|
v Facts About Botulism
Botulism is a muscle-paralyzing disease caused by a toxin made by a bacterium called Clostridium botulinum .
There are three main kinds of botulism:
Foodborne botulism occurs when a person ingests preformed toxin that leads to illness within a few hours to days. Foodborne botulism is a public health emergency because the contaminated food may still be available to other persons besides the patient.
Infant botulism occurs in a small number of susceptible infants each year who harbor C. botulinum in their intestinal tract.
Wound botulism occurs when wounds are infected with C. botulinum that secretes the toxin.
With foodborne botulism, symptoms begin within 6 hours to 2 weeks (most commonly between 12 and 36 hours) after eating toxin-containing food. Symptoms of botulism include double vision, blurred vision, drooping eyelids, slurred speech, difficulty swallowing, dry mouth, muscle weakness that always descends through the body: first shoulders are affected, then upper arms, lower arms, thighs, calves, etc. Paralysis of breathing muscles can cause a person to stop breathing and die, unless assistance with breathing (mechanical ventilation) is provided.
Botulism is not spread from one person to another. Foodborne botulism can occur in all age groups.
A supply of antitoxin against botulism is maintained by CDC. The antitoxin is effective in reducing the severity of symptoms if administered early in the course of the disease. Most patients eventually recover after weeks to months of supportive care.
v Botulism–Technical Information
| Clinical features | A neuroparalytic illness characterized by symmetric, descending flaccid paralysis of motor and autonomic nerves, always beginning with the cranial nerves. Symptoms include double vision, blurred vision, drooping eyelids, slurred speech, difficulty swallowing, dry mouth, and muscle weakness. If untreated, illness might progress to cause descending paralysis of respiratory muscles, arms and legs. Botulinum antitoxin (supplied by CDC) can prevent progression of illness and shorten symptoms in severe botulism cases if administered early. |
| Etiologic agent | A potent neurotoxin produced from Clostridium botulinum, an anaerobic, spore-forming bacterium. |
| Incidence | In 2001, 169 cases of botulism were reported to the CDC. Of these, 33 were foodborne, 112 were infant botulism, and 23 were cases of wound botulism. |
| Sequelae | Death can result from respiratory failure. About 5% die. Recovery takes months. Those who survive may have fatigue and shortness of breath for years. |
| Transmission | Foodborne botulism follows ingestion of toxin produced in food by C. botulinum . The most frequent source is home-canned foods, prepared in an unsafe manner. Wound botulism occurs when C. botulinum spores germinate within wounds. Infant botulism occurs when C. botulinum spores germinate and produce toxin in the gastrointestinal tract of infants. |
| Risk groups | All persons. Injection drug users are at increased risk for wound botulism. |
| Surveillance | In collaboration with state health departments, CDC maintains intensive surveillance for botulism in the United States. Every case of foodborne botulism is treated as a public health emergency because the responsible food, whether homemade or commercial, might still be available for consumption and could make unsuspecting persons ill. |
| Trends | Vehicles of transmission have included homemade salsa, baked potatoes cooked in aluminum foil, cheese sauce, garlic in oil, and traditionally prepared salted or fermented fish in Alaska. Wound botulism related to the use of black-tar heroin has increased, especially in California. |
| Challenges | Prompt recognition of clinical syndrome by physicians. |
| Opportunities | Clinician education. Consumer education about home canning. Educating Alaska natives about proper fermentation techniques. Applying tools of molecular biology. |
v Frequently Asked Questions
What is botulism?
Botulism is a rare but serious paralytic illness caused by a nerve toxin that is produced by the bacterium Clostridium botulinum . There are three main kinds of botulism. Foodborne botulism is caused by eating foods that contain the botulism toxin. Wound botulism is caused by toxin produced from a wound infected with Clostridium botulinum . Infant botulism is caused by consuming the spores of the botulinum bacteria, which then grow in the intestines and release toxin. All forms of botulism can be fatal and are considered medical emergencies. Foodborne botulism can be especially dangerous because many people can be poisoned by eating a contaminated food.
What kind of germ is Clostridium botulinum?
Clostridium botulinum is the name of a group of bacteria commonly found in soil. These rod-shaped organisms grow best in low oxygen conditions. The bacteria form spores which allow them to survive in a dormant state until exposed to conditions that can support their growth. There are seven types of botulism toxin designated by the letters A through G; only types A, B, E and F cause illness in humans.
How common is botulism?
