|
5. Hepatitis |
|
v Viral Hepatitis: A Through E and Beyond |
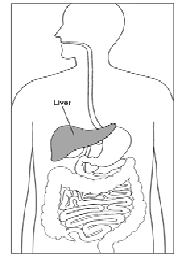 |
• jaundice (yellowing of the skin and eyes)
• fatigue
• abdominal pain
• loss of appetite
• nausea
• vomiting
• diarrhea
• low grade fever
• headache
However, some people do not have symptoms.
Hepatitis A
Disease spread. Primarily through food or water contaminated by feces from an infected person. Rarely, it spreads through contact with infected blood.
People at risk. International travelers; people living in areas where hepatitis A outbreaks are common; people who live with or have sex with an infected person; and, during outbreaks, day care children and employees, men who have sex with men, and injection drug users.
Prevention. The hepatitis A vaccine; also, avoiding tap water when traveling internationally and practicing good hygiene and sanitation.
Treatment. Hepatitis A usually resolves on its own over several weeks.
Hepatitis B
Disease spread. Through contact with infected blood, through sex with an infected person, and from mother to child during childbirth.
People at risk. People who have sex with an infected person, men who have sex with men, injection drug users, children of immigrants from disease-endemic areas, infants born to infected mothers, people who live with an infected person, health care workers, hemodialysis patients, people who received a transfusion of blood or blood products before July 1992 or clotting factors made before 1987, and international travelers.
Prevention. The hepatitis B vaccine.
Treatment. For chronic hepatitis B: drug treatment with alpha interferon, peginterferon, lamivudine, or adefovir dipivoxil. Acute hepatitis B usually resolves on its own. Very severe cases can be treated with lamivudine.
Hepatitis C
Disease spread. Primarily through contact with infected blood; less commonly, through sexual contact and childbirth.
People at risk. Injection drug users, people who have sex with an infected person, people who have multiple sex partners, health care workers, infants born to infected women, hemodialysis patients, and people who received a transfusion of blood or blood products before July 1992 or clotting factors made before 1987.
Prevention. There is no vaccine for hepatitis C; the only way to prevent the disease is to reduce the risk of exposure to the virus. This means avoiding behaviors like sharing drug needles or sharing personal items like toothbrushes, razors, and nail clippers with an infected person.
Treatment. Chronic hepatitis C: drug treatment with peginterferon alone or combination treatment with peginterferon and the drug ribavirin.
Acute hepatitis C: treatment is recommended if it does not resolve within 2 to 3 months.
Hepatitis D
Disease spread. Through contact with infected blood. This disease occurs only in people who are already infected with hepatitis B.
People at risk. Anyone infected with hepatitis B: Injection drug users who have hepatitis B have the highest risk. People who have hepatitis B are also at risk if they have sex with a person infected with hepatitis D or if they live with an infected person. Also at risk are people who received a transfusion of blood or blood products before July 1992 or clotting factors made before 1987.
Prevention. Immunization against hepatitis B for those not already infected; also, avoiding exposure to infected blood, contaminated needles, and an infected person’s personal items (toothbrush, razor, nail clippers).
Treatment. Chronic hepatitis D: drug treatment with alpha interferon.
Hepatitis E
Disease spread. Through food or water contaminated by feces from an infected person. This disease is uncommon in the United States.
People at risk. International travelers; people living in areas where hepatitis E outbreaks are common; and people who live or have sex with an infected person.
Prevention. There is no vaccine for hepatitis E; the only way to prevent the disease is to reduce the risk of exposure to the virus. This means avoiding tap water when traveling internationally and practicing good hygiene and sanitation.
Treatment. Hepatitis E usually resolves on its own over several weeks to months.
Other Causes of Viral Hepatitis
Some cases of viral hepatitis cannot be attributed to the hepatitis A, B, C, D, or E viruses. This is called non A-E hepatitis. Scientists continue to study the causes of non A-E hepatitis.
Hope Through Research
The National Institute of Diabetes and Digestive and Kidney Diseases, through its Division of Digestive Diseases and Nutrition, supports basic and clinical research into the nature and transmission of the hepatitis viruses, and the activation and mechanisms of the immune system. Results from these studies are used in developing new treatments and methods of prevention.
v Autoimmune Hepatitis ImageFilePage124
Autoimmune hepatitis is a disease in which the body’s immune system attacks liver cells. This causes the liver to become inflamed (hepatitis). Researchers think a genetic factor may predispose some people to autoimmune diseases. About 70 percent of those with autoimmune hepatitis are women, most between the ages of 15 and 40.
The disease is usually quite serious and, if not treated, gets worse over time. It’s usually chronic, meaning it can last for years, and can lead to cirrhosis (scarring and hardening) of the liver and eventually liver failure.
Autoimmune hepatitis is classified as either type I or II. Type I is the most common form in North America. It occurs at any age and is more common among women than men. About half of those with type I have other autoimmune disorders, such as type 1 diabetes, proliferative glomerulonephritis, thyroiditis, Graves’ disease, Sjögren’s syndrome, autoimmune anemia, and ulcerative colitis. Type II autoimmune hepatitis is less common, typically affecting girls ages 2 to 14, although adults can have it too.
Autoimmune Disease
One job of the immune system is to protect the body from viruses, bacteria, and other living organisms. Usually, the immune system does not react against the body’s own cells. However, sometimes it mistakenly attacks the cells it is supposed to protect. This response is called autoimmunity. Researchers speculate that certain bacteria, viruses, toxins, and drugs trigger an autoimmune response in people who are genetically susceptible to developing an autoimmune disorder.
Symptoms
Fatigue is probably the most common symptom of autoimmune hepatitis. Other symptoms include
• enlarged liver
• jaundice
• itching
• skin rashes
• joint pain
• abdominal discomfort
• spider angiomas (abnormal blood vessels) on the skin
• nausea
• vomiting
• loss of appetite
• dark urine
• pale or gray colored stools
People in advanced stages of the disease are more likely to have symptoms such as fluid in the abdomen (ascites) or mental confusion. Women may stop having menstrual periods.
Symptoms of autoimmune hepatitis range from mild to severe. Because severe viral hepatitis or hepatitis caused by a drug—for example, certain antibiotics—has the same symptoms, tests may be needed for an exact diagnosis. Your doctor should also review and rule out all your medicines before diagnosing autoimmune hepatitis.
Diagnosis
Your doctor will make a diagnosis based on your symptoms, blood tests, and liver biopsy.
| • |
Blood tests. A routine blood test for liver enzymes can help reveal a pattern typical of hepatitis, but further tests, especially for autoantibodies, are needed to diagnose autoimmune hepatitis. Antibodies are proteins made by the immune system to fight off bacteria and viruses. In autoimmune hepatitis, the immune system makes antinuclear antibodies (ANA), antibodies against smooth muscle cells (SMA), or liver and kidney microsomes (anti-LKM). The pattern and level of these antibodies help define the type of autoimmune hepatitis (type I or type II). |
| • |
Liver biopsy. A tiny sample of your liver tissue, examined under a microscope, can help your doctor accurately diagnose autoimmune hepatitis and tell how serious it is. You will go to a hospital or outpatient surgical facility for this procedure. |
Treatment
Treatment works best when autoimmune hepatitis is diagnosed early. With proper treatment, autoimmune hepatitis can usually be controlled. In fact, recent studies show that sustained response to treatment not only stops the disease from getting worse, but also may actually reverse some of the damage.
The primary treatment is medicine to suppress (slow down) an overactive immune system.
Both types of autoimmune hepatitis are treated with daily doses of a corticosteroid called prednisone. Your doctor may start you on a high dose (20 to 60 mg per day) and lower the dose to 5 to 15 mg/day as the disease is controlled. The goal is to find the lowest possible dose that will control your disease.
Another medicine, azathioprine (Imuran) is also used to treat autoimmune hepatitis. Like prednisone, azathioprine suppresses the immune system, but in a different way. It helps lower the dose of prednisone needed, thereby reducing its side effects. Your doctor may prescribe azathioprine, in addition to prednisone, once your disease is under control.
Most people will need to take prednisone, with or without azathioprine, for years. Some people take it for life. Corticosteroids may slow down the disease, but everyone is different. In about one out of every three people, treatment can eventually be stopped.
After stopping, it is important to carefully monitor your condition and promptly report any new symptoms to your doctor because the disease may return and be even more severe, especially during the first few months after stopping treatment.
In about 7 out of 10 people, the disease goes into remission, with a lessening of severity of symptoms, within 2 years of starting treatment. A portion of persons with a remission will see the disease return within 3 years, so treatment may be necessary on and off for years, if not for life.
Side Effects
Both prednisone and azathioprine have side effects. Because high doses of prednisone are needed to control autoimmune hepatitis, managing side effects is very important. However, most side effects appear only after a long period of time.
Some possible side effects of prednisone are
• weight gain
• anxiety and confusion
• thinning of the bones (osteoporosis)
• thinning of the hair and skin
• diabetes
• high blood pressure
• cataracts
• glaucoma
Azathioprine can lower your white blood cell count and sometimes causes nausea and poor appetite. Rare side effects are allergic reaction, liver damage, and pancreatitis (inflammation of the pancreas gland with severe stomach pain).
Other Treatments
People who do not respond to standard immune therapy or who have severe side effects may benefit from other immunosuppressive agents like mycophenylate mofetil, cyclosporine or tacrolimus. People who progress to end stage liver disease (liver failure) and/or cirrhosis may need a liver transplant. Transplantation has a 1-year survival rate of 90 percent and a 5-year survival rate of 70 to 80 percent.
Hope Through Research
Scientists are studying various aspects of autoimmune hepatitis to find out who gets it and why and to discover better ways to treat it. Basic research on the immune system will expand knowledge of autoimmune diseases in general. Epidemiologic research will help doctors understand what triggers autoimmune hepatitis in some people. Research on different steroids, alternatives to steroids, and other immunosuppressants will eventually lead to more effective treatments.
Points to Remember
| • |
Autoimmune hepatitis is a long-term disease in which your body’s immune system attacks liver cells. |
| • |
The disease is diagnosed using various blood tests and a liver biopsy. |
| • |
With proper treatment, autoimmune hepatitis can usually be controlled. The main treatment is medicine that suppresses the body’s overactive immune system. |
|
v What I Need to Know About Hepatitis A
|
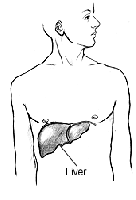 |
Hepatitis A is caused by a virus. A virus is a germ that causes sickness. (For example, the flu is caused by a virus.) People can pass viruses to each other. The virus that causes hepatitis A is called the hepatitis A virus.
|
How could I get hepatitis A? |
 |
• drinking water that has been contaminated by hepatitis A (in parts of the world with poor hygiene
and sanitary conditions)
Who can get hepatitis A?
|
Anyone can get hepatitis A. But some people are more likely to than others: |
 |
• people who travel to other countries where hepatitis A is common
What are the symptoms?
Hepatitis A can make you feel like you have the flu. You might
• feel tired
• feel sick to your stomach
• have a fever
• not want to eat
• have stomach pain
• have diarrhea
Some people have
• dark yellow urine
• light-colored stools
• yellowish eyes and skin
Some people don’t have any symptoms. If you have symptoms or think you might have hepatitis A, go to a doctor. The doctor will test your blood.
|
How is hepatitis A treated?
