men (Dean, 1996), both of whom remained uninfected despite multiple sexual exposures to HIV. Individuals whom are homozygous for a null allele of CCR5 are relatively resistant to sexually transmitted infection with HIV, indicating an important, though not absolute, role for this receptor in viral transmission. However, homozygous CCR5 mutations were not found among 14 hemophiliacs who remained uninfected with HIV after being inoculated repeatedly with HIV contaminated Factor VIII concentrate from plasma during 1980-1985. (Zagury, 1998) In this study, investigators found an overproduction of betachemokines in most of the uninfected individuals.
Several viral factors have been proposed to play a role in the transmissibility of HIV. These include phenotypic characteristics (e.g., envelope proteins required for transmission), genetic factors that control the replicative capacity and fitness of the virus, and resistance to antiviral drugs. (Vernazza, 1999) Envelope sequences can define viral quasispecies that have been phenotypically arranged according to their ability to induce syncytia formation in infected T-cells. (Paxton, 1998) It appears that the most commonly transmitted phenotype is the non-syncytia-inducing (NSI), M-tropic viral strain, which is frequently found in those who have been recently infected. During the course of HIV infection the development of a more cytopathic, syncytia-inducing (SI), T-tropic viral phenotype can be found and this is often a precursor to the development of AIDS. While some researchers have suggested that NSI isolates of HIV are preferentially transmitted (Roos, 1992), others have not been able to show preferential transmission of this isolate. (Albert, 1995) Envelope sequences can also be used to define viral subtypes, or clades, and these subtypes may also influence the transmissibility of HIV. The distribution of HIV subtypes differ according to geographic region, with A, C, D, and E predominant in sub-Saharan Africa and Asia and B predominant in the United States, the Caribbean, South America, and Western Europe. (Hu, 1996) In one study, subtype E is reported to have greater tropism for Langerhans cells than subtype B (Soto-Ramirez, 1996) and may have a greater per contact transmissibility.
Lastly, the transmission characteristics of a viral strain that is resistant to certain antiretro- viral agents may differ from transmission of wild type virus. More research is needed in this emerging field of therapy-resistant virus and its characteristics.
The natural history of HIV infection in adults has been extensively documented in the medical literature. The impact of gender on the manifestations and progression of HIV disease is still being investigated. Concerns about gender-based differences in the course of HIV infection were expressed early in the epidemic. In most industrialized countries, women tended to have lower income, be un- or under-insured for health care, know less about HIV, more likely to be Black or Hispanic, and to have a personal or partner history of injection drug or cocaine use. Women also appeared to have more rapid progression of illness than men and to present with a different constellation of opportunistic conditions than men. When sophisticated statistical methods were applied that controlled for the tendency of women to receive less care, and to present with more advanced disease, gender-based differences in HIV disease course appeared to lessen. More recently, however, with better measures of viral activity and infirmity, the issue of gender-based differences in rate of disease course and virologic parameters has again been raised. These new observations have prompted active research into the impact of gender, hormones and demographic factors on the outcome of HIV infection.
HIV infects and induces cell death in a variety of human cell lines. T-helper lymphocytes (also known as CD4 cells) are a major target of viral infection, and circulating CD4 cells become steadily depleted from peripheral blood in most untreated infected persons. Thus quantification of CD4 cells in blood is a rather simple way of determining cumulative immunologic damage due to HIV. Profound CD4 cell depletion is unusual in persons who do not have HIV infection and are usually iatrogenic or associated with severe illnesses, such as chemotherapy-induced leukopenia. (Aldrich, 2000) Other immunological parameters become altered with HIV disease progression, and though often used for research purposes, they tend to be more difficult to measure and less reliable or more costly.
Untreated HIV infection is a chronic illness that progresses through characteristic clinical stages; AIDS is an endpoint of HIV infection, resulting from severe immunological damage, loss of an effective immune response to specific opportunistic pathogens and tumors. AIDS is diagnosed by the occurrence of these specific infections and cancers or by CD4-cell depletion to less than 200/mm3.
HIV can cause a wide range of symptoms and clinical conditions that reflect varying level of immunological injury and different predisposing factors. Certain conditions tend to occur in association with each other and at specific CD4 cell counts. Staging systems for HIV disease facilitate clinical evaluation and planning therapeutic interventions, help determine the individual level of infirmity, and give prognostic information. Untreated HIV infection is a chronic illness that progresses through characteristic clinical stages that can be used to describe infirmity. Several groups have produced organized staging systems to facilitate clinical evaluation and planning therapeutic interventions. In industrialized countries, the most widely used system for classifying HIV infection and AIDS in adults and adolescents was published by the United States Centers for Disease Control in 1993. (CDC, 1992)
The case definition (Table 2-3) begins first with confirmation of HIV infection either via serologic testing (combination of a screening method such as enzyme immunoassay and more specific confirmatory test such as Western Blot), or direct detection of HIV in patient tissue by viral culture, antigen detection or other test such as polymerase chain reaction (PCR). The definition of each stage of illness is then based on two types of information: peripheral blood CD4 cell counts and clinical manifestations. CD4 cell counts are placed in three strata, ranging from relatively normal (> 500 cells/mm3) to severe CD4 depletion (< 200 cells/mm3).
The clinical manifestations of HIV infection are also placed in three strata, generally in accordance with the level of immunologic dysfunction associated with the various conditions. (Table 2-3) Category A includes persons who have minimal clinical findings, clinical findings that do not indicate immune injury (including absence of symptoms), generalized lymphadenopathy or resolved acute HIV infection. Category B includes conditions that indicate the presence of a defect in cell-mediated immunity or conditions that appear to be worsened by HIV infection. Category C includes conditions that are considered AIDS defining, even in the absence of a CD4 cell count less than 200 cell/mm3. (CDC, 1992) The addition of specific laboratory measures such as plasma HIV RNA level improves prognostic value even after the occurrence of Category C conditions. (Lyles, 1999)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acute HIV infection is a transient symptomatic illness that can be identified in 40-90% of cases of new HIV infection. It is characterized by a high rate of HIV replication, high titers of virus in blood and lymphoid organs (up to several million copies of HIV RNA per mm3 of plasma), and initiation of an HIV-specific immune response. The amount of virus present in blood and tissues begins to fall after appearance of cytotoxic (killer) lymphocytes that specifically react with HIV antigens; the vigor of this response varies among individuals and is associated with subsequent rate of disease progression. (Cao, 1995) A pool of persistently infected CD4 cells (latent reservoirs) emerges early in the course of HIV infection and persists indefinitely. (Chun, 1998)
Symptoms have been identified from 5-30 days after a recognized exposure to HIV. (Schacker, 1998) The signs and symptoms of acute HIV infection are not specific; fever, fatigue, rash, headache, lymphadenopathy, pharyngitis, mild gastrointestinal upset, night sweats, aseptic meningitis and oral ulcerations are most frequently reported. Because the clinical signs of acute HIV infection resemble those of many acute viral illnesses, the correct diagnosis is often missed. Because early treatment at the time of acute infection may be especially beneficial, early suspicion of and evaluation for HIV infection should be encouraged. (Kahn, 1998)
Regardless of whether the syndrome of acute HIV infection is recognized or not, after the HIV-specific immunological response begins to control the intensity of viremia, a so-called viral set-point is established, which varies by individual. With exceedingly rare exceptions, the immunological response to HIV does not eliminate infection, but rather establishes a steady state between viral replication and elimination. (Henrad, 1995) A variable level of viremia is attained, which can be measured via quantification of the number of copies of HIV RNA present in blood (viral load). Although the viral load within the first 120 days of HIV infection is not of prognostic value (Schacker, 1998), in most patients a relatively stable viral load is attained after recovery from acute infection, and this viral set point is highly predictive of the rate of future progression of illness, at least as determined in studies that were largely focused on men. In the case of a high viral load set point (i.e., values ranging up from 40,000 copies per mm3) more rapid decline in CD4 cell counts and more rapid occurrence of Clinical Class B and C conditions will occur. Some individuals have viral load set points that are low (below 500 copies per mm3), which indicates a better prognosis; no evidence of progression (CD4 cell depletion or HIV-diseases) is seen for long periods of time in a small subset of patients (see section on long-term progression, below). The viral set point is likely influenced by several factors such as presence of other infections at the time of HIV exposure, genetic characteristics (particularly the type of HIV binding receptors present on lymphocytes), viral characteristics, age and perhaps gender (see below). (Kahn, 1998)
During the period of clinical stability acute illnesses and other events that can stimulate the immune system, such as influenza, herpes simplex outbreaks, and Tuberculosis as well as routine vaccinations, have been demonstrated to result in 10-1000 fold increases in viral load; these increases are transient and most often resolve within two months. (Stanley, 1996; Staprans, 1995) Thus, determination of viral load for prognostic purposes should not be done during or shortly after an acute illness.