In the United States an average of 110 cases of botulism are reported each year. Of these, approximately 25% are foodborne, 72% are infant botulism, and the rest are wound botulism. Outbreaks of foodborne botulism involving two or more persons occur most years and are usually caused by eating contaminated home-canned foods. The number of cases of foodborne and infant botulism has changed little in recent years, but wound botulism has increased because of the use of black-tar heroin, especially in California.
What are the symptoms of botulism?
The classic symptoms of botulism include double vision, blurred vision, drooping eyelids, slurred speech, difficulty swallowing, dry mouth, and muscle weakness. Infants with botulism appear lethargic, feed poorly, are constipated, and have a weak cry and poor muscle tone. These are all symptoms of the muscle paralysis caused by the bacterial toxin. If untreated, these symptoms may progress to cause paralysis of the arms, legs, trunk and respiratory muscles. In foodborne botulism, symptoms generally begin 18 to 36 hours after eating a contaminated food, but they can occur as early as 6 hours or as late as 10 days.
How is botulism diagnosed?
Physicians may consider the diagnosis if the patient’s history and physical examination suggest botulism. However, these clues are usually not enough to allow a diagnosis of botulism. Other diseases such as Guillain-Barré syndrome, stroke, and myasthenia gravis can appear similar to botulism, and special tests may be needed to exclude these other conditions. These tests may include a brain scan, spinal fluid examination, nerve conduction test (electromyography, or EMG), and a tensilon test for myasthenia gravis. The most direct way to confirm the diagnosis is to demonstrate the botulinum toxin in the patient’s serum or stool by injecting serum or stool into mice and looking for signs of botulism. The bacteria can also be isolated from the stool of persons with foodborne and infant botulism. These tests can be performed at some state health department laboratories and at CDC.
How can botulism be treated?
The respiratory failure and paralysis that occur with severe botulism may require a patient to be on a breathing machine (ventilator) for weeks, plus intensive medical and nursing care. After several weeks, the paralysis slowly improves. If diagnosed early, foodborne and wound botulism can be treated with an antitoxin which blocks the action of toxin circulating in the blood. This can prevent patients from worsening, but recovery still takes many weeks. Physicians may try to remove contaminated food still in the gut by inducing vomiting or by using enemas. Wounds should be treated, usually surgically, to remove the source of the toxin-producing bacteria. Good supportive care in a hospital is the mainstay of therapy for all forms of botulism. Currently, antitoxin is not routinely given for treatment of infant botulism.
Are there complications from botulism?
Botulism can result in death due to respiratory failure. However, in the past 50 years the proportion of patients with botulism who die has fallen from about 50% to 8%. A patient with severe botulism may require a breathing machine as well as intensive medical and nursing care for several months. Patients who survive an episode of botulism poisoning may have fatigue and shortness of breath for years and long-term therapy may be needed to aid recovery.
How can botulism be prevented?
Botulism can be prevented. Foodborne botulism has often been from home-canned foods with low acid content, such as asparagus, green beans, beets and corn. However, outbreaks of botulism also occur from more unusual sources such as chopped garlic in oil, chile peppers, tomatoes, improperly handled baked potatoes wrapped in aluminum foil, and home-canned or fermented fish. Persons who do home canning should follow strict hygienic procedures to reduce contamination of foods. Oils infused with garlic or herbs should be refrigerated. Potatoes which have been baked while wrapped in aluminum foil should be kept hot until served or refrigerated. Because the botulism toxin is destroyed by high temperatures, persons who eat home-canned foods should consider boiling the food for 10 minutes before eating it to ensure safety. Instructions on safe home canning can be obtained from county extension services or from the US Department of Agriculture. Because honey can contain spores of Clostridium botulinum and this has been a source of infection for infants, children less than 12 months old should not be fed honey. Honey is safe for persons 1 year of age and older. Wound botulism can be prevented by promptly seeking medical care for infected wounds and by not using injectable street drugs.
What are public health agencies doing to prevent or control botulism?
