|
12. Hemochromatosis |
v
Hemochromatosis
Hemochromatosis, the most common form of iron overload disease, is an
inherited disorder that causes the body to absorb and store too much iron.
The extra iron builds up in organs and damages them. Without treatment, the
disease can cause these organs to fail.
Iron is an essential nutrient found in many foods. The greatest amount is
found in red meat and iron-fortified bread and cereal. In the body, iron
becomes part of hemoglobin, a molecule in the blood that transports oxygen
from the lungs to all body tissues.
Healthy people usually absorb about 10 percent of the iron contained in the
food they eat to meet the body needs. People with hemochromatosis absorb
more than the body needs. The body has no natural way to rid itself of
excess iron, so extra iron is stored in body tissues, especially the liver,
heart, and pancreas.
Causes
Genetic or hereditary hemochromatosis is mainly associated with a defect in
a gene called HFE, which helps regulate the amount of iron absorbed from
food. There are two known important mutations in HFE, named C282Y and H63D.
C282Y is the most important. When C282Y is inherited from both parents, iron
is overabsorbed from the diet and hemochromatosis can result. H63D usually
causes little increase in iron absorption, but a person with H63D from one
parent and C282Y from the other may rarely develop hemochromatosis.
The genetic defect of hemochromatosis is present at birth, but symptoms
rarely appear before adulthood. A person who inherits the defective gene
from both parents may develop hemochromatosis. A person who inherits the
defective gene from only one parent is a carrier for the disease but usually
does not develop it. However, carriers might have a slight increase in iron
absorption.
Scientists hope that further study of HFE will reveal how the body normally
metabolizes iron. They also want to learn how iron injures cells and whether
it contributes to organ damage in other diseases, such as alcoholic liver
disease, hepatitis C, porphyria cutanea tarda, heart disease, reproductive
disorders, cancer, autoimmune hepatitis, diabetes, and joint disease.
Juvenile hemochromatosis and neonatal hemochromatosis are two forms of the
disease that are not caused by an HFE defect. Their cause is unknown. The
juvenile form leads to severe iron overload and liver and heart disease in
adolescents and young adults between the ages of 15 and 30, and the neonatal
form causes the same problems in newborn infants.
Risk Factors
|
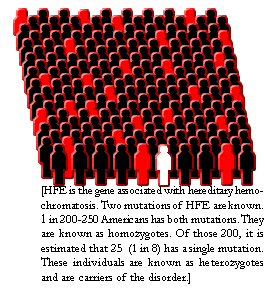
Hereditary hemochromatosis is one of the most common genetic disorders in
the United States. It most often affects Caucasians of Northern European
descent, although other ethnic groups are also affected. About 5 people in
1,000 (0.5 percent) of the U.S. Caucasian population carry two copies of the
hemochromatosis gene and are susceptible to developing the disease. One
person in 8 to 12 is a carrier of the abnormal gene. Hemochromatosis is less
common in African Americans, Asian Americans, Hispanic Americans, and
American Indians. |
 |
Symptoms
Joint pain is the most common complaint of people with hemochromatosis.
Other common symptoms include fatigue, lack of energy, abdominal pain, loss
of sex drive, and heart problems. Symptoms tend to occur in men between the
ages of 30 and 50 and in women over age 50. However, many people have no
symptoms when they are diagnosed.
If the disease is not detected early and treated, iron may accumulate in
body tissues and may eventually lead to serious problems such as
• arthritis
• liver disease, including an enlarged liver, cirrhosis, cancer, and liver
failure
• damage to the pancreas, possibly causing diabetes
• heart abnormalities, such as irregular heart rhythms or congestive heart
failure
• impotence
• early menopause
• abnormal pigmentation of the skin, making it look gray or bronze
• thyroid deficiency
• damage to the adrenal gland
Diagnosis
A thorough medical history, physical examination, and routine blood tests
help rule out other conditions that could be causing the symptoms. This
information often provides helpful clues, such as a family history of
arthritis or unexplained liver disease.
Blood tests can determine whether the amount of iron stored in the body is
too high. The transferrin saturation test determines how much iron is bound
to the protein that carries iron in the blood. The serum ferritin test shows
the level of iron in the liver. If either of these tests shows higher than
normal levels of iron in the body, doctors can order a special blood test to
detect the HFE mutation, which will help confirm the diagnosis. (If the
mutation is not present, hereditary hemochromatosis is not the reason for
the iron buildup, and the doctor will look for other causes.) A liver
biopsy, in which a tiny piece of liver tissue is removed and examined under
a microscope, may be needed. It will show how much iron has accumulated in
the liver and whether the liver is damaged.