For most HIV-infected persons, viral quasispecies evolve over time. Transition for the non-syncytia-inducing macrophage-tropic viral strains, that are commonly present after transmission to syncytia-inducing T-lymphocyte tropic strains occurs in many hosts. While variation of viral quasispecies with time is usual, the mechanism by which this process occurs has not been defined. However, transitions in viral quasispecies and cellular tropism has been observed to coincide with key clinical events such as CD4 cell depletion and development of symptomatic illness. These virologic changes may reflect evolution of a virus that is tailored to an individual’s immune response or other genetic characteristics. Interventions that prevent evolution of quasispecies in a host may yield effective therapies in the future.
The HIV RNA level in tissues does not correlate in a linear fashion with blood levels, so even in patients with undetectable plasma HIV RNA, intracellular and tissue HIV RNA can still be detected with more sophisticated techniques. (Hockett, 1999) Thus HIV replication continues at varying pace among infected persons, even those who can control viremia well.
HIV is also frequently present in the genital tract (Fiore, 1999; Iverson, 1998), where expression of inflammatory mediators, and lymphocyte receptors differ from blood and may influence the rate of viral replication and numbers of virions present. (Anderson, 1998; Hladik, 1999) While the quantities of HIV present in cervicovaginal fluid are generally similar to blood (Hart, 1999; Shaheen, 1999), they differ in some individuals. The finding that HIV isolates from the lower genital tract can have different genotypic markers than blood isolates from a single host (DiStefano, 1999; Shaheen, 1999), supports the concept that the lower genital tract sometimes functions as a separate virologic compartment.
In most studies of seroconverters, (persons for whom the date of the HIV infection can be estimated), 50-60% of adults will be diagnosed with an AIDS-defining condition within 10 years of infection (for the pre-HAART treatment era). Forty-eight percent of seroconverters die (due to any cause) after 10 years of infection. Increasing age is the factor most consistently associated with rate of progression and death in most groups of patients studied to date. (Alioum, 1998; UK Register of HIV Seroconverters Steering Committee, 1998; Pezzott, 1999; Prins, 1999) Date of infection also influences time from infection to an AIDS diagnosis, at least in some locations, demonstrating that even in the pre-HAART era, improvements in treatment resulted in tangible benefits. (Webber, 1998)
A large number of laboratory tests have been evaluated as prognostic indicators in HIV infection. For the most part, the tests can be divided into three groups: A. measures of HIV replication, B. measures of immune function and C. measures of inflammation. Group A is specific to HIV infection, Group B when indicating severe CD4 cell depletion is relatively specific to HIV infection, and Group C is generally not specific to HIV infection. HIV RNA quantitation, performed on fresh or fresh-frozen plasma or serum, is a powerful and accurate prognostic indicator in HIV infection, and is uniquely useful in determining response to Antiretroviral therapy. (Saag, 1996) In general the best measures of prognosis and staging include combinations of HIV RNA level, CD4 cell count and perhaps lymphocyte function (cytotoxic lymphocyte response to HIV). (Spijkerman, 1997; Vlahov, 1998)
In untreated adults the median time from HIV infection to AIDS in developed countries is 8-10 years. However, approximately 8-15% of HIV infected persons (most studies focus on men) remain symptom-free for much longer periods of time, a phenomenon that has been named long-term survival (LTS). Among these individuals who remain clinically stable without treatment for 5-8 years, two groups can be discerned, those who have stable CD4 cell counts and those who have low CD4 cell counts, but no AIDS defining conditions. (Schrager, 1994) Several factors have been found to be associated with long-term survival including host characteristics such as the presence of specific anti-HIV cytotoxic lympho-cyte responses, and viral characteristics such as defective genes and gene products. (Kirchhoff, 1995) LTS patients tend to have consistently lower levels of HIV RNA after the period of acute infection suggesting better control of viral replication. (Vesanen, 1996) For example, viral growth in peripheral mononuclear cells taken from LTS was markedly less than in PBMCs taken from healthy HIV-uninfected donors. (Cao, 1995)
In general, the predictors of the rate of HIVdisease progression and survival among women are the same as in men. CD4 cell count depletion and higher HIV RNA level are strong pre-dictors of progression and survival in women. (Anastos, 1996b) Several recent reports, however, describe gender-based differences in HIV RNA level and in rate of CD4 cell depletion; women had HIV RNA levels that were 30-50% lower than men who had comparable CD4 cell counts. (Bush, 1996; Evans, 1997; Farzadegan, 1998) Similar results occurred when analysis was restricted to seroconverters or when HIV culture was used to quantify viremia rather than RNA assays. (Lyles, 1998; Sterling, 1999) Intuitively, lower levels of circulating HIV RNA, which suggest lower steady state level of viremia, should be associ-ated with better outcome. However, the findings of several recent studies suggest that the lower HIV RNA level does not provide benefit to women. Women experienced more rapid CD4 cell depletion and faster progression to AIDS and death than men at similar HIV-RNA levels, even when race and age were taken into consideration. (Anastos, 1999a; Farzadegan, 1998)
Determination of the effect of gender on the rate of progression, time until occurrence of an AIDS defining condition and death, is a complicated process. Unless the date of HIV infection can be established, duration of infection becoPezzotti, 1999; Santoro-Lopes, 1998) Several studies have reported an excess proportion of infections or deaths due to bacterial infection, often pneumonia (Feldman, 1999), among women compared with men. (Melnich, 1994; Weisser, 1998)
In countries that are able to provide highly affective antiretroviral treatments (HAART), HIV-associated morbidity and mortality have declined significantly. (Michales, 1998; Miller, 1999a; Miller 1999b; Palella, 1998; Pezotti, 1999). These population findings, based on regional surveillance systems, were preceded by a multitude of clinical trials that demonstrated clinical and virologic benefits of HAART. (Bartlett, 1996; Collier, 1996; Deeks, 1997; Hammer, 1997) Despite the promise and documented benefits of HAART, clinical progression continues to occur among recipients, particularly among persons who received antiretroviral treatment prior to initiation of HAART. (Ledergerber, 1999)
Viral resistance to HAART components can occur via several mechanisms, which for the most part involve mutation of viral target proteins. (Richman, 1996; Schapiro, 1999) The emergence of antiretroviral resistance is a function of several factors: prior treatment, pre-treatment level of viremia, drug levels (adherence to medication regimens, bioavailability of medications, adequate dosing) and specifics of the regimen. (Guilick, 1998; Ledergerber, 1999; Shafer, 1998) Multiple daily doses, side effects and in some cases, dietary restrictions aggravate the problem of achieving optimal drug levels since protease inhibitor agents are relatively poorly bioavailable. Suppression of viral replication and prevention of resistance are directly related to level of antiretroviral drug. Persistent viral replication provides opportunity of occurrence of resistance mutations, and selective pressure to support continued presence of such mutants. (Condra, 1998; Feinberg, 1997; Wong, 1997) Besides clinical treatment failure, emergence of antiretroviral resistance is now associated with transmission of resistant virus to previously uninfected persons, a finding that could portend significant limits to the effectiveness of these treatments in populations over long periods of time. (Boden, 1999; Brodine, 1999; Yerly, 1999)
Albert, J, Fiore, J, Fenyo, EM, et al. Biological phenotype of HIV-1 and transmission [letter]. AIDS, 9(7): 822-3, 1995.