Public education about botulism prevention is an ongoing activity. Information about safe canning is widely available for consumers. State health departments and CDC have persons knowledgeable about botulism available to consult with physicians 24 hours a day. If antitoxin is needed to treat a patient, it can be quickly delivered to a physician anywhere in the country. Suspected outbreaks of botulism are quickly investigated, and if they involve a commercial product, the appropriate control measures are coordinated among public health and regulatory agencies. Physicians should report suspected cases of botulism to a state health department.
v Botulism in the United States
Introduction
Botulism is a neuroparalytic illness resulting from the action of a potent toxin produced by the organism Clostridium botulinum. This microbe was first described in 1897 by E. van Ermengem after his investigation of a foodborne outbreak in Ellezelles, Belgium. Foodborne botulism is rare but it may kill rapidly, and contaminated products may expose many persons. Foodborne botulism, therefore, represents a medical and a public health emergency that places a premium on rapid, effective communication between clinicians and public health officials. (1,2)"
"Botulism" comes from the Latin "botulus," meaning sausage. When botulism was first recognized in Europe, many cases were caused by home-fermented sausages. This derivation, although historically important, has lost much of its significance, since plant rather than animal products are more common vehicles. Sausage now is rarely the cause of botulism in the United States.
Four distinct forms of botulism can occur, depending on the mode of acquisition of the toxin. Foodborne botulism results from the ingestion of food containing preformed toxin. Wound botulism is caused by organisms that multiply and produce toxin in a contaminated wound. Infant botulism is due to the endogenous production of toxin by germinating spores of C. botulinum in the intestine of the infant. Child or adult botulism from intestinal colonization is represented by those cases in which no food vehicle can be identified, there is no evidence of wound botulism, and there is the possibility of intestinal colonization in a person older than 1 year of age.
C. botulinum is a group of culturally distinct organisms that are alike only in that they are clostridia and produce antigenically distinct neurotoxins with a similar pharmacologic action. C. botulinum organisms are straight to slightly curved, gram-positive (in young cultures), motile, anaerobic rods, 0.5-2.0 µm in width, 1.6-22.0 µm in length, with oval, subterminal spores. (3) The seven types of C. botulinum (A-G) are distinguished by the antigenic characteristics of the neurotoxins they produce. Types A, B, E, and in rare cases, F cause disease in humans. Types C and D cause disease in birds and mammals. Type G, identified in 1970, has not yet been confirmed as a cause of illness in humans or animals. Important epidemiologic features and some clinical characteristics distinguish the types of botulism that cause human illness. Rare cases of infant and adult botulism have been confirmed to be the result of intestinal colonization by nonbotulinum Clostridium species that produced botulinum neurotoxin.
The ability of C. botulinum to cause food poisoning in humans is directly related to the production of heat-resistant spores that survive preservation methods that kill nonsporulating organisms.(5) The heat resistance of spores varies from type to type and even from strain to strain within each type; although some strains will not survive at 80oC, spores of many strains require temperatures above boiling to ensure destruction. (6,7) The thermal resistance of spores also increases with higher pH and lower salt content of the medium in which the spores are suspended.(8)
In many cases, it is impractical or undesirable to treat a food product in a manner to eliminate all C. botulinum spores. As a result, most control methods focus on the inhibition of growth and toxin production. The main limiting factors for growth of C. botulinum in foods are: (1) temperature, (2) pH, (3) water activity, (4) redox potential, (5) food preservatives, and (6) competing microorganisms. All of these factors are interrelated and so changing one factor influences the effect of other factors. The interaction of factors may have a positive or negative effect on the inhibition of C. botulinum. In general, proteolytic strains grow optimally at 40°C; the lower limit is 10°C, upper limit is 45-50°C. Nonproteolytic strains, including type E can continue to grow even at 3.3°C. The minimum pH range for growth of proteolytic strains is 4.6-4.8; the limit is pH 5.0 for nonproteolytic strains. However, some food proteins, such as soy and beef, may have a protective effect on C. botulinum at or below pH 4.6. In addition, certain food preparations may contain low-acid "pockets" in which the pH may be high enough to support the production of toxin. Low water activity (aw) inhibits the growth of C. botulinum. A minimum aw of ~0.94 is needed to support growth and toxin production. Water activity can be limited by dehydration, but is in general controlled by the addition of NaCl. The minimum aw of 0.94 corresponds to an approximate 10% NaCl solution. High redox potential (Eh) is usually due to the presence of O2. The optimum Eh for growth of C. botulinum is low (~-350 mV) but toxin production has been observed at Eh of +250 mV. Because of this range, C. botulinum growth and toxin production can occur even in products considered to have a high oxygen level. In addition, vacuum-packaging used to lower Eh to preserve food increases anaerobic conditions and so may support the production of toxin. A number of food preservatives (nitrite, sorbic acid, parabens, phenolic antioxidants, polyphosphates and ascorbates) inhibit the growth of C. botulinum and limit toxin production. Lactic acid bacteria such as Lactobacillus, Pediococcus, and Lactococcus have been shown to produce acid and so inhibit C. botulinum. (5)9
Foodborne Botulism
A. Incidence
For the purpose of surveillance, we defined a foodborne outbreak as one or more cases of botulism in which a contaminated food source was implicated. In the period 1899-1949, 477 foodborne botulism outbreaks were recorded in the United States, and in the period from 1950 through1996, an additional 444 outbreaks were reported to CDC for a total of 921. The average number of outbreaks per year has changed little, 9.7 per year for the earlier time period and 9.4 per year since 1950.