Hemochromatosis is often undiagnosed and untreated. It is considered rare
and doctors may not think to test for it. The initial symptoms can be
diverse and vague and can mimic the symptoms of many other diseases. Also,
doctors may focus on the conditions caused by hemochromatosis—arthritis,
liver disease, heart disease, or diabetes—rather than on the underlying iron
overload. However, if the iron overload caused by hemochromatosis is
diagnosed and treated before organ damage has occurred, a person can live a
normal, healthy life.
Hemochromatosis is usually treated by a specialist in liver disorders (hepatologist),
digestive disorders (gastroenterologist), or blood disorders (hematologist).
Because of the other problems associated with hemochromatosis, several other
specialists may be on the treatment team, such as an endocrinologist,
cardiologist, or rheumatologist. Internists or family practitioners can also
treat the disease.
v
Diagnosis–How Do You Find Out
To diagnose hemochromatosis is an easy affair. Basically there are three
tests that confirm an iron overload. First there is Transferrin Saturation
(TS) or as it is called in some labs Percentage of Saturation:
Test # 1: After a 12-hour fast, measure Total Iron Binding Capacity (TIBC)
and the Serum Iron (SI). To achieve the percentage of Saturation you divide
the TIBC into SI.
| Serum Iron | SI = Yields Transferrin Saturation (TS) = or in some labs Percentage of Saturation |
|
Total Iron Binding Capacity Safe range = 12-44% |
TIBC |
Any values above this range must be considered diagnostic for
hemochromatosis and should cause immediate protocol treatment. Any values
far below this range may be a sign of bleeding ulcers, chronic infection or
cancer. Physicians should look for the cause of anemia.
Test # 2: Using the blood from the first draw, next check the amount of
storage iron– Serum Ferritin (SF)
Safe range = 5-150
A hemochromatosis patient needs to be at the lowest end of this range. We
say below 10. This needs to be the treatment goal.
Test # 3: This next test is given less frequently. It is initialized as UIBC.
It stands for Unbound Iron Binding Capacity.
Safe range is above = 146
If a patient checks below this test value, then he or she needs to be
treated for their hemochromatosis or their other iron overload condition.
If these tests measure out of safe ranges then aggressive treatment is
indicated. Diagnosis without treatment is useless. The patient must be
motivated to off-load the iron as fast as possible. The physician should not
watch these values over time or ignore them thinking they will improve on
their own. Once iron is absorbed in excess it will not correct itself. Iron
is not excreted. Its only exit from the body is by frequent bleeding or
chelation.
Some iron overloaded patients will present with a normal saturation and
still have an overload of iron.
If there is family history or symptoms or elevated ferritin over time, the
patient may be involved with this problem. In this case we recommend a
course of trial treatment. If the patients can tolerate the protocol, then
the treatment was justified. There are safety factors built into the proper
treatment that will disqualify the patient if they are not truly iron
overloaded. The physician sets the hematocrit level on the prescription for
the blood bank, for instance.
If protocol treatment is tolerated after 4-6 weeks without the patient’s
hematocrit or hemoglobin crashing, (below 30% or 10 respectively ), then
that in itself is further confirmation of the hemochromatosis or the iron
overload.
Treatment and Maintenance
The logic of the protocol treatment is to induce a mild anemia and maintain
it until the storage iron is greatly reduced. Serum ferritin is the measure
of storage iron and this number needs to come down below 10. This is
accomplished by bloodletting–therapeutic phlebotomies. By phlebotomy we
mean removal of a full unit of blood from the patient, approximately 500 mls.
This should be done in a medical setting. The schedule of this treatment
should be twice a week or at least once a week. The patient must be
motivated to off-load the iron as fast as possible. The best outcomes are
achieved by aggressive treatment. Timid treatment does not work–these
phlebotomies must be at least weekly. The attending physician writes a
prescription that tells the blood bank to remove a full unit of blood
according to schedule as long as the patient has a qualifying hematocrit of
30% or more. Some locations might prefer hemoglobin which should be set for
10 or above.