Aldrich, J, Gross, R, Adler, M, et al. The effect of acute severe illness on CD4+ lymphocyte counts in nonimmunocompromised patients. Arch Int Med, 160:715-6,2000.
Alioum, A, Leroy, V, Commenges, D, Dabis, F, and Salamon, R. Effect of gender, age, transmission category, and Antiretroviral therapy on the progression of human immunodeficiency virus infection using multistate Markov models. Epidemiol, 9: 605-612, 1998.
Alliegro, MB, Dorrucci, M, Phillips, AN, et al. Incidence and consequences of preg-nancy in women with known duration of HIV infection. Italian seroconversion study group. Arch Int Med, 157: 2585 2590, 1997.
Anastos, K, Gange, SJ, Lau, B, et al. The Women s Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS). Gender Specific Differences in QuantitativeHIV-1 RNA levels. Paper presented at the 6th Conference on Retroviruses and Opportunistic infections, Chicago, IL, 1999a.
Anastos, K, Kalish, LA, Hessol, N, et al. The relative value of CD4 cell count and quantitative HIV-1 RNA in predicting survival in HIV-1-infected women: results of the womens interagency HIV study. AIDS(13), 1999b.
Anderson, DJ, Politch, JA, Tucker, LD, et al. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res Hum Retroviruses, 145: 543-549, 1998.
Ankrah, TC, Roberts, MA, Antwi, P, et al. The African AIDS case definition and HIV serology in medical in-patients at Komfo Anoyke Teaching Hospital, Kumasi, Ghana. W African J Med, 13(2): 98-101, 1994.
Balter, M. HIV incidence: more serious than we imagined. Science, 280: 1864, 1998.
Balter, M. AIDS now Worlds fourth biggest killer. Science, 284: 1101, 1999.
Bartlett, JG. Protease inhibitors for HIV infection. Ann Intern Med, 124(12): 1086, 1996.
Bessinger, R, Clark, R, Kissinger, P, Rice, J, and Coughlin, S. Pregnancy is not associated with the progression of HIV disease in women attending an HIV oupatient program. Am J Epidemiol, 147(5): 434-40, 1998.
Blanche, S, Rouzioux, C, Moscato, ML, et al. A prospective study of infants born to women seropositive for human immunodeficiency virus type 1. HIV Infection in Newborns French Collaborative Study Group. N Engl J Med, 320(25):1643-8, 1989.
Boden, D, Hurley, A, Zhang, L, et al. HIV-1 drug resistance in newly infected individuals. J Am Med Assoc, 282: 1135 1141, 1999.
Bongaarts, J. Global population growth: demographic consequences of declining fertility. Science, 282: 419-420, 1998.
Brettle, RP, Gore, SM, Bird, AG, and McNeil, AJ. Clinical and epidemiological implications of the Centers for Disease Control/World Health Organization reclassification of AIDS cases. AIDS, 7: 531-539, 1993.
Brettle, RP, Raab, GM, Ross, A, et al. HIV infection in women: immunological markers and the influence of pregnancy. AIDS, 9: 1177-1184, 1995.
Brodine, SK, Shaffer, RA, Starkey, MJ, et al. Drug resistance patterns, genetic subtypes, clinical features, and risk factors in military personnel with HIV-1 seroconversion. Ann Intern Med, 131: 502-506, 1999.
Buchbinder, SP, Katz, MH, Hessol, NA, O Malley, PM, and Holmberg, SD. Long-term HIV-1 infection with immunologic progression. AIDS, 8: 1123-1128, 1994.
Bush, CE, Donovan, RM, Markowitz, N, et al. Gender is not a factor in serum human immunodeficiency virus type 1 RNA levels in patients with viremia. J Clin Microbiol, 34(4): 970-972, 1996.
Buskin, SE, Diamond, C, and Hopkins, SG. HIV-infected pregnant women and progression of HIV disease. Arch Int Med, 158: 1277-1278, 1998.
Cameron, D. W., Simonsen, J. N., LJ, D. Costa, et al. Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet, 2(8660): 403-7, 1989.
Cao, Y, Qin, L, Zhang, L, Safrit, J, and Ho, DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med, 332(4): 201-208, 1995.
Carre, N, Boufassa, F, Hubert, JB, et al. Predictive value of viral load and other markers for progression to clinical AIDS after CD4+ cell count falls below 200/ml. Int J Epidemiol, 27: 897-903, 1998.
CDC. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Wkly Rep, RR-17, 1992.
CDC. Management of possible sexual, injecting-drug-use, or other nonoccupational exposure to HIV, including considerations related to Antiretroviral therapy. Morbid Mortal Wkly Rep, 47: (RR-17);1-14, 1998.
CDC. Administration of zidovudine during late pregnancy and delivery to prevent perinatal HIV transmission Thailand, 1996-1998. Morbid Mortal Wkly Rep, 47:151-4, 1998.
CDC. HIV/AIDS Surveillance Report . Atlanta, Georgia: U.S. Department of Health and Human Services, 1999.
Chun, T-W, Engel, D, Berrey, MM, et al. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci, 95: 8869-8873, 1998.
Collier, AC, Coombs, RW, Schoenfeld, DA, et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N Engl J Med, 334 (16): 1011-7, 1996.
Condra, JH. Resisting resistance: maximizing the durability of Antiretroviral therapy. Ann Intern Med, 128(11): 951-953, 1998.
Consten, EC, van Lanschot, JJ, Henny, PC, Tinnemans, JG, and van der Meer, JT. A prospective study on the risk of exposure to HIV during surgery in Zambia. AIDS, 9(6): 585-8, 1995.
Coombs, RW, Collier, AC, Allain, J-P, et al. Plasma viremia in human immunodeficiency infection. N Engl J Med, 321: 1626-1631, 1989.
Coombs, RW, Speck, CE, Hughes, JP, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis, 177(2): 320-30, 1998.
Cu Uvin, S, Caliendo, AM, Reinert, SE, et al. HIV-1 in the female genital tract and the effect of Antiretroviral therapy. AIDS, 12(7): 826-7, 1998.
Dallabetta, GA, Miotti, PG, Chiphangwi, JD, et al. Traditional vaginal agents: use and association with HIV infection in Malawian women. AIDS, 9(3): 293-7, 1995.
Daly, CC, Helling-Giese, GE, Mati, JK, and Hunter, DJ. Contraceptive methods and the transmission of HIV: implications for family planning. Genitourin Med, 70(2): 110-7, 1994.