For the period 1899-1949, 1281 cases of botulism were reported, and in the time from 1950 through 1996, and additional 1087 cases were reported, bringing the total to 2368 cases. The average number of cases per outbreak has remained constant: 2.6 cases/outbreak in the first half of this century and 2.5 cases/outbreak so far in the second half.
B. Mortality
For the period 1899-1949, the case-fatality ratio was high at approximately 60%, but since about 1950, mortality has gradually decreased. For the period 1950-1996, the case fatality ratio was 15.5%. This decline in case-fatality ratio is due primarily to improvements in supportive and respiratory intensive care and perhaps to the prompt administration of antitoxin. The case-fatality ratio has generally declined over the years for all toxin types.

|
C. Geographic distribution
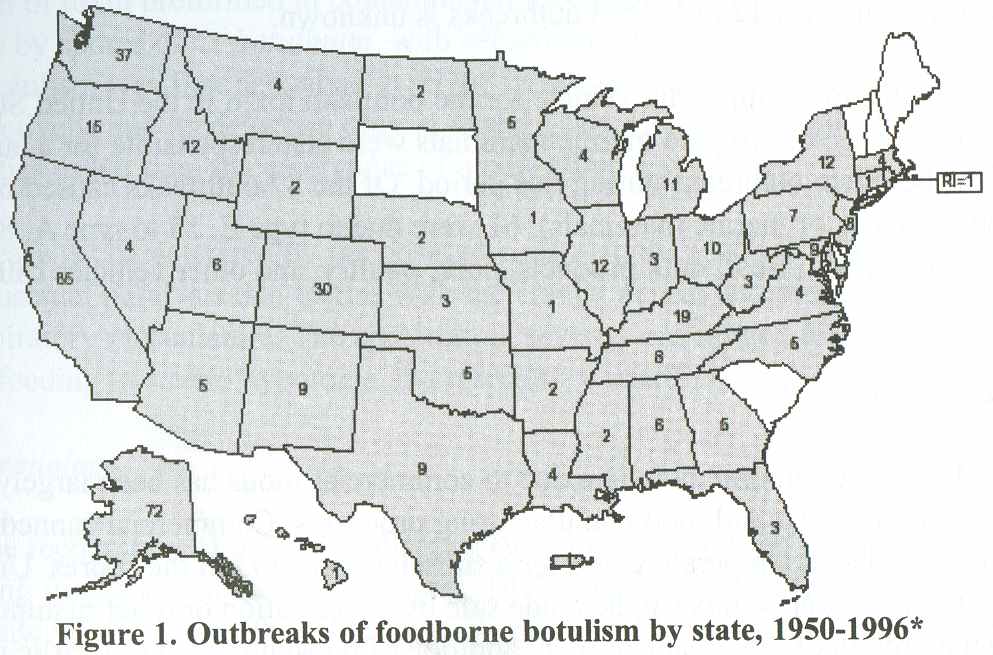
Foodborne botulism outbreaks have been reported from 46 states, Puerto Rico, and Washington D.C. from 1899 through 1996; only four states have never reported any foodborne botulism: Delaware, New Hampshire, South Carolina, and Vermont. Five western states (California, Washington, Colorado, Oregon, and Alaska) have accounted for more than half (53.8%) of all reported foodborne outbreaks since 1950. (Figure 1). Alaska alone accounts for 16.2% of outbreaks nationwide. This is because of the distinctive public health problem among the Alaska Native population, in which all botulism cases have been associated with improper preparation and storage of traditional Alaska Native foods. (1,9)
D. Food sources and products causing outbreaks
Until the early 1960s nearly all outbreaks of botulism in which toxin types were determined were caused by type A or B toxins and were usually associated with ingestion of home-canned vegetables, fruits, and meat products. Type E botulism was not recognized as a major problem in the United States until 1963, when 22 cases were reported. (13,14) Sixty-one of the 67 outbreaks of type E botulism reported from 1950 through 1996 have been traced to marine products (fish or marine mammals); several cases have been attributed to beaver. The remainder are of an undetermined source. Only three outbreaks of Type F botulism have been reported in this country with one being traced to home-prepared venison jerky.(14-16) From 1950 through 1996, 289 (65.1%) botulism outbreaks have been traced to homeprocessed foods and 31 (7%) to commercially processed foods, including foods served in restaurants. The type of food processing responsible for 124 (27.9%) outbreaks is unknown.