How can I protect myself? |
 |
|
You can get the hepatitis A vaccine. A vaccine is a drug that you take when you are healthy that keeps you from getting sick. Vaccines teach your body to attack certain viruses, like the hepatitis A virus. |
 |
You need all of the shots to be protected. If you are traveling to other countries, make sure you get all the shots before you go. If you miss a shot, call your doctor or clinic right away to set up a new appointment.
You can protect yourself and others from hepatitis A in these ways, too:
|
• |
Always wash your hands after using the toilet and before fixing food or eating. |
 |
|
• |
Wear gloves if you have to touch other people’s stool. Wash your hands afterwards. |
|
|
• |
Drink bottled water when you are in another country. (And don’t use ice cubes or wash fruits and vegetables in tap water.) |
v What I Need to Know About Hepatitis B
What is hepatitis B?
Hepatitis B is a liver disease. Hepatitis (HEP-ah-TY-tis) makes your liver swell and stops it from working right.
You need a healthy liver. The liver does many things to keep you alive. The liver fights infections and stops bleeding. It removes drugs and other poisons from your blood. The liver also stores energy for when you need it.
What causes hepatitis B?
Hepatitis B is caused by a virus. A virus is a germ that causes sickness. (For example, the flu is caused by a virus.) People can pass viruses to each other. The virus that causes hepatitis B is called the hepatitis B virus.
How could I get hepatitis B?
Hepatitis B spreads by contact with an infected person’s blood, semen, or other body fluid.
You could get hepatitis B by
• having sex with an infected person without using a condom
• sharing drug needles
• having a tattoo or body piercing done with dirty tools that were used on someone else
• getting pricked with a needle that has infected blood on it (health care workers can get hepatitis B this way)
• living with someone who has hepatitis B
• sharing a toothbrush or razor with an infected person
• traveling to countries where hepatitis B is common
An infected woman can give hepatitis B to her baby at birth or through her breast milk.
You can NOT get hepatitis B by
• shaking hands with an infected person
• hugging an infected person
• sitting next to an infected person
What are the symptoms?
Hepatitis B can make you feel like you have the flu.
You might
|
• feel tired • feel sick to your stomach • have a fever • not want to eat • have stomach pain • have diarrhea Some people have • dark yellow urine • light-colored stools • yellowish eyes and skin |
 |
Some people don’t have any symptoms.
If you have symptoms or think you might have hepatitis B, go to a doctor. The doctor will take some blood to check for hepatitis B.
|
What are the tests for hepatitis B? |
 |
v Vaccinations for Hepatitis A and B
Candidates for Hepatitis A Vaccination
Routine Vaccination
• Children living in areas with high incidence rates of hepatitis A (above the national average). Check with
your health department to see if this applies to your area.
High-Risk Populations
• Travelers to developing countries with high rates of hepatitis A, including Mexico
• Men who have sex with men
• Users of illegal drugs
• People who work with hepatitis A virus in research settings
• People who work with infected nonhuman primates
• Recipients of clotting factor concentrates
• People with chronic liver disease (because of risk of fulminant hepatitis A)
| Doses and Schedules: Hepatitis A |
| Havrix * |
|
Age
# of Doses
Schedule
Dose Children age 2to 18 years 2 0 and 6 to 12 months 720 ELISA units (0.5 mL) |
| Adults 18 years and older 2 0 and 6 to 12 months 1440 ELISA units (1.0 mL) |
| * Inactivated vaccine. Manufactured by SmithKline Beecham Biologicals. |
| VAQTA** |
| Age # of Doses Schedule Dose |
| Children age 2to 17 years 2 0 and 6 to 18 months 25 units (0.5 mL) |
| Adults 17 years and older 2 0 and 6 months 50 units (1.0 mL) |
| ** Inactivated vaccine. Manufactured by Merck & Company, Inc. |
Postexposure Prophylaxis
Immune globulin (IG) can provide temporary immunity to hepatitis A when given within 2 weeks of exposure to the hepatitis A virus. The dose is 0.02 mL/kg injected into the gluteal muscle in adults or the anterolateral thigh muscle in children under 2 years. Concurrent hepatitis A vaccination may also be appropriate in people 2 years and older. IG protects against the hepatitis A virus for 3 to 5 months, depending on dosage.
Candidates for Hepatitis B Vaccination
Routine Vaccination
• All infants, children, and adolescents
High-Risk Populations
• People with multiple sex partners and those who have been recently diagnosed with a sexually transmitted
disease
• Sex partners and household contacts of HBV carriers
• Men who have sex with men
• Household contacts of adoptees from countries with high rates of hepatitis B
• Injection drug users
• Travelers to countries with high rates of hepatitis B (staying longer than 6 months)
• People with occupational exposure to blood
• Clients and staff in institutions for the developmentally disabled
• Patients with chronic kidney failure (including those on chronic hemodialysis)
• Patients receiving clotting factor concentrates
• Inmates of long-term correctional facilities
| Doses and Schedules: Hepatitis B |
| Age # of Doses Schedule DoseRecombivax HB* DoseEnergix-B** |
|
Infants with HBs
3
0 to 2, 1 to 4, and
5.0 µg(0.5 mL)
10 µg(0.5 mL) Ag-negative mother 6 to 18 months |
|
Infants with HBs
3
Hepatitis B immune
5.0 µg(0.5 mL)
10 µg(0.5 mL) Ag-positive mother globulin and vaccination within 12 hours of birth, then vaccine at 1 to 2 and 6 months |
|
Children and adolescents 3
0, 1 to 2, and 4 to 6 months 5.0 µg(0.5 mL)
10 µg(0.5 mL) age 1 to 19 years |
| Adolescents 11 to 15 years 2 0 and 4 to 6 months 10 µg(1.0 mL) N/A |
| Adults 20 years and older 3 0, 1 to 2, and 4 to 6 months 10 µg(1.0 mL) 20 µg(1.0 mL) |
|
Immuno-compromised
3
0, 1, and 6 months
40 µg(1.0 mL)
N/A adults 4 0, 1, 2, and 6 months N/A 40 µg(2.0 mL) |
|
Note: There should be at least 1 month between the first and second doses, at least 2 months between the second and third doses, and at least 4 months between the first and third doses. For infants, the third dose should not be given before 6 months of age. *Recombinant vaccine. Manufactured by Merck & Company, Inc. **Recombinant vaccine. Manufactured by SmithKline Beecham Biologicals. |
Postexposure Prophylaxis
Prophylactic treatment for exposure to hepatitis B virus involves either hepatitis B immune globulin (HBIG), hepatitis B vaccine, or a combination of both. The HBIG dose equals 0.06 mL/kg. Efficacy ranges from 70 to 95 percent for different types of exposure.
| Exposure | Treatment |
| Perinatal | 1 dose of HBIG given with the first hepatitis B vaccine dose. |
| Percutaneous or permucosal | HBIG and vaccination depending on vaccination and exposure status. |
| Sexual | HBIG with or without vaccination for exposure to acute hepatitis B; vaccination alone for chronic exposure. |
| Household contact | HBIG with vaccination for acute hepatitis B in infants under age 12 months; vaccination alone for chronic. |
Combination Vaccine
Twinrix* is a vaccine for both hepatitis A and hepatitis B. It combines two FDA-approved vaccines—Havrix, for hepatitis A, and Engerix-B, for hepatitis B. It protects individuals 18 years of age or older against diseases caused by hepatitis A and hepatitis B viruses. The vaccine is recommended for travelers whose occupation or behavior puts them at high risk for exposure to hepatitis B virus, or who are visiting countries with a high or intermediate rate of both hepatitis viruses, as defined by the Centers for Disease Control and Prevention.
|
Twinrix*
Age
# of Doses
Schedule
Dose *Manufactured by SmithKline Beecham Pharmaceuticals. |
References
|
1. |
Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 1999;48(RR-12). |
|
2. |
Centers for Disease Control and Prevention. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 1991;40(RR-13). |
|
3. |
Food and Drug Administration. 2001. New Combination Vaccine Approved for Protection Against Two Hepatitis Viruses. FDA Talk Paper. Available at: www.fda.gov/bbs/topics/ANSWERS/2001/ANS01084.html. Accessed February 9, 2004. |
|
|
 |
What causes hepatitis C?
Hepatitis C is caused by a virus. A virus is a germ that causes sickness. (For example, the flu is caused by a virus.) People can pass viruses to each other. The virus that causes hepatitis C is called the hepatitis C virus.
How could I get hepatitis C?
Hepatitis C is spread by contact with an infected person’s blood. You could get hepatitis C by
| • |
sharing drug needles Image |
 |
| • |
getting pricked with a needle that has infected blood on it (hospital workers can get hepatitis C this way) |
|
| • |
having sex with an infected person, especially if you or your partner has other sexually transmitted diseases |
|
| • |
being born to a mother with hepatitis C |
In rare cases, you could get hepatitis C by
• getting a tattoo or body piercing with unsterilized, dirty tools
You can NOT get hepatitis C by
• shaking hands with an infected person
• hugging an infected person
• kissing an infected person
• sitting next to an infected person
|
Could I get hepatitis C from a blood transfusion? |
 |
What are the symptoms?
Many people with hepatitis C don’t have symptoms. However, some people with hepatitis C feel like they have the flu.
|
So, you might • feel tired • feel sick to your stomach • have a fever • not want to eat • have stomach pain • have diarrhea Some people have • dark yellow urine • light-colored stools • yellowish eyes and skin |
 |
If you have symptoms or think you might have hepatitis C, go to a doctor.
|
What are the tests for hepatitis C? |
 |
|
How is hepatitis C treated? |
 |
How can I protect myself?
You can protect yourself and others from hepatitis C.
| • |
Don’t share drug needles with anyone. |
 |
| • |
Wear gloves if you have to touch anyone’s blood. |
|
| • |
If you have several sex partners, use a condom during sex. |
|
| • |
Don’t use an infected person’s toothbrush, razor, or anything else that could have blood on it. |
|
| • |
If you get a tattoo or body piercing, make sure it is done with clean tools. |
|
| • |
If you have hepatitis C, don’t give your blood or plasma. The person who receives it could become infected with the virus. |
v Chronic Hepatitis C: Current Disease Management
Introduction
The hepatitis C virus (HCV) is one of the most important causes of chronic liver disease in the United States. It accounts for about 15 percent of acute viral hepatitis, 60 to 70 percent of chronic hepatitis, and up to 50 percent of cirrhosis, end-stage liver disease, and liver cancer. Almost 4 million Americans, or 1.8 percent of the U.S. population, have antibody to HCV (anti-HCV), indicating ongoing or previous infection with the virus. Hepatitis C causes an estimated 10,000 to 12,000 deaths annually in the United States.
A distinct and major characteristic of hepatitis C is its tendency to cause chronic liver disease. At least 75 percent of patients with acute hepatitis C ultimately develop chronic infection, and most of these patients have accompanying chronic liver disease.
Chronic hepatitis C varies greatly in its course and outcome. At one end of the spectrum are patients who have no signs or symptoms of liver disease and completely normal levels of serum liver enzymes. Liver biopsy usually shows some degree of chronic hepatitis, but the degree of injury is usually mild, and the overall prognosis may be good. At the other end of the spectrum are patients with severe hepatitis C who have symptoms, HCV RNA in serum, and elevated serum liver enzymes, and who ultimately develop cirrhosis and end-stage liver disease. In the middle of the spectrum are many patients who have few or no symptoms, mild to moderate elevations in liver enzymes, and an uncertain prognosis.