De Cock, KM, Lucas, S, Coulibaly, D, Coulibaly, I-M, and Soro, B. Expansion of surveillance case definition for AIDS in resource-poor countries. Lancet, 342: 437-438, 1993.
De Cock, KM, Selick, R, Soro, B, Gayle, H, and Colebunders, RL. AIDS surveillance in Africa: a reappraisal of case definitions. Br Med J, 303: 1185-1188, 1991.
de Martino, M., Tovo, P. A., Galli, L., et al. HIV-I infection in perinatally exposed siblings and twins. The Italian Register for HIV Infection in Children. Arch Dis Child, 66(10): 1235-8, 1991.
Deacon, NJ, Tsykin, A, Solomon, A, et al. Genomic structure of an attenuated quasispecies of HIV-1 from a blood transfusion donor and recipients. Science, 270: 988-991, 1995.
Dean, M., Carrington, M., Winkler, C., et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science, 273(5283): 1856-62, 1996.
Deeks, SG, Smith, M, Holodniy, M, and Kahn, JO. HIV-1 protease inhibitors: a review for clinicians. J Am Med Assoc, 277: 145-153, 1997.
Del Amo, J, Petruckevitch, A, Phillips, A, et al. Disease progression and survival in HIV-1 infected Africans in London. AIDS, 12: 1203-1209, 1998.
Di Stefano, M, Fiore, JR, Monno, L, et al. Detection of multiple drug-resistance-associated pol mutations in cervicovaginal secretions. AIDS, 13: 992-994, 1999.
European Collaborative Study Group. Maternal viral load and vertical transmission of HIV-1: an important factor but not the only one. AIDS, 13: 1377-85, 1999.
European Study Group. Comparision of female to male and male to female transmission of HIV in 563 stable couples. European Study Group on Heterosexual Transmission of HIV. BMJ, 304(6830): 809-13, 1992.
Evans, JS, Nims, T, Cooley, J, et al. Serum levels of virus burden in early-stage human immunodeficiency virus type 1 disease in women. J Infect Dis, 175(4): 795-800, 1997.
Fahey, JL, Taylor, JMG, Detels, R, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med, 322: 166-172, 1990.
Farzadegan, H, Hoover, DR, Astemborski, J, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet, 352: 1510-1514, 1998.
Feinberg, M. Hidden dangers of incompletely suppressive Antiretroviral therapy. Lancet, 349: 1408-1409, 1997.
Feldman, C, Glatthaar, M, Morar, R, et al. Bacteremic pneumococcal pneumonia in HIV-seropositive and HIV-seronegative adults. Chest, 116: 107-114, 1999.
Fraser, VJ, and Powderly, WG. Risks of HIV infection in the healthcare setting. Annu Rev Med, 46: 203-11, 1995.
French, R, and Brocklehurst, P. The effect of pregnancy on survival in women infected with HIV: a systematic review of the literature and meta-analysis. Br J Obstet Gynecol, 105: 827-835, 1998.
Gallant, JE, Eldred, LJ, Leslie, JM, Chaisson, RE, and Quinn, TC. Impact of the 1993 revision of the CDC case definition on the performance of the W.H.O. and PAHO clinical case definitions for AIDS. AIDS, 7(10): 1396-1397,1993.
Gallant, JE, Somani, J, Chaisson, RE, et al. Diagnostic accuracy of three clinical case definitions for advanced HIV diseases. AIDS, 6(3): 295-299, 1992.
Ghys, P. D., Fransen, K., Diallo, M. O., et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote dIvoire. AIDS, 11(12): F85-93, 1997.
Gilks, C. F., and Wilkinson, D. Reducing the risk of nosocomial HIV infection in British health workers working overseas: role of post-exposure prophylaxis. BMJ, 316(7138): 1158-60, 1998.
Gilliam, B. L., Dyer, J. R., Fiscus, S. A., et al. Effects of reverse transcriptase inhibitor therapy on the HIV-1 viral burden in semen. J Acquir Immune Defic Syndr HumRetrovirol, 15(1): 54-60, 1997.
Giorgi, JV, Ho, HN, Hirji, K, and al, et. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38-CD8+ cells is associated with subsequent stable CD4+ cell levels. J Infect Dis, 170:775-781, 1994.
Gollub, EL, and Metzger, D. Community-level HIV intervention work for women means restructing society and culture. Am J Public Health, 89(11): 1762, 1999.
Greenspan, D, and Greenspan, JS. HIV-related oral disease. Lancet, 348: 729-733, 1996.
Gulick, RM, Mellors, JW, Havlir, D, et al. Similtaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100-week follow-up. J Am Med Assoc, 280: 35-41, 1998.
Gupta, P, Mellors, J, Kingsley, L, et al. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol, 71(8):6271-5, 1997.
Gwinn, M, and Wortley, PM. Epidemiology of HIV infection in women and newborns. Clin Obstet Gynecol, 39(2): 292-304, 1996.
Hamed, KA, Winters, MA, Holodniy, M, Katzenstein, DA, and Merigan, TC. Detection of human immunodeficiency virus type 1 in semen: effects of disease stage and nucleoside therapy. J Infect Dis, 167(4): 798-802, 1993.
Hammer, SM, Squires, KE, Hughes, MD, et al. A controlled tiral of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med, 337(11):725-33, 1997.
Harrer, T, Harrer, E, Kalams, SA, et al. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection: breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J Immunol, 156: 2616-2623, 1996.
Hart, CE, Lennox, JL, Pratt-Palmore, M, et al. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis, 179:871-882, 1999.
Henrad, DR, Phillips, JF, Muenz, LR, et al. Natural history of HIV-1 cell-free viremia. J Am Med Assoc, 274(7): 554-558, 1995.
Hessol, NA, Lifson, AR, and Rutherford, GW. Natural history of human immunodeficiency virus infection and key predictors of HIV disease progression. AIDS Clin Rev: 69-93, 1989.
Hladik, F, Lentz, G, Delpit, E, McElroy, A, and McElrath, MJ. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J Immunol, 163: 2306-2313, 1999.
Hocke, C, Morlat, P, Chene, G, Dequae, L, and Dabis, F. Prospective cohort study of the effect of pregnancy on the progression of human immunodeficiency virus infection. The Grouped Epidaemiologie Clinique Du SIDA en Aquitaine. Obstet Gynecol, 86: 886-891, 1995.
Hockett, RD, Kilby, JM, Derdeyn, CA, et al. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J Exp Med, 189(10): 1545-1554, 1999.
Hu, DJ, Dondero, TJ, Rayfield, MA, et al. The emerging genetic diversity of HIV. The importance of global surveillance for diagnostics, research, and prevention. JAMA, 275(3): 210-6, 1996.
International AIDS Society. Place of antiretroviral drugs in the treatment of HIV-infected people in Africa. AIDS, 13(2): 1151-3, 1999.
Jacobs, L. UNAIDS chief warns against complacency in battle against AIDS in Asia. Paper presented at the 5th International Congress on AIDS in Asia and the Pacific, Kuala Lumpur, 1999.
Kahn, JO, and Walker, BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med, 339(1): 33-39, 1998.
Kanki, PJ, Hamel, DJ, Sankale, J-L, et al. Human immunodeficiency virus type 1 sub-types differ in disease progression. J Infect Dis, 179: 68-73, 1999.
Kaplan EH, Heimer R. A model-based estimate of HIV infectivity via needle sharing. JAIDS, 5:1116-8, 1992.
Kassa, E, de Wit, R, Hailu, E, et al. Evaluation of the World Health Organization staging system for HIV infection and disease in Ethiopia: association between clinical stages and laboratory markers. AIDS, 13: 381-389, 1999.