It is important to establish an anemia and not let up on it until de-ironing
has been completed. The might take from 6 months to three years depending
on the iron burden. Age is never a reason to disqualify someone from
treatment. Frailty, small of stature and extremely old/young may require
the adjustment in amount of blood removed, but never adjust the frequency.
This process can arrest or reverse most symptoms and return the patient to a
normal lifespan. Some patients might experience a complete reversal of all
symptoms. To exclude anyone from treatment for any reason is a death
sentence.
Chelation
For those people who cannot be bled because of extreme anemia, there is
chelation. The only chelator for iron approved in the US is Desferal (desferoxomine).
This approach lacks the complete efficacy of bloodletting and should be
employed only where absolutely necessary. Declared an orphan product by the
manufacturer Norvartis , it is expensive. A course of chelator per month
$6000-$8000. For some it is infused over night with a portable pump at home
during sleep over a 12-hour period. In some cases, the infusion pump is
installed in the body of the patient.
There may be side effects for some patients. This will off-load some iron
and prolong the patient’s life. Mild anemias such as the lesser thalassemia
and some of the sideroblastic anemias may qualify for phlebotomy. A
physician considering chelation for a patient should consult an expert to
see if their patient won’t qualify for bleeding after all. For those
patients who have tried this approach and found for some reason they could
not tolerate this regimen are forced to look for an alternative.
Maintenance
After the patient has had their ferritin reduced below 10 they are declared
de-ironed. Now it is time to change the phlebotomy schedule. Usually 2-6
times a year is sufficient to keep them from reaccumulating the overload.
In this process the threshold of the hematocrit/hemoglobin can be raised
somewhat. For the first year deciding how often is a matter of trial and
error by the physician and patient. Serum ferritin should be checked yearly
to this end. Maintenance will have to be a life time affair from this point
on. To permit re-accumulation is to invite a premature death.
Venous Access
Some patients will have limited access to the veins for various reasons.
There are some things that may help with this. If the veins are small, deep
set or without tensile strength, a smaller needle might be used. A
butterfly needle–18 gauge–helps tremendously. It may take 10-15 minutes
longer in the bleeding process but helps with venous health overall. In
some medical settings a glass bottle is used and set on the floor. This
approach can cause too much vacuum on the veins and may collapse them before
a full unit is taken. Blood banks and labs that use the latest equipment
are the best treatment settings. In some extreme cases a catheter or shunt
can be installed in the shoulder for access. This method has added
maintenance problems so it should be used only if absolutely necessary. In
the list of priorities treatment needs to be at the top. Any process that
helps with patient compliance should be pursued.
Treatment–Patient Information
Currently, therapeutic phlebotomy or blood extraction is the most efficient
means of tissue iron reduction. However, preventive measures may be
incorporated into diet and behavior that can reduce the amount of iron
absorbed.
Phlebotomy
A phlebotomy is a procedure used to remove blood from a person. It is the
opposite of a transfusion, which is a way to give blood to a person. Those
with hemochromatosis (HH), also called iron overload disorder, and others
with iron loading anemias, store excess iron in their bodies and must have
phlebotomies to remove the iron.
Excess iron in a person’s body can cause damage to the liver, pancreas,
pituitary, joints and heart. As a result of this damage, one can develop
cirrhosis of the liver, diabetes, impotence, arthritis, and heart failure.
Therefore, removing the excess iron as soon as possible is critical.
People can lose small amounts of iron by simply taking half an aspirin a
day. But for those with serious iron overload, the phlebotomy is necessary
because it removes about 250 milligrams of iron with each treatment. When
iron stores are high, swift action to remove the excess is critical.
When Tests Indicate You Have Iron Overload
A doctor’s prescription or an order is needed to obtain the phlebotomies.
Usually an order is written for weekly or twice-weekly phlebotomies so long
as “pretreatment spun hematocrit is greater than 34%”. Hematocrit measures
the volume or amount of hemoglobin contained in a person’s blood.
Hemoglobin is made up of heme which is the iron containing red part of the
blood cell and globin which is a protein in the same red blood cell.
Hemoglobin carries oxygen to the body’s tissues and carbon dioxide away from
those same tissues.
Where Phlebotomy Treatments Are Done
Phlebotomies might be done at a blood donation center, as an outpatient in a
hospital or even in a doctor’s office. Your doctor will probably advise
places that provide the treatment. Consider convenience of location, cost to
do the phlebotomy, and how responsive the center is to your situation.