Chronic hepatitis C can cause cirrhosis, liver failure, and liver cancer. Researchers estimate that at least 20 percent of patients with chronic hepatitis C develop cirrhosis, a process that takes at least 10 to 20 years. After 20 to 40 years, a smaller percentage of patients with chronic disease develop liver cancer. Liver failure from chronic hepatitis C is one of the most common reasons for liver transplants in the United States. Hepatitis C is the cause of about half of cases of primary liver cancer in the developed world. Men, alcoholics, patients with cirrhosis, people over age 40, and those infected for 20 to 40 years are more likely to develop HCV-related liver cancer.
Risk Factors and Transmission
HCV is spread primarily by contact with blood and blood products. Blood transfusions and the use of shared, unsterilized, or poorly sterilized needles and syringes have been the main causes of the spread of HCV in the United States. With the introduction in 1991 of routine blood screening for HCV antibody and improvements in the test in the mid-1992, transfusion-related hepatitis C has virtually disappeared. At present, injection drug use is the most common risk factor for contracting the disease. However, many patients acquire hepatitis C without any known exposure to blood or to drug use.
The major high-risk groups for hepatitis C are
• Injection drug users, including those who used drugs briefly many years ago
• People who had blood transfusions before June 1992, when sensitive tests for anti-HCV were introduced for
blood screening
• People who have frequent exposure to blood products. These include patients with hemophilia, solid-organ
transplants, chronic renal failure, or cancer requiring chemotherapy
• Infants born to HCV-infected mothers
• Health care workers who suffer needle-stick accidents
Other groups who appear to be at slightly increased risk for hepatitis C are
• people with high-risk sexual behavior, multiple partners, and sexually transmitted diseases
• people who use cocaine, particularly with intranasal administration, using shared equipment
Maternal-Infant Transmission
Maternal-infant transmission is not common. In most studies, only 5 percent of infants born to infected women become infected. The disease in newborns is usually mild and free of symptoms. The risk of maternal-infant spread rises with the amount of virus in the mother’s blood and with complications of delivery such as early rupture of membranes and fetal monitoring. Breast-feeding has not been linked to spread of HCV.
Sexual Transmission
Sexual transmission of hepatitis C between monogamous partners appears to be uncommon. Surveys of spouses and monogamous sexual partners of patients with hepatitis C show that less than 5 percent are infected with HCV, and many of these have other risk factors for this infection. Spread of hepatitis C to a spouse or partner in stable, monogamous relationships occurs in less than 1 percent of partners per year. For these reasons, changes in sexual practices are not recommended for monogamous patients. Testing sexual partners for anti-HCV can help with patient counseling. People with multiple sex partners should be advised to follow safe sex practices, which should protect against hepatitis C as well as hepatitis B and HIV.
Sporadic Transmission
Sporadic transmission, when the source of infection is unknown, occurs in about 10 percent of acute hepatitis C cases and in 30 percent of chronic hepatitis C cases. These cases are usually referred to as sporadic or community-acquired infections. These infections may have come from exposure to the virus from cuts, wounds, or medical injections or procedures.
Unsafe Injection Practices
In many areas of the world, unsafe injection practices are an important and common cause of hepatitis C (and hepatitis B as well). Use of inadequately sterilized equipment, lack of disposable needles and syringes, and inadvertent contamination of medical infusions are unfortunately well-documented causes of transmission of hepatitis C. Careful attention to universal precautions and injection techniques should prevent this type of spread. In the United States, multiple-use vials are a frequent culprit in leading to nosocomial spread of hepatitis C.
Clinical Symptoms and Signs
Many people with chronic hepatitis C have no symptoms of liver disease. If symptoms are present, they are usually mild, nonspecific, and intermittent. They may include
• fatigue
• mild right-upper-quadrant discomfort or tenderness (“liver pain”)
• nausea
• poor appetite
• muscle and joint pains
Similarly, the physical exam is likely to be normal or show only mild enlargement of the liver or tenderness. Some patients have vascular spiders or palmar erythema.
Clinical Features of Cirrhosis
Once a patient develops cirrhosis or if the patient has severe disease, symptoms and signs are more prominent. In addition to fatigue, the patient may complain of muscle weakness, poor appetite, nausea, weight loss, itching, dark urine, fluid retention, and abdominal swelling.
Physical findings of cirrhosis may include
• enlarged liver
• enlarged spleen
• jaundice
• muscle wasting
• excoriations
• ascites
• ankle swelling
Extrahepatic Manifestations
Complications that do not involve the liver develop in 1 to 2 percent of people with hepatitis C. The most common is cryoglobulinemia, which is marked by
• skin rashes, such as purpura, vasculitis, or urticaria
• joint and muscle aches
• kidney disease
• neuropathy
• cryoglobulins, rheumatoid factor, and low complement levels in serum
Other complications of chronic hepatitis C are:
• glomerulonephritis
• porphyria cutanea tarda
Diseases that are less well documented to be related to hepatitis C are:
• seronegative arthritis
• keratoconjunctivitis sicca (Sjögren’s syndrome)
• non-Hodgkin’s type, B-cell lymphomas
• fibromyalgia
• lichen planus
Serologic Tests
Enzyme Immunoassay
Anti-HCV is detected by enzyme immunoassay (EIA). The third-generation test (EIA-3) used today is more sensitive and specific than previous ones. However, as with all enzyme immunoassays, false-positive results are occasionally a problem with the EIA-3. Additional or confirmatory testing is often helpful.
The best approach to confirm the diagnosis of hepatitis C is to test for HCV RNA using a sensitive assay such as polymerase chain reaction (PCR) or transcription mediated amplification (TMA). The presence of HCV RNA in serum indicates an active infection.
Testing for HCV RNA is also helpful in patients in whom EIA tests for anti-HCV are unreliable. For instance, immunocompromised patients may test negative for anti-HCV despite having HCV infection because they may not produce enough antibodies for detection with EIA. Likewise, patients with acute hepatitis may test negative for anti-HCV when first tested. Antibody is present in almost all patients by 1 month after onset of acute illness; thus, patients with acute hepatitis who initially test negative may need followup testing. In these situations, HCV RNA is usually present and confirms the diagnosis.
Recombinant Immunoblot Assay
Immunoblot assays can be used to confirm anti-HCV reactivity as well. These tests are also called “Western blots”; serum is incubated on nitrocellulose strips on which four recombinant viral proteins are blotted. Color changes indicate that antibodies are adhering to the proteins. An immunoblot is considered positive if two or more proteins react and is considered indeterminate if only one positive band is detected. In some clinical situations, confirmatory testing by immunoblotting is helpful, such as for the person with anti-HCV detected by EIA who tests negative for HCV RNA. The EIA anti-HCV reactivity could represent a false-positive reaction, recovery from hepatitis C, or continued virus infection with levels of virus too low to be detected (the last occurs only rarely when sensitive PCR or TMA assays are used). If the immunoblot test for anti-HCV is positive, the patient has most likely recovered from hepatitis C and has persistent antibody. If the immunoblot test is negative, the EIA result was probably a false positive.
Immunoblot tests are routine in blood banks when an anti-HCV-positive sample is found by EIA. Immunoblot assays are highly specific and valuable in verifying anti-HCV reactivity. Indeterminate tests require further followup testing, including attempts to confirm the specificity by repeat testing for HCV RNA.
Direct Assays for HCV RNA
PCR and TMA amplification can detect low levels of HCV RNA in serum. Testing for HCV RNA is a reliable way of demonstrating that hepatitis C infection is present and is the most specific test for infection. Testing for HCV RNA is particularly useful when aminotransferases are normal or only slightly elevated, when anti-HCV is not present, or when several causes of liver disease are possible. This method also helps diagnose hepatitis C in people who are immunosuppressed, have recently had an organ transplant, or have chronic renal failure. A PCR assay has now been approved by the Food and Drug Administration for general use. This assay will detect HCV RNA in serum down to a lower limit of 50 to 100 copies per milliliter (mL) which is equivalent to 25 to 50 international units (IU). A slightly more sensitive TMA test is currently under evaluation and may soon become available. Almost all patients with chronic hepatitis C will test positive by these assays.
Quantification of HCV RNA in Serum
Several methods are available for measuring the concentration or level of virus in serum, which is an indirect assessment of viral load. These methods include a quantitative PCR and a branched DNA (bDNA) test. Unfortunately, these assays are not well standardized, and different methods from different laboratories can provide different results on the same specimen. In addition, serum levels of HCV RNA can vary spontaneously by 3- to 10-fold over time. Nevertheless, when performed carefully, quantitative assays provide important insights into the nature of hepatitis C. Most patients with chronic hepatitis C have levels of HCV RNA (viral load) between 100,000 (105) and 10,000,000 (107) copies per mL. Expressed as IU, these averages are 50,000 to 5 million IU.
Viral levels as measured by HCV RNA do not correlate with the severity of the hepatitis or with a poor prognosis (as in HIV infection); but viral load does correlate with the likelihood of a response to antiviral therapy. Rates of response to a course of alpha interferon and ribavirin are higher in patients with low levels of HCV RNA. There are several definitions of a “low level” of HCV RNA, but the usual definition is below 1 million IU (2 million copies) per mL.
In addition, monitoring HCV RNA levels during the early phases of treatment may provide early information on the likelihood of a response. Yet because of the shortcomings of the current assays for HCV RNA level, these tests are not always reliable guides to therapy.
Genotyping and Serotyping of HCV
There are 6 known genotypes and more than 50 subtypes of hepatitis C. The genotype of infection is helpful in defining the epidemiology of hepatitis C. More important, knowing the genotype or serotype (genotype-specific antibodies) of HCV is helpful in making recommendations and counseling regarding therapy. Patients with genotypes 2 and 3 are two to three times more likely to respond to interferon-based therapy than patients with genotype 1. Furthermore, when using combination therapy, the recommended dose and duration of treatment depend on the genotype. For patients with genotypes 2 and 3, a 24-week course of combination treatment using interferon and 800 milligrams (mg) of ribavirin daily is adequate, whereas for patients with genotype 1, a 48-week course and full dose of ribavirin (1,000 to 1,200 mg daily) is recommended. For these reasons, testing for HCV genotype is often clinically helpful. Once the genotype is identified, it need not be tested again; genotypes do not change during the course of infection.
Biochemical Indicators of Hepatitis C Virus Infection
| • |
In chronic hepatitis C, increases in the alanine and aspartate aminotransferases range from 0 to 20 times (but usually less than 5 times) the upper limit of normal. |
| • |
Alanine aminotransferase (ALT) levels are usually higher than aspartate aminotransferase (AST) levels, but that finding may be reversed in patients who have cirrhosis. |
| • |
Alkaline phosphatase and gamma glutamyl transpeptidase are usually normal. If elevated, they may indicate cirrhosis. |
| • |
Rheumatoid factor and low platelet and white blood cell counts are frequent in patients with severe fibrosis or cirrhosis, providing clues to the presence of advanced disease. |
| • |
The enzymes lactate dehydrogenase and creatine kinase are usually normal. |
| • |
Albumin levels and prothrombin time are normal until late-stage disease. |
| • |
Iron and ferritin levels may be slightly elevated. |
Normal Serum ALT Levels
Some patients with chronic hepatitis C have normal serum alanine aminotransferase (ALT) levels, even when tested on multiple occasions. In this and other situations in which the diagnosis of chronic hepatitis C may be questioned, the diagnosis should be confirmed by testing for HCV RNA. The presence of HCV RNA indicates that the patient has ongoing viral infection despite normal ALT levels.