Kirchhoff, F, Greenough, TC, Brettler, DB, Sullivan, JL, and Desrosiers, RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med, 332(4): 228-232, 1995.
Koot, M, and et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Annals of Internal Medicine, 118: 681-688, 1993.
Krieger, JN, Coombs, RW, Collier, AC, et al. Recovery of human immunodeficiency virus type 1 from semen: minimal impact of stage of infection and current antiviral chemotherapy. J Infect Dis, 163(2): 386 8, 1991.
Laga, M, Manoka, A, Kivuvu, M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS, 7: 95-102, 1993.
Laga, M, Taelman, H, Van der Stuyft, P, et al. Advanced immunodeficiency as a risk factor for heterosexual transmission of HIV. AIDS, 3(6): 36-6, 1989.
Lazzarin, A, Saracco, A, Musicco, M, and Nicolosi, A. Man-to-woman sexual transmission of the human immunodeficiency virus. Risk factors related to sexual behavior, mans infectiousness, and womans susceptibility. Italian Study Group on HIV Heterosexual Transmission. Arch Intern Med, 151(12): 241-16, 1991.
Learmont, JC, Gecy, AF, Mills, J, et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1: a report from the Sydney blood bank cohort. N Engl J Med, 340: 1715-1722, 1999.
Ledergerber, B, Egger, M, Oprovil, M, et al. Clinical progression and virologic failure on highly active Antiretroviral therapy in HIV-1 patients: a prospective cohort study. Lancet, 353: 863-868, 1999.
Lifson, AR, Allen, S, Wolf, W, et al. Classification of HIV infection and disease in women from Rwanda: evaluation of the World Health Organization HIV staging system and recommended modifications. Ann Intern Med, 122: 262-270, 1995.
Lifson, AR, O Malley, PM, Hessol, NA, et al. HIV seroconversion in two homesexual men after receptive oral intercourse with ejaculation: implications for counseling concerning safe sexual practices. Am J Public Health, 80(12): 1509-11, 1990.
Lyles, CM, Vlahov, D, Farzadegan, H, et al. Comparisons of two measures of human immunodeficiency virus (HIV) type 1 load in HIV risk groups. J Clin Microbiol, 36(12): 3647-3652, 1998.
Lyles, RH, Chu, C, Mellors, JW, et al. Prognostic value of plasma HIV RNA in the natural history of Pneu- mocystis carinii pneumonia, cytomegalovirus and Mycobacterium avium complex. AIDS, 13: 341-349, 1999.
Marmor, M, Weiss, LR, Lyden, M, et al. Possible female-to-female transmission of human immunodeficiency virus. Ann Intern Med, 105(6): 969, 1986.
Martin, MP, Dean, M, Smith, MW, et al. Genetic acceleration of AIDS progression by a promoter variant of CCR5. Science, 282: 1907-1911, 1998.
Mastro TD, de Vincenzi I. Probabilities of sexual HIV-1 transmission. AIDS 10 (suppl A): 75-82, 1996.
Mati, JK, Mbugua, S, and Wanderi, P. Cervical cancer in Kenya: prospects for early detection at primary level. Int J Gynaecol Obstet, 47(3): 261-7, 1994.
McFarland, W, Mvere, D, Shandera, W, and Reingold, A. Epidemiology and prevention of transfusion-associated human immunodeficiency virus transmission in sub-Saharan Africa. Vox Sang, 72(2): 85-92, 1997.
Melnick, SL, Sherer, R, Louis, TA, and al, et. Survival and disease progression according to gener of patients with HIV infection: the Terry Beirn Community Programs for Clinical Research on AIDS. J Am Med Assoc, 272: 1915-1921, 1994.
Michaels, SH, Clark, R, and Kissinger, P. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med, 339(6): 405-406, 1998.
Miller, V, Mocroft, A, Reiss, P, et al. Relations among CD4 lymphocyte count nadir, anti-retroviral therapy, and HIV-1 disease progression: results from the EuroSIDA Study. Ann Intern Med, 130: 570-577, 1999a.
Miller, V, Staszewski, S, Nisius, G, et al. Risk of new AIDS diseases in people on triple therapy. Lancet, 353: 463, 1999b.
Monini, P, Rotola, A, DeLellis, L, et al. Latent BK virus infection and Kaposis sarcoma pathogenesis. Int J Cancer, 66: 717-722, 1996.
Monzon, OT, and Capellan, JM. Female-to-female transmission of HIV. Lancet, 2(8549):40-1, 1987.
Morgan, D, Maude, GH, Malamba, SS, et al. HIV-1 disease progression and AIDS-defining disorders in rural Uganda. Lancet, 350: 245-250, 1997.
Morgan, D, Ross, A, Mayanja, B, Malamba, S, and Whitworth, J. Early manifestations (pre-AIDS) of HIV-1 infection in Uganda. AIDS, 12: 591-596, 1998.
Moses, S, Plummer, FA, Bradley, JE, et al. The association between lack of male circumcision and risk for HIV infection: a review of the epidemiological data. Sex Trans Dis, 21(4): 201-10, 1994.
Mostad, SB, Jackson, S, Overbaugh, J, et al. Cervical and vaginal shedding of human immunodeficiency virus type 1-infected cells throughout the menstrual cycle. J Infect Dis, 178(4): 983-991, 1998.
Munoz, A, Kirby, AJ, He, YD, et al. Long-term survivors with HIV-1 infection: incubation period and longitudinal patterns of CD4+ lymphocytes. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 8: 496-505, 1995.
Musicco, M, Lazzarin, A, Nicolosi, A, et al. Antiretroviral treatment of men infected with human immunodeficiency virus type 1 reduces the incidence of heterosexual transmission. Italian Study Group on HIV Heterosexual Transmission. Arch Intern Med, 154(17): 1971-6, 1994.
Nair, P, Alger, L, Hines, S, et al. Maternal and neonatal characteristics associated with HIV infection in infants of seropositive women. J Acquir Immune Defic Syndr, 6(3): 298-302, 1993.
Nicolosi, A, Correa Leite, ML, Musicco, M, et al. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Italian Study Group on HIV Heterosexual Transmission. Epidemiology, 5(6): 570-5, 1994.
O’Brien, WA, Hartigan, PM, Martin, D, et al. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N Engl J Med, 334: 426-431, 1996.
Palasanthiran, P., Ziegler, J. B., Stewart, G. J., et al. Breast-feeding during primary maternal human immunodeficiency virus infection and risk of transmission from mother to infant. J Infect Dis, 167(2): 441-4, 1993.
Palella, FJ, Delaney, KM, Moorman, AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med, 338(13): 853-860, 1998.
Paxton, WA, and Kang, S. Chemokine receptor allelic polymorphisms: relationships to HIV resistance and disease progression. Semin Immunol, 10(3): 187-94, 1998.
Perry, S, Jacobsberg, L, and Fogel, K. Orogenital transmission of human immunodeficiency virus (HIV). Ann Intern Med, 111(11): 951-2, 1989.
Pezotti, P, Napoli, PA, Acciai, S, et al. Increasing survival time after AIDS in Italy: the role of new combination antiretroviral therapies. AIDS, 13: 249-255, 1999.
Pezzotti, P, Galai, N, Vlahov, D, et al. Direct comparison of time to AIDS and infectious disease death between HIV seroconverter injection drug users in Italy and the United States: results from the ALIVE and ISS studies. J Acquired Immune Defi Syndr Hum Retrovirol, 20: 275-282, 1999.
Phoolchareon, W. HIV/AIDS prevention in Thailand: success and challenges. Science, 280: 1873-1874, 1998.