Vegetables were the most important vehicle for the botulism toxin in the Untied States from 1950 through 1996. Fish and marine mammals were also responsible for a large number of the botulism outbreaks during this period. Of the 87 outbreaks caused by marine products (fish, or marine mammals), 61 were due to type E, 15 to type A, 8 to type B, and 3 were unknown. Beef, milk products, pork, poultry, and other vehicles caused fewer outbreaks.
E. Prevention and control
In the United States, foodborne botulism due to commercial foods has been largely controlled by safe canning and food manufacturing processes. Commercial canned foods are heated to a sufficient temperature and for a sufficient time to kill the spores. Unheated commercial foods in cans or jars can be made safe by acidification or other manipulations that inhibit the growth of the organism (e.g., addition of phosphoric acid to garlic in oil). Occasionally, commercial foods still cause botulism if they are prepared in a way that permits toxin production.
Many outbreaks of foodborne botulism in the United States result from eating improperly preserved home-canned foods. (18) Persons doing home canning and other food preservation should be educated about the proper time, pressure, and temperature required to destroy spores, the need for adequately refrigerated storage of incompletely processed f oods, and the effectiveness of boiling, with stirring, home-canned vegetables to destroy botulinum toxins. (19) A pressure cooker must be used to can vegetables at home safely because it can reach temperatures above boiling [>212°F [>100°C]), which is necessary to kill botulism spores. (19) Although botulism spores are heat stable, botulinum toxin is heat labile. Botulinum toxin can be inactivated by heating to 176°F (80°C). Therefore, heating home-canned foods before consumption can reduce the risk of botulism intoxication.
C. botulinum may cause container lids to bulge and the contents to have "off-odors." Commercial cans or home-canned products with bulging lids should not be opened, and foods with off-odors should not be eaten or "taste tested." (19) For more information about safe homecanning procedures, contact your local county extension home economist or see the website the Extension Service of the U.S. Department of Agriculture (USDA) at http://ext.usu.edu/publica/foodpubs.htm .(20)
Infant Botulism
Since 1980, infant botulism has been the most common form of botulism reported in the United States. It is epidemiologically distinct from foodborne botulism, representing not ingestion of toxin preformed in contaminated foods but colonization (infection) of the intestine by spores of C. botulinum, with subsequent in vivo toxin production. (21) Although infant botulism was first described in 1976,(22,23) earlier cases have been identified retrospectively, and its detection only in recent years might reflect advances in diagnostic capabilities rather than the emergence of a new clinical syndrome.
The disease is characterized by the onset of constipation, which is followed shortly by neuromuscular paralysis that begins with the cranial nerves and progresses to peripheral and respiratory musculature. The spectrum in severity has ranged from mild lethargy and slowed feeding to severe hypotonia and respiratory insufficiency.
A. Epidemiology
Since the recognition of infant botulism in 1976, cases have been identified with increasing frequency. In the United States, 1442 cases were reported to CDC from 46 states between 1976 and 1996. Type A accounted for 46.5% of these cases and type B for 51.9%. Since reporting began to stabilize in 1980, the average annual incidence of reported infant botulism in the United States has been approximately 1.9/100,000 live births. Since 1976, 47.2% of all infant botulism cases have been reported from California. Between 1976 and 1994, the incidence of infant botulism was highest in Delaware, Hawaii, Utah, and California (9.0, 8.8, 6.3 and 5.7 per 100,000 live births, respectively). The reasons for the apparent geographic variation are unknown.