Liver Biopsy
Liver biopsy is not necessary for diagnosis but is helpful for grading the severity of disease and staging the degree of fibrosis and permanent architectural damage. Hematoxylin and eosin stains and Masson’s trichrome stain are used to grade the amount of necrosis and inflammation and to stage the degree of fibrosis. Specific immunohistochemical stains for HCV have not been developed for routine use. Liver biopsy is also helpful in ruling out other causes of liver disease, such as alcoholic liver injury or iron overload.
HCV causes the following changes in liver tissue:
| • |
Necrosis and inflammation around the portal areas, so-called “piecemeal necrosis” or “interface hepatitis.” |
| • |
Necrosis of hepatocytes and focal inflammation in the liver parenchyma. |
| • |
Inflammatory cells in the portal areas (“portal inflammation”). |
| • |
Fibrosis, with early stages being confined to the portal tracts, intermediate stages being expansion of the portal tracts and bridging between portal areas or to the central area, and late stages being frank cirrhosis characterized by architectural disruption of the liver with fibrosis and regeneration. Several scales are used to stage fibrosis, most commonly a scale from 0 to 4 where 0 indicates none and 4 indicates cirrhosis. Stage 1 and 2 fibrosis is limited to the portal and periportal areas. Stage 3 fibrosis is characterized by bridges of fibrosis bands linking up portal and central areas. |
| • |
Grading and staging of hepatitis by assigning scores for severity are helpful in managing patients with chronic hepatitis. The degree of inflammation and necrosis can be assessed as none, minimal, mild, moderate, or severe. The degree of fibrosis can be similarly assessed. Scoring systems are particularly helpful in clinical studies on chronic hepatitis. |
Serum Markers of Hepatic Fibrosis
Liver biopsy is an invasive procedure that is expensive and not without complications. At least 20 percent of patients have pain requiring medications after liver biopsy. More uncommon complications include puncture of another organ, infection, and bleeding. Significant bleeding after liver biopsy occurs in 1/100 to 1/1,000 cases, and deaths are reported in 1/5,000 to 1/10,000 cases. Obviously, noninvasive means of grading and staging liver disease would be very helpful.
ALT levels, particularly if tested over an extended period, are reasonably accurate reflections of disease activity. Thus, patients with repeatedly normal ALT levels usually have mild necroinflammatory activity on liver biopsy. Furthermore, patients who maintain ALT levels above 5 times the upper limit of normal usually have marked necroinflammatory activity. But for the majority of patients with mild-to-moderate ALT elevations, the actual level is not very predictive of liver biopsy findings.
More important is a means to stage liver disease short of liver biopsy. Unfortunately, serum tests are not reliable in predicting fibrosis, particularly earlier stages (0, 1, and 2). When patients develop bridging (stage 3) fibrosis and cirrhosis (stage 4), serum tests may be helpful. The “danger signals” that suggest the presence of advanced fibrosis include an aspartate aminotransferase (AST) that is higher than ALT (reversal of the ALT/AST ratio), a high gamma glutamyl transpeptidase or alkaline phosphatase, a low platelet count (which is perhaps the earliest change), rheumatoid factor, elevations in globulins, and, of course, abnormal bilirubin, albumin or prothrombin time. Physical findings of a firm liver, or enlarged spleen or prominent spider angionata or palmar erythema, are also danger signals. While none of these findings are perfect, their presence should raise the suspicion of significant fibrosis and lead to evaluation for treatment earlier rather than later.
Diagnosis
Hepatitis C is most readily diagnosed when serum aminotransferases are elevated and anti-HCV is present in serum. The diagnosis is confirmed by the finding of HCV RNA in serum.
Acute Hepatitis C
Acute hepatitis C is diagnosed on the basis of symptoms such as jaundice, fatigue, and nausea, along with marked increases in serum ALT (usually greater than 10-fold elevation), and presence of anti-HCV or de novo development of anti-HCV.
Diagnosis of acute disease can be problematic because anti-HCV is not always present when the patient develops symptoms and sees the physician. In 30 to 40 percent of patients, anti-HCV is not detected until 2 to 8 weeks after onset of symptoms. In this situation, testing for HCV RNA is helpful, as this marker is present even before the onset of symptoms and lasts through the acute illness. Another approach to diagnosis of acute hepatitis C is to repeat the anti-HCV testing a month after onset of illness. Of course, a history of an acute exposure is also helpful in establishing the diagnosis.
Chronic Hepatitis C
Chronic hepatitis C is diagnosed when anti-HCV is present and serum aminotransferase levels remain elevated for more than 6 months. Testing for HCV RNA (by PCR) confirms the diagnosis and documents that viremia is present; almost all patients with chronic infection will have the viral genome detectable in serum by PCR.
Diagnosis is problematic in patients who cannot produce anti-HCV because they are immunosuppressed or immunoincompetent. Thus, HCV RNA testing may be required for patients who have a solid-organ transplant, are on dialysis, are taking corticosteroids, or have agammaglobulinemia. Diagnosis is also difficult in patients with anti-HCV who have another form of liver disease that might be responsible for the liver injury, such as alcoholism, iron overload, or autoimmunity. In these situations, the anti-HCV may represent a false-positive reaction, previous HCV infection, or mild hepatitis C occurring on top of another liver condition. HCV RNA testing in these situations helps confirm that hepatitis C is contributing to the liver problem.
Differential Diagnosis
The major conditions that can be confused clinically with chronic hepatitis C include
• autoimmune hepatitis
• chronic hepatitis B and D
• alcoholic hepatitis
• nonalcoholic steatohepatitis (fatty liver)
• sclerosing cholangitis
• Wilson’s disease
• alpha-1-antitrypsin-deficiency-related liver disease
• drug-induced liver disease
Treatment
The therapy for chronic hepatitis C has evolved steadily since alpha interferon was first approved for use in this disease more than 10 years ago. At the present time, the optimal regimen appears to be a 24- or 48-week course of the combination of pegylated alpha interferon and ribavirin.
Alpha interferon is a host protein that is made in response to viral infections and has natural antiviral activity. Recombinant forms of alpha interferon have been produced, and several formulations (alfa-2a, alfa-2b, consensus interferon) are available as therapy for hepatitis C. These standard forms of interferon, however, are now being replaced by pegylated interferons (peginterferons). Peginterferon is alpha interferon that has been modified chemically by the addition of a large inert molecule of polyethylene glycol. Pegylation changes the uptake, distribution, and excretion of interferon, prolonging its half-life. Peginterferon can be given once weekly and provides a constant level of interferon in the blood, whereas standard interferon must be given several times weekly and provides intermittent and fluctuating levels. In addition, peginterferon is more active than standard interferon in inhibiting HCV and yields higher sustained response rates with similar side effects. Because of its ease of administration and better efficacy, peginterferon has been replacing standard interferon both as monotherapy and as combination therapy for hepatitis C.
Ribavirin is an oral antiviral agent that has activity against a broad range of viruses. By itself, ribavirin has little effect on HCV, but adding it to interferon increases the sustained response rate by two- to threefold. For these reasons, combination therapy is now recommended for hepatitis C, and interferon monotherapy is applied only when there are specific reasons not to use ribavirin.
Two forms of peginterferon have been developed and studied in large clinical trials: peginterferon alfa-2a (Pegasys: Hoffman La Roche: Nutley, NJ) and peginterferon alfa-2b (Pegintron: Schering-Plough Corporation, Kenilworth, NJ). These two products are roughly equivalent in efficacy and safety, but have different dosing regimens. Peginterferon alfa-2a is given subcutaneously in a fixed dose of 180 micrograms (mcg) per week. Peginterferon alfa-2b is given subcutaneously weekly in a weight-based dose of 1.5 mcg per kilogram per week (thus in the range of 75 to 150 mcg per week).
Ribavirin is an oral medication, given twice a day in 200-mg capsules for a total daily dose based upon body weight. The standard dose of ribavirin is 1,000 mg for patients who weigh less than 75 kilograms (165 pounds) and 1,200 mg for those who weigh more than 75 kilograms. In certain situations, an 800-mg dose (400 mg twice daily) is recommended (see below).
Combination therapy leads to rapid improvements in serum ALT levels and disappearance of detectable HCV RNA in up to 70 percent of patients. However, long-term improvement in hepatitis C occurs only if HCV RNA disappears during therapy and stays undetectable once therapy is stopped. Among patients who become HCV RNA negative during treatment, a proportion relapse when therapy is stopped. The relapse rate is lower in patients treated with combination therapy compared with monotherapy.
Thus, a 48-week course of combination therapy using peginterferon and ribavirin yields a sustained response rate of approximately 55 percent. A similar course of peginterferon monotherapy yields a sustained response rate of only 35 percent. A response is considered “sustained” if HCV RNA remains undetectable for 6 months or more after stopping therapy.
The optimal duration of treatment varies depending on whether interferon monotherapy or combination therapy is used, as well as by HCV genotype. For patients treated with peginterferon monotherapy, a 48-week course is recommended, regardless of genotype. For patients treated with combination therapy, the optimal duration of treatment depends on viral genotype. Patients with genotypes 2 and 3 have a high rate of response to combination treatment (70 to 80 percent), and a 24-week course of combination therapy yields results equivalent to those of a 48-week course. In contrast, patients with genotype 1 have a lower rate of response to combination therapy (40 to 45 percent), and a 48-week course yields a significantly better sustained response rate. Again, because of the variable responses to treatment, testing for HCV genotype is clinically useful when using combination therapy.
In addition, the optimal dose of ribavirin appears to vary depending on genotype. For patients with genotypes 2 or 3, a dose of 800 mg daily appears adequate. For patients with genotype 1, the full dose of ribavirin (1,000 or 1,200 mg daily depending on body weight) appears to be needed for an optimal response.
Who Should Be Treated?
Patients with anti-HCV, HCV RNA, elevated serum aminotransferase levels, and evidence of chronic hepatitis on liver biopsy, and with no contraindications, should be offered therapy with the combination of alpha interferon and ribavirin. The National Institutes of Health Consensus Development Conference Panel recommended that therapy for hepatitis C be limited to those patients who have histological evidence of progressive disease. Thus, the panel recommended that all patients with fibrosis or moderate to severe degrees of inflammation and necrosis on liver biopsy should be treated and that patients with less severe histological disease be managed on an individual basis. Patient selection should not be based on the presence or absence of symptoms, the mode of acquisition, the genotype of HCV RNA, or serum HCV RNA levels.
Patients with cirrhosis found through liver biopsy can be offered therapy if they do not have signs of decompensation, such as ascites, persistent jaundice, wasting, variceal hemorrhage, or hepatic encephalopathy. However, interferon and combination therapy have not been shown to improve survival or the ultimate outcome in patients with preexisting cirrhosis.
Patients older than 60 years also should be managed on an individual basis, since the benefit of treatment in these patients has not been well documented and side effects appear to be worse in older patients. However, even patients in their late seventies have been successfully treated for hepatitis C.
The role of interferon therapy in children with hepatitis C remains uncertain. Ribavirin has yet to be evaluated adequately in children, and pediatric doses and safety have not been established. Thus, if children with hepatitis C are treated, monotherapy is recommended, and ribavirin should not be used outside of controlled clinical trials.
People with both HCV and HIV infection should be offered therapy for hepatitis C as long as there are no contraindications. Indeed, hepatitis C tends to be more rapidly progressive in patients with HIV co-infection, and end-stage liver disease has become an increasingly common cause of death in HIV-positive persons. For these reasons, therapy for hepatitis C should be recommended even in HIV-infected patients with early and mild disease. Once HIV infection becomes advanced, complications of therapy are more difficult and response rates are less. The decision to treat people co-infected with HIV must take into consideration the concurrent medications and medical conditions. The efficacy of peginterferon and ribavirin in HIV-infected people has been tested in only a small number of patients. Ribavirin may still have significant interactions with other antiretroviral drugs.