Planella, T, Cortes, M, Martinez-Bru, C, et al. The predictive value of several markers in the progression to acquired immunodeficiency syndrome. Clin Chem Lab Med, 36:169-173, 1998.
Plourde, PJ, Pepin, J, Agoki, E, et al. Human immunodeficiency virus type 1 seroconversion in women with genital ulcers. J Infect Dis, 170(2): 313-7, 1994.
Plummer, FA, Simonsen, JN, Cameron, DW, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis, 163(2): 233-9, 1991.
Plummer, FA. Heterosexual transmission of human immunodeficiency virus type 1 (HIV): interactions of conventional sexually transmitted diseases, hormonal contraception and HIV-1. AIDS Res Human Retroviruses, Suppl: 5-10, 1998.
Prins, M, Brettle, RP, Robertson, JR, et al. Geographical variation in disease progression in HIV-1 sero- converted injecting drug users in Europe? Int J Epidemiol, 28: 541-549, 1999.
Quinn, TC. Global burden of the HIV pandemic. Lancet, 348(9020): 99-106, 1996.
Quinn, TC, Wawer, MJ, Sewankambo, N, et al. Viral load and risk of heterosexual transmission of HIV-1 among sexual partners. 7th Conference on Retroviruses and Opportunistic infections, San Francisco, January 30-February 2, 2000.
Rabeneck, L, Hartigan, PM, Huang, IW, Souchek, J, and Wray, N. Predicting progression to AIDS: an evaluation of two approaches. J Gen Intern Med, 11: 622-624, 1996.
Rich, JD, Buck, A, Tuomala, RE, and Kazanjian, PH. Transmission of human immunodeficiency virus infection presumed to have occurred via female homesexual contact. Clin Infect Dis, 17(6): 1003-5, 1993.
Richman, DD. Antiretroviral drug resistance: mechanisms, pathogenesis, clinical significance. Antiviral Chemother, 4: 383-395, 1996.
Roos, MT, Lange, JM, de Goede, RE, et al. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J Infect Dis, 165(3):427-32, 1992.
Roques, P, Marce, D, Courpotin, C, et al. Correlation between HIV provirus burden and in utero transmission. AIDS, 7 Suppl 2: 39-43, 1993.
Royce, RA, Sena, A, Cates, W, Jr, and Cohen, MS. Sexual transmission of HIV. N Engl J Med, 336(15): 1072-8, 1997.
Saag, MS, Holodniy, M, Kurtizkes, DR, et al. HIV viral load markers in clinical practice. Nature Med, 2(6): 625-629, 1996.
Saah, AJ, Hoover, DR, Weng, S, et al. Association of HLA profiles with early plasma viral load, CD4+ cell counts and rate of progression to AIDS following acute HIV-1 infection. AIDS, 12: 2107-2113, 1998.
Sabatini, MT, Patel, K, and Hirschman, R. Kaposi s sarcoma and T-cell lymphoma in an immunodeficient woman: a case report. AIDS Res, 1(2): 135-7, 1983.
Samuel, MC, Hessol, N, Shiboski, S, et al. Factors associated with human immunodeficiency virus seroconversion in homesexual men in three San Francisco cohort studies, 1984-1989. J Acquir Immune Defic Syndr, 6(3): 303-12, 1993.
Santoro-Lopes, G, Harrison, LH, Moulton, LH, et al. Gender and survival after AIDS in Rio de Janeiro, Brazil. J Acquired Immune Defi Syndr Hum Retrovirol, 19: 403-407, 1998.
Satcher, D. The global HIV/AIDS epidemic. J Am Med Assoc, 281: 1479, 1999.
Schacker, TW, Hughes, JP, Shea, T, Coombs, RW, and Corey, L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med, 128(8): 613-620, 1998.
Schapiro, JM, Lawrence, J, Speck, R, et al. Resistance mutations to zidovudine and saquinavir in patients receiving zidovudine plus saquinavir or zidovudine and zalcitabine plus saquinavir in AIDS clincial trials group 229. J Infect Dis, 179:249-253, 1999.
Schechter, MT, Le, N, Craib, KJP, et al. Use of the Markov model to estimate the waiting times in a modified W.H.O. staging system for HIV infection. J Acquired Immun Defi Synd Hum Retrovirol, 8: 474-479, 1995.
Schrager, LK, Young, JM, Fowler, MG, Mathieson, BJ, and Vermund, SH. Long-term survivors of HIV-1 infection: definitions and research challenges. AIDS, 8(suppl 1): 595-5108, 1994.
Seidlin, M, Vogler, M, Lee, E, Lee, YS, and Dubin, N. Heterosexual transmission of HIV in a cohort of couples in New York City. AIDS, 7(9): 1247-54, 1993.
Sewankambo, N, Gray, RH, Wawer, MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet, 350: 546-550, 1997.
Shafer, RW, Winters, MA, Palmer, S, and Merigan, TC. Multiple concurrent reverse transcriptase and protease mutations and multidrug resistance of HIV-1 isolates from theavily treated patients. Ann Intern Med, 128(11): 906 911, 1998.
Shaheen, F, Sison, AV, McIntosh, L, Mukhtar, M, and Pomerantz, RJ. Analysis of HIV-1in the cervicovaginal secretions and blood of pregnant and nonpregnant women. J Hum Virol, 2: 154-166, 1999.
Soto-Ramirez, LE, Renjifo, B, McLane, MF, et al. HIV-1 Langerhans cell tropism associated with heterosexual transmission of HIV. Science, 271(5253): 1291-3, 1996.
Speck, C. E., Coombs, R. W., Koutsky, L. A., et al. Risk factors for HIV-1 shedding in semen. Am J Epidemiol, 150(6): 622-31,1999.
Spijkerman, IJB, Prins, M, Goudsmit, J, et al. Early and late HIV-1 RNA level and its association with other markers and disease progression in long-term AIDS-free homesexual men. AIDS, 11: 1383-1388, 1997.
St Louis, ME, Kamenga, M, Brown, C, et al. Risk for perinatal HIV-1 transmission according to maternal immunologic, virologic, and placental factors. JAMA, 269(22): 2853-9, 1993.
Stanley, SK, Ostrowski, MA, Justement, JS, et al. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med, 334: 222-1230, 1996.
Staprans, SI, Hamilton, BL, Follansbee, SE, et al. Activation of virual replication after vaccination of HIV-1-infected individuals. J Exp Med, 182(6): 1727-37, 1995.
Sterling, TR, Lyles, CM, Vlahov, D, et al. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis, 180:666-72, 1999.
Taha, TE, Gray, RH, Kumwenda, NI, et al. HIV infection and disturbances in vaginal flora during pregnancy. JAIDS, 20(1): 52-9, 1999.
Tindall, B, Evans, L, Cunningham, P, et al. Identification of HIV-1 in semen following primary HIV-1 infection. AIDS, 6(9): 949-52, 1992.
Tokars JI, Marcus R, Culver DH , et al, for the CDC Cooperative Needlestick Surveillance Group. Surveillance of HIV infection and zidovudine use among healthcare workers after occupational exposure to HIV-infected blood. Ann Intern Med 118:913-9, 1993.
UK register of HIV seroconverters steering committee. The AIDS incubation period in the UK estimated from a national register of HIV seroconverters. AIDS, 12:659-667, 1998.
van Benthem, BHB, Veuglers, PJ, Cornelisse, PGA, et al. Is AIDS a floating point between HIV seroconversion and death? Insights from the tricontinental seroconverter study. AIDS, 12: 1039-1045, 1998.