Once you have determined the facility that will provide treatment, a trip to
the lab is required. Before the phlebotomy may be done, hemoglobin and
hematocrit will be checked. Usually centers have labs on site; the results
will be forwarded to the attending nurse. These preliminary numbers help
assure you do not become seriously anemic (not enough iron); the desire is
to become only slightly anemic as a result of treatment. A person is
entitled to the results of any lab tests.
Keep Good Records.....
You may request a copy of lab work from the office manager in charge of
records in the doctor’s office. Obtaining lab results is highly recommended
so that a journal may be compiled. Journals will become a valuable tool if
you have to move to another town or seek treatment from another doctor.
Knowing about your disorder and understanding the diagnostic process helps
to speed recovery and avoid future health setbacks.
The Procedure
After the preliminary tests for hemoglobin and hematocrit are finished, a
nurse prepares you for the phlebotomy. Usually you will stretch out on a
comfortable recliner chair. The nurse takes your blood pressure, temperature
and heart rate (pulse). These numbers will be recorded on your medical chart
for future reference. The nurse then waits for the lab to call with
hemoglobin and hematocrit readings. After being notified levels are within a
safe range, your arm will be prepared for blood extraction.
A special band is tied around the upper part of the arm. This helps the vein
to stand up. You may have to squeeze a soft rubber ball or make a fist
several times to help the vein remain accessible. The nurse then swabs an
iodine-based antiseptic on the vein and all around the area near the vein.
This is to disinfect the area where the needle is to be inserted and to make
certain no bacteria gets into your system during treatment.
A special needle is then inserted into the vein. You might feel a little
pinch, but it lasts only a second. A piece of tape is placed over the needle
to keep it stable; you just sit back and relax.
Some like to bring a headset with earphones or a good book to read during
treatment. While relaxing, the blood flows from the needle, into a tube, and
then into the blood bag. The blood bag sits on a special scale that measures
the weight of the blood.
Myth #1. Taking two vials of blood from the arm is the same as a phlebotomy.
Incorrect: A true phlebotomy treatment involves removal of about 450cc of
blood or a full bag.
When the bag is sufficiently filled, about one pint, the phlebotomy is
complete. The speed with which the blood bag fills depends on the
thickness/thinness of your blood. Drinking adequate amounts of water, at
least 6-8 glasses a day for two weeks before the phlebotomy will help. If
you are still in school, you may want to get permission to carry bottled
water and drink at least 6 ounces each hour.
Myth #2. Iron can be removed by several methods.
Incorrect: Health food store products which claim to remove heavy metals
will not remove iron. Only two methods remove iron from the body: phlebotomy
and desferrioxamine which is a chelator used for those with conditions of
ironloading anemia.
While the blood is flowing out of your arm, you might think about all the
iron that is leaving your system. About 250 mgs of iron are removed with
each extraction. Think about how well you will be and the dreadful diseases
you may avoid by having this procedure. You may be finished in as few as ten
minutes or as many as thirty; again, it depends on your vein and thickness
of your blood.
After the treatment. After the phlebotomy, the nurse will remove the needle
from your arm. You may need to keep the area bandaged or you may need to
apply mild pressure if bleeding continues. You should rest for about 20
minutes following therapy. This is a precaution to insure you do not get
weak or dizzy. You may be given a snack while you are resting and it is
suggested you eat something after your therapy.
Your blood will be discarded regardless of where you have the phlebotomy. HH
blood is currently handled in the same way as contaminated blood. Efforts
are being made to change this. Don’t get frustrated or take it personally;
your blood may be labeled contaminated, but you are not.
Between phlebotomies. You might consider learning to drink at least eight
glasses of water a day, taking extra B12 with folic acid and vitamin E.
These supplements help to build red blood cells, which assure adequate
hemoglobin and hematocrit levels. Your doctor should recommend the amount
you take of these vitamins because the dosage will depend on your weight and
age.
It is important to remember that just because a supplement is beneficial,
taking more than the recommended dose does not provide a greater benefit;
indeed, it may cause damage. Also, gulping great amounts of water prior to
therapy is not wise; you may actually cause yourself to become water
intoxicated, a serious condition. Use wisdom; implement diet changes slowly
and with knowledge of the potential dangers associated with these changes.