In many of these indefinite situations, the indications for therapy should be reassessed at regular intervals. In view of the rapid developments in hepatitis C today, better therapies may become available within the next few years, at which point expanded indications for therapy would be appropriate.
Patients with acute hepatitis C are a major challenge to management and therapy. Because such a high proportion of patients with acute infection develop chronic hepatitis C, prevention of chronicity has become a focus of attention. In small studies, 83 to 100 percent of persons treated within 1 to 4 months of onset have had resolution of the infection. What is unclear is what dose, duration, and regimen of treatment to use. A practical regimen is peginterferon monotherapy for 24 weeks. The possible role for ribavirin, for short courses of therapy, and for lower doses of peginterferon are under evaluation.
In patients with clinically significant extrahepatic manifestations, such as cryoglobulinemia and glomerulonephritis, therapy with alpha interferon can result in remission of the clinical symptoms and signs. However, relapse after stopping therapy is common. In some patients, long-term or maintenance alpha interferon therapy can be used despite persistence of HCV RNA in serum if clinical symptoms and signs resolve on therapy.
Who Should Not Be Treated?
Therapy is inadvisable outside of controlled trials for patients who have
• clinically decompensated cirrhosis because of hepatitis C
• normal aminotransferase levels
• a kidney, liver, heart, or other solid-organ transplant
• specific contraindications to either monotherapy or combination therapy
Contraindications to alpha interferon therapy include severe depression or other neuropsychiatric syndromes, active substance or alcohol abuse, autoimmune disease (such as rheumatoid arthritis, lupus erythematosus, or psoriasis) that is not well controlled, bone marrow compromise, and inability to practice birth control. Contraindications to ribavirin and thus combination therapy include marked anemia, renal dysfunction, and coronary artery or cerebrovascular disease, and, again, inability to practice birth control.
Alpha interferon has multiple neuropsychiatric effects. Prolonged therapy can cause marked irritability, anxiety, personality changes, depression, and even suicide or acute psychosis. Patients particularly susceptible to these side effects are those with preexisting serious psychiatric conditions and patients with neurological disease.
Strict abstinence from alcohol is recommended during therapy with interferon. Interferon therapy can be associated with relapse in people with a previous history of drug or alcohol abuse. Therefore, alpha interferon should be given with caution to a patient who has only recently stopped alcohol or substance abuse. Typically a 6-month abstinence is recommended before starting therapy, but this should be applied only to patients with a history of alcohol abuse, not to social drinkers. Patients with continuing alcohol or substance abuse problems should only be treated in collaboration with alcohol or substance abuse specialists or counselors. Patients can be successfully treated while on methadone or in an active substance abuse program. Indeed, the rigor and regular monitoring that accompany methadone treatment provide a structured format for combination therapy. The dose of methadone may need to be modified during interferon-based therapy for hepatitis.
Alpha interferon therapy can induce autoantibodies, and a 24- to 48-week course triggers an autoimmune condition in about 2 percent of patients, particularly if they have an underlying susceptibility to autoimmunity (high titers of antinuclear or antithyroid antibodies, for instance). Exacerbation of a known autoimmune disease (such as rheumatoid arthritis or psoriasis) occurs commonly during interferon therapy.
Alpha interferon has bone marrow suppressive effects. Therefore, patients with bone marrow compromise or cytopenias, such as low platelet count (< 75,000 cells/mm3) or neutropenia (< 1,000 cells/mm3) should be treated cautiously and with frequent monitoring of cell counts. These side effects appear to be more common with peginterferon than standard interferon.
Ribavirin causes red cell hemolysis to a variable degree in almost all patients. Therefore, patients with a preexisting hemolysis or anemia (hemoglobin < 11 grams [g] or hematocrit < 33 percent) should not receive ribavirin. similarly, patients who have significant coronary or cerebral vascular disease should not receive ribavirin, as the anemia caused by treatment can trigger significant ischemia. fatal myocardial infarctions and strokes have been reported during combination therapy with alpha interferon and ribavirin.
Growth factors such as erythropoietin to raise red blood cell counts or granulocyte stimulating factor to raise neutrophil counts have been used successfully to treat patients with cytopenias during combination therapy. The proper role, dose, and side effects of these adjunctive therapies have yet to be defined.
Ribavirin is excreted largely by the kidneys. Patients with renal disease can develop hemolysis that is severe and even life-threatening. Patients who have elevations in serum creatinine above 2.0 mg per deciliter (dL) should not be treated with ribavirin.
Finally, ribavirin causes birth defects in animal studies and should not be used in women or men who are not practicing adequate means of birth control. Alpha interferon also should not be used in pregnant women, as it has direct antigrowth and antiproliferative effects.
Combination therapy should therefore be used with caution. Patients should be fully informed of the potential side effects before starting therapy.
Side Effects of Treatment
Common side effects of alpha interferon and peginterferon (occurring in more than 10 percent of patients) include
• fatigue
• muscle aches
• headaches
• nausea and vomiting
• skin irritation at the injection site
• low-grade fever
• weight loss
• irritability
• depression
• mild bone marrow suppression
• hair loss (reversible)
Most of these side effects are mild to moderate in severity and can be managed. They are worse during the first few weeks of treatment, especially with the first injection. Thereafter, side effects diminish. Acetaminophen may be helpful for the muscle aches and low-grade fever. Fatigue and depression are occasionally so troublesome that the dose of interferon should be decreased or therapy stopped early. Depression and personality changes can occur on interferon therapy and be quite subtle and not readily admitted by the patient. These side effects need careful monitoring. Patients with depression may benefit from antidepressant therapy using selective serotonin reuptake inhibitors. Generally, the psychiatric side effects resolve within 2 to 4 weeks of stopping combination therapy.
Ribavirin also causes side effects, and the combination is generally less well tolerated than interferon monotherapy. The most common side effects of ribavirin are
• anemia
• fatigue and irritability
• itching
• skin rash
• nasal stuffiness, sinusitis, and cough
Ribavirin causes a dose-related hemolysis of red cells; with combination therapy, hemoglobin usually decreases by 2 to 3 g/dL and the hematocrit by 5 to 10 percent. The amount of decrease in hemoglobin is highly variable. The decrease starts between weeks 1 and 4 of therapy and can be precipitous. Some patients develop symptoms of anemia, including fatigue, shortness of breath, palpitations, and headache.
The sudden drop in hemoglobin can precipitate angina pectoris in susceptible people, and fatalities from acute myocardial infarction and stroke have been reported in patients receiving combination therapy for hepatitis C. For these important reasons, ribavirin should not be used in patients with preexisting anemia or with significant coronary or cerebral vascular disease. If such patients require therapy for hepatitis C, they should receive alpha interferon monotherapy.
Ribavirin has also been found to cause itching and nasal stuffiness. These are histamine-like side effects; they occur in 10 to 20 percent of patients and are usually mild to moderate in severity. In some patients, however, sinusitis, recurrent bronchitis, or asthma-like symptoms become prominent. It is important that these symptoms be recognized as attributable to ribavirin, because dose modification (by 200 mg per day) or early discontinuation of treatment may be necessary.
Uncommon side effects of alpha interferon, peginterferon, and combination therapy (occurring in less than 2 percent of patients) include:
• autoimmune disease (especially thyroid disease)
• severe bacterial infections
• marked thrombocytopenia
• marked neutropenia
• seizures
• depression and suicidal ideation or attempts
• retinopathy (microhemorrhages)
• hearing loss and tinnitus
Rare side effects include acute congestive heart failure, renal failure, vision loss, pulmonary fibrosis or pneumonitis, and sepsis. Deaths have been reported from acute myocardial infarction, stroke, suicide, and sepsis.
A unique but rare side effect is paradoxical worsening of the disease. This is assumed to be caused by induction of autoimmune hepatitis, but its cause is really unknown. Because of this possibility, aminotransferases should be monitored. If ALT levels rise to greater than twice the baseline values, therapy should be stopped and the patient monitored. Some patients with this complication have required corticosteroid therapy to control the hepatitis.
|
Algorithm for Treatment
i
i
i
i
i
i
i
|
Before Starting Therapy
| • |
Do a liver biopsy to confirm the diagnosis of HCV, assess the grade and stage of disease, and rule out other diagnoses. In situations where a liver biopsy is contraindicated, such as clotting disorders, combination therapy can be given without a pretreatment liver biopsy. |
| • |
Test for serum HCV RNA to document that viremia is present. |
| • |
Test for HCV genotype (or serotype) to help determine the duration of therapy and dose of ribavirin. |
| • |
Measure blood counts and aminotransferases to establish a baseline for these values. |
| • |
Counsel the patient about the relative risks and benefits of treatment. Side effects should be thoroughly discussed. |
During Therapy
| • |
Measure blood counts and aminotransferases at weeks 1, 2, and 4 and at 4- to 8-week intervals thereafter. |
| • |
Adjust the dose of ribavirin downward (by 200 mg at a time) if significant anemia occurs (hemoglobin less than 10 g/dL or hematocrit < 30 percent) and stop ribavirin if severe anemia occurs (hemoglobin < 8.5 g/dl or hematocrit < 26 percent). |
| • |
Adjust the dose of peginterferon downward if there are intolerable side effects such as severe fatigue, depression, or irritability or marked decreases in white blood cell counts (absolute neutrophil count below 500 cells/mm3) or platelet counts (decrease below 30,000 cells/mm3). When using peginterferon alfa-2a, the dose can be reduced from 180 to 135 and then to 90 mcg per week. When using peginterferon alfa-2b, the dose can be reduced from 1.5 to 1.0 and then to 0.5 mcg per kilogram per week. |
| • |
In patients with genotype 1, measure HCV RNA level immediately before therapy and again (by the same method) at week 12. Therapy can be stopped early if HCV RNA levels have not decreased by at least two log10 units, as studies have shown that genotype 1 patients without this amount of decrease in HCV RNA are unlikely to have a sustained response (likelihood is < 1 percent). in situations where hcv rna levels are not obtainable, repeat testing for hcv rna by pcr (or tma) should be done at 24 weeks and therapy stopped if hcv rna is still present, as a sustained response is unlikely. |
| • |
Reinforce the need to practice strict birth control during therapy and for 6 months thereafter. |
| • |
Measure thyroid-stimulating hormone levels every 3 to 6 months during therapy. Patients with genotypes 2 or 3 can stop therapy at 24 weeks. Patients with genotype 1 and a drop in HCV RNA by 12 weeks should continue therapy for 48 weeks. |
At the end of therapy, test HCV RNA by PCR to assess whether there is an end-of-treatment response.
After Therapy
| • |
Measure aminotransferases every 2 months for 6 months. |
| • |
Six months after stopping therapy, test for HCV RNA by PCR. If HCV RNA is still negative, the chance for a long-term “cure” is excellent; relapses have rarely been reported after this point. |
Options for Patients Who Do Not Respond to Treatment
Few options exist for patients who either do not respond to therapy or who respond and later relapse. Patients who relapse after a course of interferon monotherapy may respond to a course of combination therapy, particularly if they became and remained HCV RNA negative during the period of monotherapy. The response rates and optimal dose (800 vs. 1,000 mg to 1,200 mg of ribavirin) and duration (24 or 48 weeks) of peginterferon and ribavirin for relapse or previous nonresponder patients have not been defined. The algorithm for treatment given above is for treatment of naive patients.