The characteristics of infant botulism cases have been clarified in recent years. Infants hospitalized with the disease tend to have had higher birth weights than other infants, and their mothers tend to be white, older, and better educated than mothers in the general population. Affected infants are also more commonly breast-fed, (24,25) and breast-feeding is associated with an older age at onset in type B cases.(25) In general, affected infants are the product of normal gestation and delivery. No congenital abnormalities have been shown to be associated with infant botulism, and the children were generally healthy until the onset of botulism. Approximately the same number of males as females have been affected. The mean age at onset has been 13 weeks, the range from 1 to 63 weeks. Clustering of cases of infant botulism has been noted in some suburban areas in the eastern United States and in some small towns and rural areas in the West. (26-29)
B. Source of C. botulinum
Infant botulism occurs when an infant ingests spores (less likely vegetative cells) of C. botulinum which in turn colonize the intestinal tract and produce toxin. (21) In a prototypical case, type B organisms, but no toxin, were isolated from honey fed to an infant with infant botulism whose fecal specimens contained type B organisms and toxin. Family members who ate some of the same honey did not become ill. In several studies, more than 20% of affected infants had ingested honey before the onset of botulism.(25,29,30) In many cases, C. botulinum spores of the same type were cultured from honey in the same households.
However, since most infants with infant botulism have had no exposure to honey, the risk factors and vehicles of transmission of C. botulinum for the majority of cases remain unclear. (21,31) A survey of foods commonly fed to infants revealed C. botulinum in specimens of corn syrup as well as honey, but in no other category of foods tested. (32) In other studies, the same types of C. botulinum that caused disease were isolated from soil in an infant’s yard and from vacuum cleaner dust. Investigators have also frequently noted environmental conditions that might expose infants directly to environmental sources of C. botulinum spores, such as a shared crib, dusty or windy locales, nearby building construction, or outdoor activities. (26,28) These exposures have not, however, been fully evaluated by controlled studies. Infants hospitalized with botulism have also more typically been breast-fed than have control infants. (25,31,33,34) Breast-feeding is known to affect the fecal flora differently than formula feeding; in mice, the fecal flora have been shown to be an important susceptibility factor in challenge experiments with C. botulinum type A spores.(35)
C. Prevention and control
The risk factors for infant botulism are poorly described, but possible sources of spores include foods and dust. Honey should not be fed to infants because it has been identified as a food source. (19)
Wound Botulism
Wound botulism is a rare disease resulting from the growth of C. botulinum spores in a contaminated wound with in vivo toxin production.(36) Neurologic findings are indistinguishable from those seen in foodborne botulism; however, gastrointestinal symptoms do not occur. Wounds might not be obvious or grossly infected. Between 1943, when the syndrome was first recognized, through 1985, 33 cases of wound botulism were reported in the United States. Of these, 25 were laboratory confirmed; 17 cases were type A, 7 type B, and 1 a mixture of type A and type B organisms. (37) The median age of patients was 21 years (range 6-44 years); 81% were male. The wounds were usually deep and contained avascular areas; many patients had compound fractures, and four had extensive crush injuries of the hand. The median incubation period in cases of trauma was 7 days (range 4-21 days). (38) Since 1980, wound botulism cases have occurred in persons who used illicit drugs; these were associated either with needle puncture sites or with nasal or sinus lesions due to chronic cocaine sniffing. (39) From 1986 through 1996, 78 cases of wound botulism were reported in the United States; the majority were linked to injectable drug use, particularly with so-called "black tar heroin." Sixty-six cases were type A, 9 were type B, and the remaining 3 were of unknown toxin type. The median age of patients was 38 years (range 5-65 years); 60% were male.
Child or Adult Botulism From Intestinal Colonization
Isolated cases of botulism in which extensive investigation failed to implicate a specific food as the cause of the disease have been recorded by CDC since 1978 as cases of "undetermined origin" rather than of foodborne botulism; through 1996, all of these cases have been in adults. Although there has been speculation since the 1920s, careful investigation has now demonstrated that some of these cases are caused by colonization of the gastrointestinal tract by C. botulinum or C. baratii with in vivo production of toxin, analogous to the pathogenesis of infant botulism. (40,41) Support for the diagnosis of botulism from intestinal colonization is provided by the demonstration of the prolonged excretion of toxin and C. botulinum in the stool, and by the demonstration of spores of C. botulinum but not preformed toxin in suspected foods. In some cases of botulism strongly suspected of representing intestinal colonization, the patients had a history of gastrointestinal surgery or illnesses such as inflammatory bowel disease, which might have predisposed them to enteric colonization. (42) No other specific risk factors have been identified.