Vernazza, PL, Eron, JJ, Fiscus, SA, and Cohen, MS. Sexual transmission of HIV: infectiousness and prevention. AIDS, 13(2): 155-66, 1999.
Vernazza, PL, Gilliam, BL, Dyer, J, et al. Quantification of HIV in semen: correlation with antiviral treatment and immune status. AIDS, 11(8): 987-93, 1997.
Vesanen, M, Stevens, CE, Taylor, PE, Rubinstein, P, and Saksela, K. Stability in controlling viral replication identifies long-term nonprogressors as a distinct subgroup among human immunodeficiency virus type 1-infected persons. J Virol, 70(12):9035-9040, 1996.
Vittinghoff, E, Douglas, J, Judson, F, et al. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol, 150(3): 306-11, 1999.
Vlahov, D, Graham, N, Hoover, D, et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: plasma viral load and CD4+ cell count. J Am Med Assoc, 279(1): 35-40, 1998.
Webber, MP, Schoenbaum, EE, Gourevitch, MN, et al. Temporal trends in the progression of human immunodeficiency virus disease in a cohort of drug users. Epidemiol, 9: 613-617, 1998.
Weisser, M, Rudin, C, Battegay, M, et al. Does pregnancy influence the course of HIV infection? Evidence from two large Swiss cohort studies. J Acquired Immune Defi Syndr Hum Retrovirol, 17: 404-410, 1998.
Weniger, BG, Quinhaoes, EP, Sereno, AB, et al. A simplified surveillance case definition of AIDS derived from empirical clinical data. The Clinical AIDS Study Group, and the Working Group on AIDS case definition. J Acquired Immun Defi Synd Hum Retrovirol, 5(12): 1212-1223, 1992.
W.H.O. International Collaborating Group for the Study of the W.H.O. Staging. Proposed World Health Organization Staging System for HIV-Infection and Disease:preliminary testing by an international collaborative cross-sectional study. AIDS, 7:711-718, 1993.
Willerford, DM, Bwayo, JJ, Hensel, M, et al. Human immunodeficiency virus infection among high-risk seronegative prostitutes in Nairobi. J Infect Dis, 167(6): 1414-7, 1993.
Winkler, C, Modi, W, Smith, MW, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. Science, 279: 389-393, 1998.
Wong, JK, Gunthard, HF, Havlir, DV, et al. Reduction of HIV-1 in blood and lymphnodes following potent Antiretroviral therapy and the virologic correlates of treatment failure. Proc Natl Acad Sci, USA, 94: 12574-12579, 1997.
Yerly, S, Kaiser, L, Race, E, et al. Transmission of antiretroviral-drug-resistant HIV-1 variants. Lancet, 354: 729-733, 1999.
Zagury, D, Lachgar, A, Chams, V, et al. C-C chemokines, pivotal in protection against HIV type 1 infection. Proc Natl Acad Sci U S A, 95(7): 3857-61, 1998.
Zimmerman, PA, Buckler-White, A, Alkhatib, G, et al. Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Molecular Med, 3(1): 23-36, 1997.
HIV infection among U.S. women has increased significantly over the last decade, especially in communities of color. CDC estimates that, in the United States, between 120,000 and 160,000 adult and adolescent females are living with HIV infection, including those with AIDS.
Between 1992 and 1998, the number of persons living with AIDS increased in all groups, as a result of the 1993 expanded AIDS case definition and, more recently, improved survival among those who have benefited from the new combination drug therapies. During that 6-year period, a growing proportion of women were living with AIDS, reflecting the ongoing shift in populations affected by the epidemic. In 1992, women accounted for 14% of persons living with AIDS–by 1998, the proportion had grown to 20%.
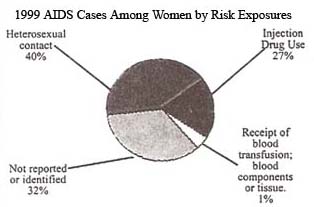
In just over a decade, the proportion of all AIDS cases reported among adult and adolescent women more than tripled, from 7% in 1985 to 23% in 1999. The epidemic has increased most dramatically among women of color. African American and Hispanic women together represent less than one-fourth of all U.S. women, yet they account for more than three-fourths (77%) of AIDS cases reported to date among women in our country. In 1999 alone (see chart above), women of color represented an even higher proportion of cases.
While AIDS-related deaths among women were decreasing as of 1998, largely as a result of recent advances in HIV treatment, HIV/AIDS remains among the leading causes of death for U.S. women aged 25-44. And among African American women in this same age group, AIDS was the third leading case of death in 1998.
In 1999 most women(40%)reported with AIDS were infected through heterosexual exposure to HIV, injection drug use accounted for 27%. In addition to the direct risks associated with drug injection (sharing needles), drug use also is fueling the heterosexual spread of the epidemic. A large proportion of women infected heterosexually were infected through sex with an injection drug user. Reducing the toll of the epidemic among women will require efforts to combat substance abuse, in addition to reducing HIV risk behaviors.
Many HIV/AIDS cases among women in the United States are initially reported without risk information, suggesting that women may be unaware of their partners’ risk factors or that healthcare providers are not documenting their risk. Historically, more than two-thirds of AIDS cases among women initially reported without identified risk were later reclassified as heterosexual transmission, and just over one-fourth were attributed to injection drug use.
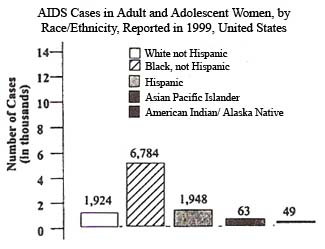
In the United States, the impact of HIV and AIDS in the African American community has been devastating. Through December 1999, CDC had received reports of 733,374 AIDS cases - of those, 272,881 cases occurred among African Americans. Representing only an estimated 12% of the total U.S. population, African Americans make up almost 37% of all AIDS cases reported in this country.
Researchers estimate that 240,000-325,000 African Americans - about 1 in 50 African American men and 1 in 160 African American women - are infected with HIV. Of those infected with HIV, it is estimated that almost 118,000 African Americans were living with AIDS at the end of 1998.
In 1999, more African Americans were reported with AIDS than any other racial/ethnic group
Data on HIV and AIDS diagnoses in 25 states with integrated reporting systems show these trends are continuing. In these states, during the period from January 1996 through June 1999, African Americans represented a high proportion (50%) of all AIDS diagnoses, but an even greater proportion (57%) of all HIV diagnoses. And among young people (ages 13 to 24), 65% of the HIV diagnoses were among African Americans.
Adult/Adolescent Men . Among African American men with AIDS, men who have sex with men (MSM) represent the largest proportion (37%) of reported cases since the epidemic began. The second most common exposure category for African American men is injection drug use (34%), and heterosexual exposure accounts for 8% of cumulative cases.
Adult/Adolescent Women . Among African American women, injection drug use has accounted for 42% of all AIDS case reports since the epidemic began, with 38% due to heterosexual contact.
Looking at select seroprevalence studies among high-risk populations gives an even clearer picture of why the epidemic continues to spread in communities of color. The data suggest that three interrelated issues play a role–the continued health disparities between economic classes, the challenges related to controlling substance abuse, and the intersection of substance abuse with the epidemic of HIV and other sexually transmitted diseases (STDs).
It is clear that the public sector alone cannot successfully combat HIV and AIDS in the African American community. Overcoming the current barriers to HIV prevention and treatment requires that local leaders acknowledge the severity of the continuing epidemic among African Americans and play an even greater role in combating HIV/AIDS in their own communities. Additionally, HIV prevention strategies known to be effective (both behavioral and biomedical) must be available and accessible for all populations at risk.