An experimental approach to treatment of non-responders is the use of long-term or maintenance interferon, which is feasible only if the peginterferon is well tolerated and has a clear-cut effect on serum aminotransferases or liver histology, despite lack of clearance of HCV RNA. This approach is now under evaluation in long-term clinical trials in the United States. New medications and approaches to treatment are needed. Most promising for the future are the use of other cytokines and the development of newer antivirals, such as RNA polymerase, helicase, or protease inhibitors.
Hope Through Research
Basic Research
A major focus of hepatitis C research is developing a tissue culture system that will enable researchers to study HCV outside the human body. Animal models and molecular approaches to the study of HCV are also important. Understanding how the virus replicates and how it injures cells would be helpful in developing a means of controlling it and in screening for new drugs that would block it.
Diagnostic Tests
More sensitive and less expensive assays for measuring HCV RNA and antigens in the blood and liver are needed. Although current tests for anti-HCV are quite sensitive, a small percentage of patients with hepatitis C test negative for anti-HCV (false-negative reaction), and a percentage of patients who test positive are not infected (false-positive reaction). Also, there are patients who have resolved the infection but still test positive for anti-HCV. Convenient tests to measure HCV in serum and to detect HCV antigens in liver tissue would be helpful. Clinically, noninvasive tests that would reliably predict liver fibrosis would be a very valuable advance.
New Treatments
Most critical for the future is the development of new antiviral agents for hepatitis C. Most interesting will be specific inhibitors of HCV-derived enzymes such as protease, helicase, and polymerase inhibitors. Drugs that inhibit other steps in HCV replication may also be helpful in treating this disease, by blocking production of HCV antigens from the RNA (IRES inhibitors), preventing the normal processing of HCV proteins (inhibitors of glycosylation), or blocking entry of HCV into cells (by blocking its receptor). In addition, nonspecific cytoprotective agents might be helpful for hepatitis C by blocking the cell injury caused by the virus infection. Further, molecular approaches to treating hepatitis C are worthy of investigation; these consist of using ribozymes, which are enzymes that break down specific viral RNA molecules, and antisense oligonucleotides, which are small complementary segments of DNA that bind to viral RNA and inhibit viral replication. All of these approaches remain experimental and few have been applied to humans. The serious nature and the frequency of hepatitis C in the population make the search for new therapies of prime importance.
Prevention
At present, the only means of preventing new cases of hepatitis C are to screen the blood supply, encourage health professionals to take precautions when handling blood and body fluids, and inform people about high-risk behaviors. Programs to promote needle exchange offer some hope of decreasing the spread of hepatitis C among injection drug users. Furthermore, all drug users should receive instruction in safer injection techniques, simple interventions that can be life-saving. Vaccines and immunoglobulin products do not exist for hepatitis C, and development seems unlikely in the near future because these products would require antibodies to all the genotypes and variants of hepatitis C. Nevertheless, advances in immunology and innovative approaches to immunization make it likely that some form of vaccine for hepatitis C will eventually be developed.
Selected Review Articles and References
Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Seminars in Liver Disease. 2000;20(1):17-35.
Centers for Disease Control and Prevention. Hepatitis A to E. Available at: www.cdc.gov/ncidod/diseases/hepatitis/slideset/httoc.htm (accessed Nov. 25, 1996).
Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HVC) infection and HVC-related chronic disease. Morbidity and Mortality Weekly Report. 1998;47:1-39.
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C infection. New England Journal of Medicine. 2002;347:972-982.
Lauer GM, Walker BD. Hepatitis C virus infection. New England Journal of Medicine. 2001;345:41-52.
Liang TJ, Reherman B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Annals of Internal Medicine. 2000;132:296-305.
Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965.
McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling M-H, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. New England Journal of Medicine. 1998;339(21):1485-1492.
Proceedings of the June 10-12 “Management of Hepatitis C: 2002. National Institutes of Health Consensus Development Conference Update.” Hepatology. 2002;36(5, part 2).
Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai M-Y, Gane E, O’Grady J, Reichen J, Diago M,
Lin A, Hoffman J, Brunda MJ. Peginterferon alfa-2a in patients with chronic hepatitis C. New England Journal of Medicine. 2000;343:1666-1672.
v Cirrhosis of the Liver
The liver, the largest organ in the body, is essential in keeping the body functioning properly. It removes or neutralizes poisons from the blood, produces immune agents to control infection, and removes germs and bacteria from the blood. It makes proteins that regulate blood clotting and produces bile to help absorb fats and fat-soluble vitamins. You cannot live without a functioning liver.
In cirrhosis of the liver, scar tissue replaces normal, healthy tissue, blocking the flow of blood through the organ and preventing it from working as it should. Cirrhosis is the twelfth leading cause of death by disease, killing about 26,000 people each year. Also, the cost of cirrhosis in terms of human suffering, hospital costs, and lost productivity is high.
Causes
Cirrhosis has many causes. In the United States, chronic alcoholism and hepatitis C are the most common ones.
| • |
Alcoholic liver disease. To many people, cirrhosis of the liver is synonymous with chronic alcoholism, but in fact, alcoholism is only one of the causes. Alcoholic cirrhosis usually develops after more than a decade of heavy drinking. The amount of alcohol that can injure the liver varies greatly from person to person. In women, as few as two to three drinks per day have been linked with cirrhosis and in men, as few as three to four drinks per day. Alcohol seems to injure the liver by blocking the normal metabolism of protein, fats, and carbohydrates. |
| • |
Chronic hepatitis C. The hepatitis C virus ranks with alcohol as a major cause of chronic liver disease and cirrhosis in the United States. Infection with this virus causes inflammation of and low grade damage to the liver that over several decades can lead to cirrhosis. |
| • |
Chronic hepatitis B and D. The hepatitis B virus is probably the most common cause of cirrhosis worldwide, but it is less common in the United States and the Western world. Hepatitis B, like hepatitis C, causes liver inflammation and injury that over several decades can lead to cirrhosis. Hepatitis D is another virus that infects the liver, but only in people who already have hepatitis B. |
| • |
Autoimmune hepatitis. This disease appears to be caused by the immune system attacking the liver and causing inflammation, damage, and eventually scarring and cirrhosis. |
| • |
Inherited diseases. Alpha-1 antitrypsin deficiency, hemochromatosis, Wilson’s disease, galactosemia, and glycogen storage diseases are among the inherited diseases that interfere with the way the liver produces, processes, and stores enzymes, proteins, metals, and other substances the body needs to function properly. |
| • |
Nonalcoholic steatohepatitis (NASH). In NASH, fat builds up in the liver and eventually causes scar tissue. This type of hepatitis appears to be associated with diabetes, protein malnutrition, obesity, coronary artery disease, and treatment with corticosteroid medications. |
| • |
Blocked bile ducts. When the ducts that carry bile out of the liver are blocked, bile backs up and damages liver tissue. In babies, blocked bile ducts are most commonly caused by biliary atresia, a disease in which the bile ducts are absent or injured. In adults, the most common cause is primary biliary cirrhosis, a disease in which the ducts become inflamed, blocked, and scarred. Secondary biliary cirrhosis can happen after gallbladder surgery if the ducts are inadvertently tied off or injured. |
| • |
Drugs, toxins, and infections. Severe reactions to prescription drugs, prolonged exposure to environmental toxins, the parasitic infection schistosomiasis, and repeated bouts of heart failure with liver congestion can all lead to cirrhosis. |
Symptoms
Many people with cirrhosis have no symptoms in the early stages of the disease. However, as scar tissue replaces healthy cells, liver function starts to fail and a person may experience the following symptoms:
• exhaustion
• fatigue
• loss of appetite
• nausea
• weakness
• weight loss
• abdominal pain
• spider-like blood vessels (spider angiomas) that develop on the skin
As the disease progresses, complications may develop. In some people, these may be the first signs of the disease.
Complications of Cirrhosis
Loss of liver function affects the body in many ways. Following are the common problems, or complications, caused by cirrhosis.
| • |
Edema and ascites. When the liver loses its ability to make the protein albumin, water accumulates in the legs (edema) and abdomen (ascites). |
| • |
Bruising and bleeding. When the liver slows or stops production of the proteins needed for blood clotting, a person will bruise or bleed easily. The palms of the hands may be reddish and blotchy with palmar erythema. |
| • |
Jaundice. Jaundice is a yellowing of the skin and eyes that occurs when the diseased liver does not absorb enough bilirubin. |
| • |
Itching. Bile products deposited in the skin may cause intense itching. |
| • |
Gallstones. If cirrhosis prevents bile from reaching the gallbladder, gallstones may develop. |
| • |
Toxins in the blood or brain. A damaged liver cannot remove toxins from the blood, causing them to accumulate in the blood and eventually the brain. There, toxins can dull mental functioning and cause personality changes, coma, and even death. Signs of the buildup of toxins in the brain include neglect of personal appearance, unresponsiveness, forgetfulness, trouble concentrating, or changes in sleep habits. |
| • |
Sensitivity to medication. Cirrhosis slows the liver’s ability to filter medications from the blood. Because the liver does not remove drugs from the blood at the usual rate, they act longer than expected and build up in the body. This causes a person to be more sensitive to medications and their side effects. |
| • |
Portal hypertension. Normally, blood from the intestines and spleen is carried to the liver through the portal vein. But cirrhosis slows the normal flow of blood through the portal vein, which increases the pressure inside it. This condition is called portal hypertension. |
| • |
Varices. When blood flow through the portal vein slows, blood from the intestines and spleen backs up into blood vessels in the stomach and esophagus. These blood vessels may become enlarged because they are not meant to carry this much blood. The enlarged blood vessels, called varices, have thin walls and carry high pressure, and thus are more likely to burst. If they do burst, the result is a serious bleeding problem in the upper stomach or esophagus that requires immediate medical attention. |
| • |
Insulin resistance and type 2 diabetes. Cirrhosis causes resistance to insulin. This hormone, produced by the pancreas, enables blood glucose to be used as energy by the cells of the body. If you have insulin resistance, your muscle, fat, and liver cells do not use insulin properly. The pancreas tries to keep up with the demand for insulin by producing more. Eventually, the pancreas cannot keep up with the body’s need for insulin, and type 2 diabetes develops as excess glucose builds up in the bloodstream. |
| • |
Liver cancer. Hepatocellular carcinoma, a type of liver cancer commonly caused by cirrhosis, starts in the liver tissue itself. It has a high mortality rate. |
| • |
Problems in other organs. Cirrhosis can cause immune system dysfunction, leading to infection. Fluid in the abdomen (ascites) may become infected with bacteria normally present in the intestines. Cirrhosis can also lead to impotence, kidney dysfunction and failure, and osteoporosis. |
Diagnosis
The diagnosis of fatty liver is based on liver biopsy. Even though alcoholic fatty liver is associated with palpable enlargement of the liver in more than 90 percent of patients, fatty liver alone cannot be distinguished from fatty liver with alcoholic hepatitis or fibrosis with cirrhosis without a biopsy. In fact, one of the problems in diagnosing fatty liver in the chronic alcoholic is being able to differentiate it from alcoholic hepatitis with severe steatosis.
The definitive diagnosis of alcoholic hepatitis requires liver biopsy as well. Signs and symptoms are not specific for the diagnosis of alcoholic hepatitis. Anorexia, nausea, or weight loss are present in 70 percent of the patients and abdominal pain in 50 percent. Jaundice, fever, and elevated white blood cell count are also commonly present. If the results of a liver biopsy are not available, a probable diagnosis can be made with a history of chronic as well as current heavy alcohol consumption, a very large liver, serum aspartate aminotransferase activity higher than that of serum alanine aminotransferase, elevated serum alkaline phosphatase activity, and no evidence of viral or other drug-induced hepatitis. The sensitivity and specificity of this definition are not known.