Clinical Syndrome
The clinical syndrome of botulism, whether foodborne, infant, wound, or intestinal colonization, is dominated by the neurologic symptoms and signs resulting from a toxin-induced blockade of the voluntary motor and autonomic cholinergic junctions and is quite similar for each cause and toxin type. (21,34,38,43) Incubation periods for foodborne botulism are reported to be as short as 6 hours or as long as 10 days,(44) but generally the time between toxin ingestion and onset of symptoms ranges from 18 to 36 hours.(45) The ingestion of other bacteria or their toxins in the improperly preserved food or changes in bowel motility are likely to account for the abdominal pain, nausea and vomiting, and diarrhea that often precede or accompany the neurologic symptoms of foodborne botulism. Dryness of the mouth, inability to focus to a near point (prompting the patient to complain of "blurred vision"), and diplopia are usually the earliest neurologic complaints. If the disease is mild, no other symptoms may develop and the initial symptoms will gradually resolve. The person with mild botulism may not come to medical attention. In more severe cases, however, these initial symptoms may be followed by dysphonia, dysarthria, dysphagia, and peripheral-muscle weakness. If illness is severe, respiratory muscles are involved, leading to ventilatory failure and death unless supportive care is provided. Recovery follows the regeneration of new neuromuscular connections. A 2- to 8-week duration of ventilatory support is common, although patients have required ventilatory support for up to 7 months before the return of muscular function. (43) Death occurs in 5%-10% of cases of foodborne botulism; early deaths result from a failure to recognize the severity of disease or from secondary pulmonary or systemic infections, whereas deaths after 2 weeks are usually from the complications of long-term mechanical ventilatory management. (43)
Perhaps because infants are not able to complain about the early effects of botulinum intoxication, the neurologic dysfunction associated with infant botulism often seems to develop suddenly. The major manifestations are poor feeding, diminished suckling and crying ability, neck and peripheral weakness (the infants are often admitted as "floppy babies"), and ventilatory failure.(21,24,34) Constipation is also often seen in infants with botulism, and in some, has preceded the onset of neurologic abnormalities by many days. Loss of facial expression, extraocular muscle paralysis, dilated pupils, and depression of deep tendon reflexes have been reported more frequently with type B than with type A infant botulism.(34) Treatment with aminoglycoside antimicrobial agents may promote neuromuscular weakness in infant botulism(46) and has been associated with an increased likelihood of the requirement of mechanical ventilation.(21,34) Fewer than 2% of reported cases of infant botulism result in death.
Diagnosis
Botulism is probably substantially underdiagnosed. The diagnosis is not difficult when it is strongly suspected, as in the setting of a large outbreak, but since cases of botulism most often occur singularly, the diagnosis may pose a more perplexing problem. Findings from many outbreaks have suggested that early cases are commonly misdiagnosed. They may be diagnosed only retrospectively after death, when the subsequent clustering of cases of botulism-like illness finally alerts public health personnel to an outbreak of botulism.(47-49) Other cases are undoubtedly missed entirely. Entire outbreaks may even go undetected despite severe illness in patients; for example, one outbreak was recognized retrospectively only after a second cluster of cases was associated with the same vehicle.(44)
Botulism should be suspected in any adult with a history of acute onset of gastrointestinal, autonomic (e.g., dry mouth, difficulty focusing), and cranial nerve (diplopia, dysarthria, dysphagia) dysfunction or in any infant with poor feeding, diminished sucking and crying ability, neck and peripheral muscle weakness, and/or ventilatory distress. (50) The demonstration of bilateral cranial nerve findings and the documentation of neurologic progression (descending peripheral muscle weakness, ventilatory compromise) increase the level of suspicion. The diagnosis is even more likely if an adult patient has recently eaten home-canned foods or if family members are similarly ill, or both. If the typical clinical syndrome is present and no food item can be pinpointed as a means of transmission, a contaminated wound should be sought. If the typical syndrome is seen and a wound is identified, the wound should be explored and specimens taken for culture and toxicity testing even if the wound appears clean.
The differential diagnosis includes myasthenia gravis, stroke, Guillain-Barré syndrome, bacterial and chemical food poisoning, tick paralysis, chemical intoxication (e.g., from carbon monoxide, barium carbonate, methyl chloride, methyl alcohol, organic phosphorus compound, or atropine), mushroom poisoning, medication reactions (e.g., from antibiotics such as neomycin, streptomycin, kanamycin, or gentamicin), poliomyelitis, diphtheria, and psychiatric illness. In infant botulism, sepsis (especially meningitis), electrolyte-mineral imbalance, metabolic encephalopathy, Reye syndrome, Werdnig-Hoffman disease, congenital myopathy, and Leigh disease should also be considered.