Diet tips. You also may consider eating more fiber, refrain from cooking in
an iron skillet, and avoiding Vitamin C at mealtime. Fiber impedes iron
absorption while vitamin C enhances iron absorption. Drinking tea with meals
is helpful as the tannin in tea also impedes iron absorption. Decaffeinated
tea might be the better choice; some physicians believe that too much
caffeine can be unhealthy. Eat foods like fruit and juice high in vitamin C
between meals. You should be aware that tobacco is rich in iron and that
inhalation of this smoke directly or indirectly adds to your iron stores.
Exercise is a good idea. Regular, intense exercise or taking aspirin daily
will cause some blood loss and thus iron loss. However, you should consult
your doctor before incorporating any of these practices into your daily
routine. Aspirin can be dangerous for youths with fever and it can interact
with some drugs. Your pharmacist may be able to provide you with drug
interaction advise; if not, contact your doctor.
Each person responds to treatment in a unique way. You may need many
phlebotomies or only a few. Much depends on age, the extent of saturation,
one’s physical condition including symptoms, and the speed with which an
individual unloads iron. Your physician will help to determine this with a
rough estimate of mobilizable iron.
Note: The following calculation method provides a rough estimate only; it is
not an exact science. Ferritin levels can be unreliable or skewed when low
or very high, which can lead to distortion of estimated number of
extractions. Also, individuals with liver damage such as cirrhosis will
unload faster than those without liver damage.
Other factors that may skew ferritin include presence of inflammation or
infection. Used as a guideline to establish some benchmark of ferritin
levels however, this rough calculation helps your physician determine a
strategy for when ferritin can be measured so de-ironing progress can be
evaluated and undue anemia can be avoided.
Initial ferritin x 10= approximation of mobilizable iron in milligrams (mgs)
in your body. Since each phlebotomy removes about 250 milligrams of iron,
you can estimate how close you are getting to an acceptable level.
Example: Ferritin of 195 x 10=an estimated 1950 milligrams of stored iron
that can be removed with phlebotomy. The goal is to reach a safe ferritin
range of 25-75ng/mL. Using the estimated mobilizable iron of 1950 minus 450
(to avoid total depletion of ferritin) you can calculate about how many
treatments you might require. Example: 1950-450= 1500 mgs of iron needed to
be removed. Each treatment removes about 250mgs of iron, therefore:1500
divided by 250=6 treatments needed to reach a safe ferritin range. Your
ferritin may actually drop below the 25-75ng/mL range; a ferritin below
20ng/mL is considered anemia. In the course of treatment, your physician may
recommend that your target ferritin be lower than the 25ng/mL whereby you
might experience symptoms of anemia. Iron Disorders Institute considers this
practice outdated and suggests de-ironing not include forced-sustained
anemia to achieve de-ironed status.
Additional research and study must be done to determine benefits of
de-ironing practices. Since Iron Disorders Institute is mindful of
discomfort and problems related to anemia, IDI suggests a more conservative
step down process of treatment using phases.
Prior to treatment, a patient will have demonstrated a fasting ferritin
greater than 200ng/mL (females) or 300ng/mL (males) with an accompanying
transferrin iron saturation percentage value greater than 45%.
Phase one: When ferritin is above 1000ng/mL phlebotomy treatments will be
aggressive usually as frequent as twice weekly while tolerable and until
ferritin drops below 1000ng/mL. Using the estimated number of extractions (ferritin
X10, etc.) your physician will have an approximate timetable for when
ferritin might be nearing phase two or when ferritin is below 750-800ng/mL.
Phase two: When patient has had an estimated number of treatments sufficient
to drop ferritin below 1000ng/mL (preferably 750-800ng/mL) ferritin should
be measured to confirm patient’s unloading pattern. Frequency of treatment
may slow down from twice weekly to once a week or even to every other week
depending upon judgment of attending physician. During phase two
pretreatment hemoglobin of 12.0g/dL -13.0-g/dL (females) and 12.5g/dL to
13.5g/dL (males) is best as red blood cell production is better challenged
when hemoglobin levels are within this range.
During phase two: Patient might consider incorporating routine exercise
(twenty minute walk three times week minimum), adequate water intake (6-8
8oz glasses per day) and vitamin supplementation. Vitamin supplementation
might include: B complex (without C) plus extra B6, folic acid and B12.