The definitive diagnosis of cirrhosis also requires histologic examination of the liver. Diagnostic evaluation of the patient suspected to have cirrhosis takes advantage of all possible information sources–patient history, physical examination, laboratory tests, and special procedures. However, the only reliable evidence of cirrhosis is based on the gross appearance of the liver (at laparoscopy, laparotomy, or autopsy) and the microscopic examination of the liver tissue. Because liver biopsy is an invasive procedure, it is reserved for symptomatic individuals and is not suitable for general population studies.
Ultrasonography, because of ease of use and no known risk to the patient, is a frequent screening tool in patients with hepatic dysfunction but lacks the sensitivity and specificity to detect asymptomatic cirrhosis in the general population. In recent years, computerized tomography (CT) scanning has begun to provide a valuable adjunct in differentiating certain types of liver conditions. The sensitivity and specificity of CT scanning in detecting asymptomatic cirrhosis in the population at large has not been examined. In addition, at present this modality is too prohibitively expensive to be recommended for use in epidemiologic studies. Radionuclide imaging and angiography are also useful techniques ‘in differentiating liver lesions but, again, are too invasive, time consuming and expensive to be useful in population studies.
No single laboratory blood test is sufficiently sensitive or specific enough to diagnose cirrhosis, although combinations of tests may point to one condition or another. Marked elevation of a combination of commonly performed serum measures such as alkaline phosphatase, alanine aminotransferase, and bilirubin is diagnostic of biliary obstruction or liver disease but not necessarily cirrhosis. Mild elevation of a single measure, however, is not specific for liver disease. In an evaluation of 100 blood donors with elevated alanine aminotransferase activity, only 6 were found to have identifiable chronic liver disease.
The doctor may diagnose cirrhosis on the basis of symptoms, laboratory tests, the medical history, and a physical examination. For example, during a physical examination, the doctor may notice that the liver feels harder or larger than usual and order blood tests that can show whether liver disease is present.
If looking at the liver is necessary to check for signs of disease, the doctor might order a computerized axial tomography (CAT) scan, ultrasound, magnetic resonance imaging (MRI), or a scan of the liver using a radioisotope (a harmless radioactive substance that highlights the liver). Or the doctor might look at the liver using a laparoscope, an instrument that is inserted through the abdomen and relays pictures back to a computer screen.
A liver biopsy will confirm the diagnosis. For a biopsy, the doctor uses a needle to take a tiny sample of liver tissue, then examines it under the microscope for scarring or other signs of disease.
Treatment
Liver damage from cirrhosis cannot be reversed, but treatment can stop or delay further progression and reduce complications. Treatment depends on the cause of cirrhosis and any complications a person is experiencing. For example, cirrhosis caused by alcohol abuse is treated by abstaining from alcohol. Treatment for hepatitis-related cirrhosis involves medications used to treat the different types of hepatitis, such as interferon for viral hepatitis and corticosteroids for autoimmune hepatitis. Cirrhosis caused by Wilson’s disease, in which copper builds up in organs, is treated with medications to remove the copper. These are just a few examples—treatment for cirrhosis resulting from other diseases depends on the underlying cause. In all cases, regardless of the cause, following a healthy diet and avoiding alcohol are essential because the body needs all the nutrients it can get, and alcohol will only lead to more liver damage. Light physical activity can help stop or delay cirrhosis as well.
Treatment will also include remedies for complications. For example, for ascites and edema, the doctor may recommend a low-sodium diet or the use of diuretics, which are drugs that remove fluid from the body. Antibiotics will be prescribed for infections, and various medications can help with itching. Protein causes toxins to form in the digestive tract, so eating less protein will help decrease the buildup of toxins in the blood and brain. The doctor may also prescribe laxatives to help absorb the toxins and remove them from the intestines.
For portal hypertension, the doctor may prescribe a blood pressure medication such as a beta-blocker. If varices bleed, the doctor may either inject them with a clotting agent or perform a so-called rubber-band ligation, which uses a special device to compress the varices and stop the bleeding.
When complications cannot be controlled or when the liver becomes so damaged from scarring that it completely stops functioning, a liver transplant is necessary. In liver transplantation surgery, a diseased liver is removed and replaced with a healthy one from an organ donor. About 80 to 90 percent of patients survive liver transplantation. Survival rates have improved over the past several years because of drugs such as cyclosporine and tacrolimus, which suppress the immune system and keep it from attacking and damaging the new liver.
v Primary Biliary Cirrhosis
Primary biliary cirrhosis is a liver disease that slowly destroys the bile ducts in the liver. Bile, a substance that helps digest fat, leaves the liver through these ducts. When the ducts are damaged, bile builds up in the liver and damages liver tissue. Over time, the disease can cause cirrhosis and may make the liver stop working.
The cause of primary biliary cirrhosis is unknown. The disease affects women more often than men, and usually occurs between the ages of 30 and 60 years. Some research suggests that the disease might be caused by a problem within the immune system.
The most common symptoms of primary biliary cirrhosis are itchy skin and fatigue. Other symptoms include jaundice (yellowing of the eyes and skin), cholesterol deposits on the skin, fluid retention, and dry eyes or mouth. Some people with primary biliary cirrhosis also have osteoporosis, arthritis, and thyroid problems.
Primary biliary cirrhosis is diagnosed through laboratory tests, x rays, and in some cases, a liver biopsy (a simple operation to remove a small piece of liver tissue). Treatment may include taking vitamin and calcium supplements, hormone therapy, and medicines to relieve symptoms. Ursodiol is beneficial for patients with primary biliary cirrhosis although it does not cure the disease. A liver transplant may be necessary if the liver is severely damaged.
v Primary Sclerosing Cholangitis
In primary sclerosing cholangitis (PSC), the bile ducts inside and outside the liver become inflamed and scarred. As the scarring increases, the ducts become blocked. The ducts are important because they carry bile out of the liver. Bile is a liquid that helps break down fat in food. If the ducts are blocked, bile builds up in the liver and damages liver cells. Eventually, PSC can cause liver failure.
Researchers do not know what causes PSC. Among the theories under investigation are the possible role of bacteria, viruses, and immune system problems. PSC appears to be associated with ulcerative colitis, a type of inflammatory bowel disease.
The disease usually begins between ages 30 and 60, but the disease can also arise during childhood. PSC is more common in men than women. PSC progresses slowly, so a person can have the disease for years before symptoms develop. The main symptoms are itching, fatigue, and jaundice, which causes yellowing of the eyes or skin. An infection in the bile ducts can cause chills and fever.
PSC is diagnosed through cholangiography, which involves injecting dye into the bile ducts and taking an x ray. Cholangiography can be performed as an endoscopic procedure (endoscopic retrograde cholangiopancreatography, ERCP), through radiology or surgery, or with magnetic resonance imaging (MRI) scans. Treatment includes medication to relieve itching, antibiotics to treat infections, and vitamin supplements, as people with PSC are often deficient in vitamins A, D, and K. In some cases, surgery to open major blockages in the common bile duct is also necessary. Liver transplantation may be an option if the liver begins to fail.
v Nonalcoholic Steatohepatitis
Nonalcoholic steatohepatitis or NASH is a common, often “silent” liver disease. It resembles alcoholic liver disease, but occurs in people who drink little or no alcohol. The major feature in NASH is fat in the liver, along with inflammation and damage. Most people with NASH feel well and are not aware that they have a liver problem. Nevertheless, NASH can be severe and can lead to cirrhosis, in which the liver is permanently damaged and scarred and no longer able to work properly.
NASH affects 2 to 5 percent of Americans. An additional 10 to 20 percent of Americans have fat in their liver, but no inflammation or liver damage, a condition called “fatty liver.” Although having fat in the liver is not normal, by itself it probably causes little harm or permanent damage. If fat is suspected based on blood test results or scans of the liver, this problem is called nonalcoholic fatty liver disease (NAFLD). If a liver biopsy is performed in this case, it will show that some people have NASH while others have simple fatty liver.
Both NASH and NAFLD are becoming more common, possibly because of the greater number of Americans with obesity. In the past 10 years, the rate of obesity has doubled in adults and tripled in children. Obesity also contributes to diabetes and high blood cholesterol, which can further complicate the health of someone with NASH. Diabetes and high blood cholesterol are also becoming more common among Americans.
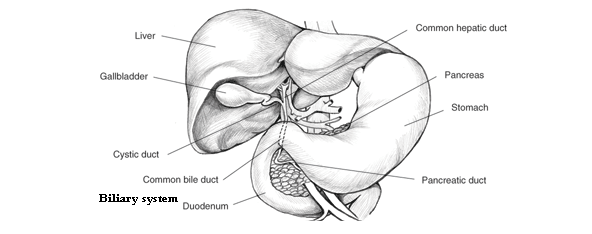 |
Diagnosis
NASH is usually first suspected in a person who is found to have elevations in liver tests that are included in routine blood test panels, such as alanine aminotransferase (ALT) or aspartate aminotransferase (AST). When further evaluation shows no apparent reason for liver disease (such as medications, viral hepatitis, or excessive use of alcohol) and when x rays or imaging studies of the liver show fat, NASH is suspected. The only means of proving a diagnosis of NASH and separating it from simple fatty liver is a liver biopsy. For a liver biopsy, a needle is inserted through the skin to remove a small piece of the liver. NASH is diagnosed when examination of the tissue under the microscope shows fat along with inflammation and damage to liver cells. If there is fat without inflammation and damage, simple fatty liver or NAFLD is diagnosed. An important piece of information learned from the biopsy is whether scar tissue has developed in the liver. Currently, no blood tests or scans can reliably provide this information.
Symptoms
NASH is usually a silent disease with few or no symptoms. Patients generally feel well in the early stages and only begin to have symptoms—such as fatigue, weight loss, and weakness—once the disease is more advanced or cirrhosis develops. The progression of NASH can take years, even decades. The process can stop and, in some cases, reverse on its own without specific therapy. Or NASH can slowly worsen, causing scarring or “fibrosis” to appear and accumulate in the liver. As fibrosis worsens, cirrhosis develops; the liver becomes seriously scarred, hardened, and unable to function normally. Not every person with NASH develops cirrhosis, but once serious scarring or cirrhosis is present, few treatments can halt the progression. A person with cirrhosis experiences fluid retention, muscle wasting, bleeding from the intestines, and liver failure. Liver transplantation is the only treatment for advanced cirrhosis with liver failure, and transplantation is increasingly performed in people with NASH. NASH ranks as one of the major causes of cirrhosis in America, behind hepatitis C and alcoholic liver disease.