The United States has a large and growing Hispanic population that is heavily affected by the HIV/AIDS epidemics. In 1999, Hispanics represented 13% of the U.S. population (including residents of Puerto Rico), but accounted for 19% of the total number of new U.S. AIDS cases reported that year (9,021 of 46,400 cases). The AIDS incidence rate per 100,000 population (the number of new cases of a disease that occur during a specific time period) among Hispanics in 1999 was 25.6, almost 3 times the rate for whites (7.6) but lower than the rate for African Americans (66.0).
Hispanics in the United States include a diverse mixture of ethnic groups and cultures. As shown in the chart at left, HIV exposure risks for U.S.-born Hispanics and Hispanics born in other countries vary greatly1, indicating a need for specifically targeted prevention efforts.
Between 1992 and 1998, the number of persons living with AIDS increased in all groups, as a result of the 1993 expanded AIDS case definition and, more recently, improved survival among those who have benefited from the new combination drug therapies. During that 6-year period, the characteristics of persons living with AIDS were changing, reflecting an expansion of the epidemic, particularly in minority populations. In 1992, 17% of those estimated to be living with AIDS were Hispanic, while in 1998, 19% were Hispanic. In comparison, non-Hispanic whites represented 49% of people estimated to be living with AIDS in 1992, but only 39% in 1998.
Cumulatively, males account for the largest proportion (81%) of AIDS cases reported among Hispanics in the United States, although the proportion of cases among women is rising. Women represent 19% of cumulative AIDS cases among Hispanics, but account for 22% of cases reported in 1999 alone. Fifty-seven percent of Hispanics reported with AIDS in 1999 were born in the U.S.; of those 43% were born in Puerto Rico.
From the beginning of the epidemic through December 1999, 107,867 Hispanic men have been reported with AIDS in the United States. Of these cases, men who have sex with men (MSM) represent 43%, injection drug users (IDUs) account for 36%, and 6% of cases were due to heterosexual contact. About 7% of cases were among Hispanic men who both had sex with men and injected drugs. Among men born in Puerto Rico, however, injection drug use accounts for a significantly higher proportion of cases than male-male sex.
For adult and adolescent Hispanic women, heterosexual contact accounts for the largest proportion (47%) of cumulative AIDS cases, most of which are linked to sex with an injection drug user. Injection drug use accounts for an additional 40% of AIDS cases among U.S. Hispanic women.
While race and ethnicity alone are not risk factors for HIV infection, underlying social and economic conditions (such as language or cultural diversity, higher rates of poverty and substance abuse, or limited access to healthcare) may increase the risk for infection in some Hispanic American communities.
To improve prevention programs in Hispanic communities across the United States, in addition to addressing underlying social and economic conditions, we must apply the lessons we have already learned about the design of culturally appropriate HIV prevention efforts for each Hispanic population.
In the United States, HIV-related death has the greatest impact on young and middle-aged adults, particularly racial and ethnic minorities. In 1998, HIV was the fifth leading cause of death for Americans between the ages of 25 and 44. Among African American men in this age group, HIV infection has been the leading cause of death since 1991. In 1998, among black women 25-44 years old, HIV infection was the third leading cause of death. Many of these young adults likely were infected in their teens and twenties. It has been estimated that at least half of all new HIV infections in the United States are among people under 25, and the majority of young people are infected sexually. In 1999, 1,813 young people (ages 13 to 24) were reported with AIDS, bringing the cumulative total to 29,629 cases of AIDS in this age group. Among young men aged 13- to 24-years, 50% of all AIDS cases reported in 1999 were among men who have sex with men (MSM); 8% were among injection drug users (IDUs); and 8% were among young men infected heterosexually. In 1999, among young women the same age, 47% of all AIDS cases reported were acquired heterosexually and 11% were acquired through injection drug use.
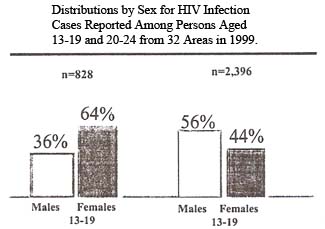
Among both males and females in this age group, the proportion of cases with exposure risk not reported or identified (25% for males and 41% for females) will decrease and the proportion of cases attributed to sexual contact and injection drug use will increase as follow-up investigations are completed and cases are reclassified into these categories. Surveillance data analyzed from 25 states with integrated HIV and AIDS reporting systems for the period between January 1996 and June 1999 indicate that young people (aged 13 to 24) accounted for a much greater proportion of HIV (13%) than AIDS cases (3%). These data also show that even though AIDS incidence (the number of new cases diagnosed during a given time period, usually a year) is declining, there has not been a comparable decline in the number of newly diagnosed HIV cases among youth.
Scientists believe that cases of HIV infection diagnosed among 13- to 24-year-olds are indicative of overall trends in HIV incidence (the number of new infections in a given time period, usually a year) because this age group has more recently initiated high-risk behaviors. Females made up nearly half (49%) of HIV cases in this age group reported from the 32 areas with confidential HIV reporting for adults and adolescents in 1999-and in young people between the ages of 13 and 19, a much greater proportion of HIV infections was reported among females (64%) than among males (36%). Cumulatively, young African Americans are most heavily affected, accounting for 56% of all HIV cases ever reported in this age group in these 32 areas.
CDC research has shown that early, clear communications between parents and young people about sex is an important step in helping adolescents adopt and maintain protective sexual behaviors. In addition, a wide range of activities must be implemented in communities to reduce the toll HIV infection and AIDS takes on young Americans.
Of the adults reported with AIDS in the United States through June 30, 2000, 22,618 had been employed in health care. These cases represented 5.1 percent of the 445,380 AIDS cases reported to CDC for whom occupational information was known (information on employment in the healthcare setting was missing for 299,723 reported AIDS cases).
The type of job is known for 21,261 (94%) of the 22,618 reported healthcare workers with AIDS. The specific occupations are as follows: 1,714 physicians, 114 surgeons, 4,928 nurses, 474 dental workers, 431 paramedics, 2,965 technicians, 1,019 therapists, and 4,985 health aides. The remainder are maintenance workers, administrative staff, etc. Overall, 74% of the healthcare workers with AIDS, including 1,345 physicians, 85 surgeons, 3,660 nurses, 374 dental workers, and 304 paramedics, are reported to have died.
CDC is aware of 56 healthcare workers in the United States who have been documented as having seroconverted to HIV following occupational exposures. Twenty-five have developed AIDS. These individuals who seroconverted include 19 laboratory workers (16 of whom were clinical laboratory workers), 23 nurses, 6 physicians, 2 surgical technicians, 1 dialysis technician, 1 respiratory therapist, 1 health aide, 1 embalmer/morgue technician, and 2 housekeepers/maintenance workers. The exposures were as follows: 48 had percutaneous (puncture/cut injury) exposure, 5 had mucocutaneous (mucous membrane and/or skin) exposure, 2 had both percutaneous and mucocutaneous exposure, and 1 had an unknown route of exposure. Forty-nine healthcare workers were exposed to HIV-infected blood, 3 to concentrated virus in a laboratory, 1 to visibly bloody fluid, and 3 to an unspecified fluid.
CDC is also aware of 138 other cases of HIV infection or AIDS among healthcare workers who have not reported other risk factors for HIV infection and who report a history of occupational exposure to blood, body fluids, or HIV-infected laboratory material, but for whom seroconversion after exposure was not documented. The number of these workers who acquired their infection through occupational exposures is unknown.