Supplement amounts would depend upon patient’s age, weight, gender, and
condition such as lactation, pregnancy, or presence of other medical
conditions and established by attending physician. Except for B12, levels
are provided in Food and Nutrition Board, Institute of Medicine-National
Academy of Sciences, Dietary Reference Intakes: Tolerable Upper Intake
Levels (UL) for Certain Nutrients and Food Components.
Phase two treatment continues until patient reaches ferritin of 300ng/mL
(male), 200ng/mL female. Ferritin might be measured at this time to evaluate
unloading pattern. Treatment frequency might be reduced once again from
monthly or every six-eight weeks, maintaining pretreatment hemoglobin above
13.3g/dL but continued until ferritin is brought down to safe range 25-75ng/mL.
Phase three: Patient may donate blood routinely as defined by attending
physician for optimum quality of health or may have periodic therapeutic
phlebotomy by doctor’s order. Frequency of donation or therapeutic
phlebotomy will depend upon patient’s personal health profile as observed by
patient and attending physician: age, weight, response to treatment,
symptoms, rate of iron unloading and general physical condition.
At anytime during treatment you experience symptoms of heart irregularities
or severe abdominal pain or symptoms of anemia, alert your physician
immediately.
Symptoms of anemia can often be misunderstood by a patient as reoccurring
iron overload. Symptoms of anemia can include: fatigue, heart arrhythmia,
headache, sensitivity to cold, shortness of breath, dizziness and restless
legs syndrome. Again, if while treatment is in progress, you experience any
of these symptoms, bring it to the attention of your physician.
The recovery phase of treatment. Recovery is a period of time when you have
been adequately de-ironed and symptoms have diminished. Unfortunately, it is
possible that not all symptoms will disappear. If excess iron has had enough
time to damage critical organs, you may never restore these damaged organs
to full function. Issues of this nature need to be discussed with the doctor
to determine if additional treatment is appropriate.
During the recovery phase, you must be attentive to any sign of repeat
symptoms. So long as you remain symptom-free, the doctor will retest your
iron levels in three months. The initial three-month exam following recovery
will provide your baseline. A baseline is the first set of numbers after a
series of phlebotomies whereby a person’s pattern of unloading can be
established. These numbers are very important to your doctor and to your
health. From the baseline data the maintenance or treatment program for an
individual will be established. Additional retests to discern baseline may
have to be done; these will usually occur in three-month intervals.
A person’s length of recovery period, treatment and maintenance program is
determined by how often that person must have a phlebotomy to keep iron
levels in a normal range. Maintenance patients are those who have reached
normal iron ranges and who can remain within those normal ranges by donating
blood periodically.
Most blood donation centers allow one donation every eight weeks. If you are
a candidate for maintenance, then a periodic blood donation will suffice. If
you are found to need treatment, needing more than one extraction in eight
weeks, the attending physician will provide you with the necessary order for
additional phlebotomies. Your gastroenterologist or hematologist may refer
you back to your family physician for the maintenance phase of your therapy.
Afterwards, you may resume a normal, happy healthy life with only a small
adjustment to your schedule: a life-saving, blood donation every now and
then.
Source: Iron Disorders Institute Inc.
P.O. Box 2031
Greenville, South Carolina 29602
www.irondisorders.org
Screening for Hemochromatosis
Screening for hemochromatosis (testing people who have no symptoms) is not a
routine part of medical care or checkups. However, researchers and public
health officials do have some suggestions:
|
• |
Brothers and sisters of people who have hemochromatosis should have their blood tested to see if they have the disease or are carriers. |
|
• |
Parents, children, and other close relatives of people who have the disease should consider testing. |
|
• |
Doctors should consider testing people who have joint disease, severe and continuing fatigue, heart disease, elevated liver enzymes, impotence, and diabetes, because these conditions may result from hemochromatosis. |
Since the genetic defect is common
and early detection and treatment are so effective, some researchers and
education and advocacy groups have suggested that widespread screening for
hemochromatosis would be cost-effective and should be conducted. However, a
simple, inexpensive, and accurate test for routine screening does not yet
exist, and the available options have limitations. For example, the genetic
test provides a definitive diagnosis, but it is expensive. The blood test
for transferrin saturation is widely available and relatively inexpensive,
but it may have to be done twice with careful handling to confirm a
diagnosis and to show that it is the consequence of iron overload.
Prepared by Iron Overload Disease Association, Inc.