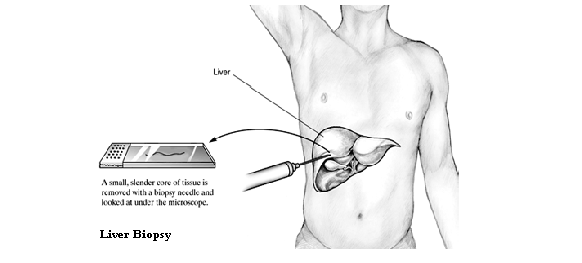 |
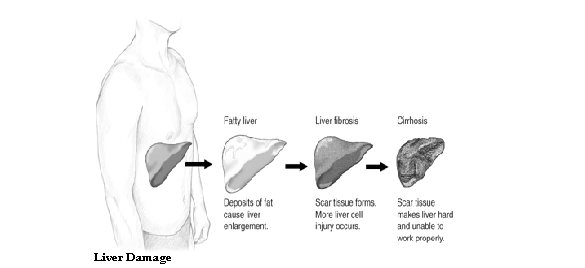 |
Causes
Although NASH has become more common, its underlying cause is still not clear. It most often occurs in persons who are middle-aged and overweight or obese. Many patients with NASH have elevated blood lipids, such as cholesterol and triglycerides, and many have diabetes or pre-diabetes, but not every obese person or every patient with diabetes has NASH. Furthermore, some patients with NASH are not obese, do not have diabetes, and have normal blood cholesterol and lipids. NASH can occur without any apparent risk factor and can even occur in children. Thus, NASH is not simply obesity that affects the liver.
While the underlying reason for the liver injury that causes NASH is not known, several factors are possible candidates:
• insulin resistance
• release of toxic inflammatory proteins by fat cells (cytokines)
• oxidative stress (deterioration of cells) inside liver cells
Treatment
It is important to stress that there are currently no specific therapies for NASH. The most important recommendations given to persons with this disease are to
• reduce their weight (if obese or overweight)
• follow a balanced and healthy diet
• increase physical activity
• avoid alcohol
• avoid unnecessary medications
These are standard recommendations, but they can make a difference. They are also helpful for other conditions, such as heart disease, diabetes, and high cholesterol.
A major attempt should be made to lower body weight into the healthy range. Weight loss can improve liver tests in patients with NASH and may reverse the disease to some extent. Research at present is focusing on how much weight loss improves the liver in patients with NASH and whether this improvement lasts over a period of time.
People with NASH often have other medical conditions, such as diabetes, high blood pressure, or elevated cholesterol. These conditions should be treated with medication and adequately controlled; having NASH or elevated liver enzymes should not lead people to avoid treating these other conditions.
Experimental approaches under evaluation in patients with NASH include antioxidants, such as vitamin E, selenium, and betaine. These medications act by reducing the oxidative stress that appears to increase inside the liver in patients with NASH. Whether these substances actually help treat the disease is not known, but the results of clinical trials should become available in the next few years.
Another experimental approach to treating NASH is the use of newer antidiabetic medications—even in persons without diabetes. Most patients with NASH have insulin resistance, meaning that the insulin normally present in the bloodstream is less effective for them in controlling blood glucose and fatty acids in the blood than it is for people who do not have NASH. The newer antidiabetic medications make the body more sensitive to insulin and may help reduce liver injury in patients with NASH. Studies of these medications—including metformin, rosiglitazone, and pioglitazone—are being sponsored by the National Institutes of Health and should answer the question of whether these medications are beneficial in NASH.
Hope Through Research
What is most needed in the management of NASH is more research to better understand the liver injury found in this disease. When the pathways that lead to the injury are fully known, safe and effective means can be developed to reverse these pathways and help patients with NASH. Recent breakthroughs in mapping the human genome and uncovering the individual steps by which insulin and other hormones regulate blood glucose and fat could provide the necessary clues.
The National Institute of Diabetes and Digestive and Kidney Diseases funds the NASH Clinical Research Network, which comprises eight clinical centers located throughout the United States and a coordinating center at Johns Hopkins University.
The NASH network researches the nature and underlying cause of NASH and conducts clinical studies on prevention and treatment. More information on the NASH Clinical Research Network and the locations of the clinical centers are available at www.nashcrn.com.
Points to Remember
Nonalcoholic steatohepatitis (NASH) is fat in the liver, with inflammation and damage.
• NASH occurs in people who drink little or no alcohol and affects 2 to 5 percent of Americans, especially
people who are middle-aged and overweight or obese.
• Nash can occur in children.
• People who have NASH may feel well and may not know that they have a liver disease.
• NASH can lead to cirrhosis, a condition in which the liver is permanently damaged and cannot work
properly.
• Fatigue can occur at any stage of NASH.
• Weight loss and weakness may begin once the disease is advanced or cirrhosis is present.
• NASH may be suspected if blood tests show high levels of liver enzymes or if scans show fatty liver.
v What I Need to Know About Liver Transplantation
What does my liver do?
Your liver helps fight infections and cleans your blood. It also helps digest food and stores energy for when you need it.
What are the signs of liver problems?
|
Some signs of liver problems are • feeling tired or weak • losing your appetite • feeling sick to your stomach • losing weight • bruising or bleeding easily, such as nosebleeds • bloating due to fluid buildup in the abdomen (ascites*) • declining mental functions |
 |
|
Also, liver problems often make the skin and the whites of the eyes turn yellow, a condition called jaundice, and may cause swelling in the legs and the abdomen.
Liver transplantation is surgery to remove a diseased liver and replace it with a healthy one. |
 |
This kind of surgery has been done for more than 38 years. Many people have had liver transplants and now lead normal lives.
What are the reasons for needing a liver transplant?
In adults, the most common reason for liver transplantation is cirrhosis. Cirrhosis is caused by many different types of liver injuries that destroy healthy liver cells and replace them with scar tissue. Cirrhosis can be caused by viruses such as hepatitis B and C, alcohol, autoimmune liver diseases, buildup of fat in the liver, and hereditary liver diseases.
In children, the most common reason for liver transplantation is biliary atresia. Bile ducts, which are tubes that carry bile out of the liver, are missing or damaged in this disease, and obstructed bile causes cirrhosis. Bile helps digest food.
Other reasons for transplantation are liver cancer, benign liver tumors, and hereditary diseases. Sometimes the cause of liver disease is not known.
 |
|
How will I know whether I need a liver transplant? |
 |
Can anyone with liver problems get a transplant?
You cannot have a transplant if you have
• cancer in another part of your body
• serious heart, lung, or nerve disease
• active alcohol or illegal drug abuse
• an active, severe infection
• inability to follow your doctor’s instructions
|
How long does it take to get a new liver? |
 |
Where do the livers for transplants come from?
Whole livers come from people who have just died. This type of donor is called a cadaveric donor. Sometimes a healthy person will donate part of his or her liver for a particular patient. This kind of donor is called a living donor.
All living donors and donated livers are tested before transplant surgery. The testing makes sure the liver is healthy, matches your blood type, and is the right size so it has the best chance of working in your body.
Health insurance
You should check your health insurance policy to be sure it covers liver transplantation and prescription medicines. You will need many prescription medicines after the surgery and for the rest of your life.
|
What happens in the hospital? The surgeon will disconnect your diseased liver from your bile ducts and blood vessels before removing it. |
 |
The blood that flows into your liver will be blocked or sent through a machine to return to the rest of your body. The surgeon will put the healthy liver in place and reconnect it to your bile ducts and blood vessels. Your blood will then flow into your new liver.
|
After Surgery |
 |
What is rejection?
Rejection occurs when your body’s natural defenses, called the immune system, damage the new liver. Your immune system keeps you healthy by fighting against things that don’t belong in your body, such as bacteria and viruses. After a transplant, it is common for your immune system to fight against the liver and try to destroy it.
How is rejection prevented?
To keep your body from rejecting the new liver, you will take medicines. These drugs, such as steroids, cyclosporine, tacrolimus, sirolimus, and mycophenolate mofetil, are called immunosuppressants. Immunosuppressants weaken your immune system’s ability to reject your new liver.
Do immunosuppressants have any side effects?
Yes. You can get infections more easily because these drugs weaken your immune system. You will need to stay away from people who are sick. These drugs may also increase your blood pressure, cause your cholesterol to rise, cause diabetes, weaken your bones, and damage your kidneys. Steroid drugs may also cause changes in how you look by causing weight gain. Your doctor and the transplant team will monitor these effects and may treat you for complications.
What are the signs of rejection?
Doctors will check your blood for liver enzymes, the first sign of rejection. Often rejection does not make you feel ill. Sometimes rejection can cause
• nausea
• pain
• fever
• jaundice
|
Often, a liver biopsy is needed to be sure that the transplanted liver is being rejected. For a biopsy,the doctor takes a small piece of the liver to view under a microscope. Other problems include |
 |
• blockage of the blood vessels going into or out of the liver
• damage to the tubes that carry bile into the intestine
What if the transplant doesn’t work?
Liver transplants usually work. About 80 to 90 percent of transplanted livers are still working after 1 year. If the new liver does not work or if your body rejects it, your doctor and the transplant team will decide whether another transplant is possible.
How do I take care of my liver after I leave the hospital?
|
After you leave the transplant center at the hospital, you will see your doctor often to be sure your new liver is working well. You will have regular blood tests to check that your new liver is not being damaged by rejection, infections, or problems with blood vessels or bile ducts. You will need to avoid sick people and report any illnesses to your doctor. You will need to eat a healthy diet, exercise, and not drink alcohol, especially if alcohol was the cause of damage to your own liver. You should use medicines, including ones you can buy without a prescription, only if your doctor says they are safe for you. It is important to do what your doctor says to take care of your new liver. Can I go back to my daily activities? |
 |
Yes. After a successful liver transplant, most people can go back to their normal daily activities. Getting your strength back will take some time, though, depending on how sick you were before the transplant. You will need to check with your doctor on how long your recovery period should be. Social workers and support groups will help you adjust to life with a new liver.
• Work. After recovery, most people are able to go back to work.
• Diet. Most people can go back to eating as they did before. Some medicines may cause you to gain weight, and others may cause diabetes or a rise in your cholesterol. Meal planning and a balanced low-fat diet can help you remain healthy.
• Exercise. Most people can engage in physical activity after a successful liver transplant.
• Sex. Most people return to a normal sex life after liver transplantation. It is important for women to avoid becoming pregnant in the first year after transplantation. You should talk to your transplant team about sex and reproduction after transplantation.
If you have any questions, you may want to check with your doctor before starting any activity.
Glossary
Ascites (uh-SY-teez): A buildup of fluid in the abdomen.
Autoimmune (AW-toh-im-YOON): A term that refers to a person’s immune system attacking his or her own body.
Biliary atresia (BILL-ee-air-ee uh-TREEZ-ya): A condition that results when the bile ducts inside or outside the liver don’t have normal openings. Bile becomes trapped in the liver, causing jaundice and cirrhosis. This condition is present from birth and without surgery may cause death.
Biopsy (BYE-op-see): Removing a small piece of tissue to view under a microscope.
Cirrhosis (sir-ROH-sis): A chronic liver condition caused by scar tissue and damage to cells. Cirrhosis makes it hard for the liver to remove poisons (toxins) like alcohol and drugs from the blood. These toxins build up in the blood and may affect the brain.
Cyclosporine (sy-klo-SPOR-in): An immunosuppressant used after transplantation to prevent rejection.
Immunosuppressants (im-you-no-suh-PRESS-unts): Medicines that stop your immune system from attacking bacteria, viruses, and transplanted organs.
Jaundice (JAWN-dus): A symptom of many disorders. Jaundice causes the skin and the whites of the eyes to turn yellow.
Mycophenolate mofetil (MY-co-PHEN-olate MOF-i-til): An immunosuppressant used after transplantation to prevent rejection.
Sirolimus (si-RAW-lih-mus): An immunosuppressant used after transplantation to prevent rejection.
Steroids (STAIR-oids): A group of immunosuppressants used after transplantation to prevent rejection.
Tacrolimus (ta-CRAW-lih-mus): An immunosuppressant used after transplantation to prevent rejection.