433 Westwind Drive, North Palm Beach, FL, 33408-5123
(561) 840-8512
www.ironoverload.org
v
Hemochromatosis and Anemia Diet
|
1. |
A low iron diet is not recommended or even possible to design. Iron is in everything and foods that contain iron also provide other essential elements to help heal and rebuild the body. Red meat is an important source of B vitamins. Fresh fruits and vegetables benefit the liver more than processed foods. |
|
2. |
Iron is not excreted. A normal metabolism for iron refrains from absorbing more than the daily need. One milligram of iron is lost daily through hair, fingernails, dead skin cells and other detritus. The average daily loss for menstruating woman is one and half milligrams. That one or one and a half is the daily need despite outdated levels recommended by the governmental agencies. |
|
3. |
Body damage from iron injury is entirely preventable. Treatment is completely benign. An individual with elevated iron should begin protocol treatment and be motivated to bring ferritin to the lowest end of normal ranges. Then the patient must continue a maintenance program to prevent the re-buildup. Each individual loads iron at a different rate. The first year is experimental. Measure the ferritin–measure of storage iron–at the end of each year, and adjust the schedule as necessary. |
|
4. |
Protocol treatment is blood removal once or twice a week at the blood bank. The patient should be well hydrated and should not skip meals. Treatment is inexpensive or free in some cases and is effective. Such treatment will return the patient to a normal lifespan and reverse most if not all symptoms. |
|
5. |
With a hemoglobin of 10 or higher and a hematocrit of 30% or higher, a full unit of blood should be drawn off once or twice a week. The blood is usable as donor blood when it meets all safety criteria. |
|
6. |
What about anemia? Anemias are iron-loading, except for anemias resulting from chronic blood loss or tumor. When iron accumulates in storage instead being used by hemoglobin, the patient’s hemoglobin will test low. Iron should not be administered. Instead the patient needs a complex of B vitamins, including B6, folate or folic acid and B12. The excess iron must be removed despite the anemia. |
|
7. |
When low iron is found, it is essential to seek the source of the blood loss or cancer. Cancer cells require iron to proliferate. It is dangerous to medicate with iron without first knowing the iron levels and then discovering the reason for low iron. |
|
8. |
When anemia is severe enough to require transfusions, physicians should be aware that this process will increase the dangerous iron burden. Iron must be simultaneously removed through the iron chelator Desferal. |
|
9. |
Preventing liver cancer is the patient’s primary goal. The patient should avoid medications where possible and protect the liver. The physicians should use medications only when absolutely necessary. |
|
10. |
Alcohol should be avoided until the de-ironing process is completed. When liver enzymes return to normal, the patient may ease back into social drinking. |
|
11. |
Tylenol ®–acetaminophen–should never be taken with alcohol. The patient who tests with elevated liver enzymes, as many do, must avoid Tylenol ® altogether. |
|
12. |
Those in protocol treatment will benefit from a B complex of vitamins, including B6, folic acid or folate and B12 Low doses of vitamin E–below 50 International Units daily–is also beneficial. |
|
13. |
Iron patients must check labels of processed foods for added iron or vitamin C. An example is that oatmeal or shredded wheat should be selected instead of the breakfast cereal Total ®. |
|
14. |
Who should take iron? The only candidate for iron supplementation is an individual who has had large portions of gut removed. |
|
15. |
The patient must not take any over-the-counter vitamin C. Vitamin C as a pill has been found to mobilize stored iron into the heart muscle where it sets up arrhythmia or heart rhythm disturbances. It can also cause people to over absorb iron even from their ordinary diets. And it can fire the iron that is stored in the joints and makes arthritis worse. The source for vitamin C should be any uncooked food. |
|
16. |
The patient must not eat or handle raw seafood. Cooked is OK, but not raw. Iron overloaded patients should also not walk on beaches barefoot. This is because of a bacterium common in all sea water called Vibrio vulnificus. This bacterium when it encounters stored iron is catastrophically toxic. Every year it is the cause of death and maiming when an undiagnosed iron overloaded patient comes in contact with this bacteria. |
|
17. |
Drinking tea can help inhibit iron absorption. Tannin is the blocking agent. The patient must not believe that this will be a replacement for protocol treatment. |
|
18. |
Excess iron stores in liver, heart, brain, pancreas, joints and everywhere. Iron oxidizes–rusts–and results in deadly and expensive disease. Symptoms of heart disease, cancer, cirrhosis, diabetes, arthritis, sexual dysfunction and others are completely preventable when they are based on iron. Maintaining low iron levels improves immunity, making iron unavailable to viruses, bacteria and cancer cells. |
|
19. |
The most common of several iron storage diseases is hemochromatosis. It is caused by the most frequently expressed genetic abnormality in any population and can result in the metabolic defect that leads to iron overload. It is estimated that 42 million Americans are at risk, including those with the double gene and those with the single gene expression. The single mutation may result in enough excess iron to cause heart attack or stroke, aside from full blown hemochromatosis. |
Prepared by Iron Overload Disease Association, Inc.
433 Westwind Drive, North Palm Beach, FL, 33408-5123
(561) 840-8512
www.ironoverload.org
v
Objections to Genetic Testing
|
• |
There are 40 known mutations of the HFE gene that can cause an overload of iron. The labs check for only the two most often expressed mutations: c282y and h63d. There are still some mutations that are yet to be discovered. Clearly this test is not ready to be used as a screening device at this time. Jerome Sullivan, MD PhD says that the genetic test can “confirm but not rule out hemochromatosis.” |
|
• |
Professional genetics organizations have said that person under the age of 18 should not be checked with genetic tests. But we know that children and even neonates can have too much iron even to the point of death. |
|
• |
Genetic testing is expensive, $150-$500 per person. All blood relatives must be checked. Think of the cost of screening an entire family. And the screening is incomplete, iron levels must still be checked. |
|
• |
There are two different naming conventions for each of these mutations. This adds to the possibility of confusion. Also there are issues of homozygosity (full blown expression) and heterozygosity (partial expression, sometimes called the carrier rate). It is possible to be a compound heterozygote which means that you have one copy of each of the tested mutations. We have literature in hand showing that people with only partial genetics can also get sick. The labs want to report the findings directly to your doctor. But your doctor is usually poorly trained to deal with genetics. It’s a highly specialized part of medicine. This information needs to be explained to the patient through a trained genetics counselor. Doctors like certainty. If there is any confusion, the doctor might back away from the diagnosis while he casts about for another explanation for the symptoms. Iron overload is fatal if not detected and treated. |
|
• |
Maybe it is the case that you can end up with an overload of iron without a genetic basis. This has not been yet ruled out. |
|
• |
The insurance companies have demonstrated a willingness to discriminate against people who have hemochromatosis. They usually refuse coverage altogether or if they do cover hemochromatosis patients it is with exorbitant premiums. If you are found with a genetic basis for excess iron you may be discriminated against even before you have an overload of iron. The U.S. Congress is trying to deal with the issue of genetic discrimination at this time. But whether they are able to come up with preventative measures that are enforceable is problematic. Remember the insurance industry has a strong lobby in place. |
|
• |
All of the above objections were based on the currently known HFE gene. Now a second HFE gene has recently been discovered in Switzerland. Of course it can be found in all populations around the world. It has been termed HFE2. Clearly as genetic science for excess iron is still evolving it is not ready to be used as a screening device. |
Sometime in the future the science
will be firmed in this area and a one-time test of your genes will make
sense. Presently Iron Overload Diseases Assn. recommends a simple blood
test called transferrin saturation (TS) or in some labs may be called
percentage of saturation. Any score above 44% will need immediate protocol
intervention. UIBC is an alternate test for screening. Safe levels are
above 146. Testing for too much iron is not a onetime check but a lifetime
survey. Everybody in the bloodline will need this test at each and every
physical no matter the age and no matter the gender. All that is needed for
good health where excess iron is concerned is vigilance and treatment where
necessary.
Source: Iron Overload Diseases Association, Inc.
Hope Through Research
Current research in hemochromatosis is concentrated in four areas:
|
• |
Genetics. Scientists are working to understand more about how the HFE gene normally regulates iron levels and why not everyone with an abnormal pair of genes develops the disease. |
|
• |
Pathogenesis. Scientists are studying how iron injures body cells. Iron is an essential nutrient, but above a certain level it can damage or even kill the cell. |
|
• |
Epidemiology. Research is under way to explain why the amounts of iron people normally store in their bodies differ. Research is also being conducted to determine how many people with the defective HFE gene go on to develop symptoms, as well as why some people develop symptoms and others do not. |
|
• |
Screening and testing. Scientists are working to determine at what age testing is most effective, which groups should be tested, and what the best tests for widespread screening are. |