Routine laboratory studies are not helpful in confirming the clinical suspicion of botulism. Serum electrolytes, renal and liver function tests, complete blood tests, urinalysis, and electrocardiograms will all be normal unless secondary complications occur. A normal cerebrospinal fluid (CSF) examination helps differentiate botulism from Guillain-Barré syndrome, although a slightly elevated CSF protein level is occasionally seen with botulism, and the protein level might be initially normal in Guillain-Barré syndrome.(43) A normal tensilon test helps to differentiate botulism from myasthenia gravis. Normal neuroradiologic studies, such as computed tomographic scans or magnetic resonance imaging, help to rule out stroke, another condition commonly confused with botulism. (44,49)
Electromyography (EMG) may be helpful in distinguishing botulism from myasthenia gravis and Guillain-Barré syndrome, diseases that botulism often mimics closely. A characteristic EMG pattern observed in adult patients with botulism has been well described. (51-53) EMG is most useful, however, when conducted with repetitive stimulation at 50 Hz. EMGs should be performed on clinically involved muscles; positive results may be obtained from only one muscle even though many are weak. Because of variations in EMG results and their interpretation, EMG is most reliable if conducted by persons with experience in this procedure.
Toxicity testing of serum specimens, culture of tissues debrided from a wound, and toxicity testing, plus culture of stool specimens or epidemiologically incriminated foods or both are the best methods for confirming the diagnosis of botulism. (54) Cultures were positive for 51% of stool specimens and toxin testing was positive for 37% of sera and 23% of stool specimens collected from 309 persons with clinically diagnosed botulism reported to CDC from 1975 to 1988; at least one laboratory test was positive for 65% of patients. (45) Collecting stool and serum samples early during the course of illness increases the likelihood of obtaining positive results. However, in any given situation, these tests may not be helpful; large outbreaks have occurred in which none (55) or a very low percentage (56) of specimens yielded positive results. In addition, laboratory results may not be reported until many hours or days after the specimens are received. The administration of antitoxin is the only specific therapy available for botulism, and evidence suggests that it is effective only if given very early in the course of neurologic dysfunction.(57) Hence, the diagnosis of this illness cannot await the results of studies that may be long delayed and be confirmatory only in some cases. The diagnosis should be made on the basis of the case history and physical findings.
Treatment
The mainstays of treatment of foodborne and wound botulism are as follows: 1) administration of botulinum antitoxin in an attempt to prevent neurologic progression of a moderate, slowly progressive illness, or to shorten the duration of ventilatory failure in those with a severe, rapidly progressive illness; 2) careful monitoring of respiratory vital capacity and aggressive respiratory care for those with ventilatory insufficiency (monitoring of respiratory vital capacity should be performed as soon as diagnosis of botulism is made); and 3) meticulous and intensive care for the duration of the often prolonged paralytic illness.
Antitoxin therapy is more effective if undertaken early in the course of illness. (57) This is not surprising when one considers that equine antitoxin neutralizes only toxin molecules yet unbound to nerve endings. (58) More than 80% of persons reported with adult botulism in the United States are treated with antitoxin. Before administration of antitoxin, skin testing should be performed to test for sensitivity to serum or antitoxin. Administration of one 10-ml vial of trivalent botulism antitoxin by the intravenous route results in serum levels of type A, B, and E antibodies capable of neutralizing serum toxin concentrations many fold in excess of those reported for botulism patients. Therefore, after skin testing for sensitivity, contrary to the antitoxin package insert, administration of one vial of antitoxin intravenously is recommended and antitoxin need not be repeated since the circulating antitoxins have a half-life of 5 to 8 days. (58)* However, treatment is not without risk, as approximately 9% of persons treated experience hypersensitivity reactions. (60) It is therefore extremely important that physicians recognize botulism as early in its course as possible, and yet not mistake other neurologic syndromes for botulism.
Equine antitoxin rarely has been used in infant botulism because of the risk of inducing lifelong hypersensitivity to equine antigens and lack of evidence of its benefit. Also, because of early concerns that anaphylactic reactions with the equine-derived product might be more severe in infants, few infants have been given the product. (21) However, a human-derived botulism antitoxin, termed "botulism immune globulin," has been prepared, and a clinical trial of its efficacy when given early in the course of illness is in progress in California.(17) In infant botulism, antibiotics are used only to treat secondary infections because lysis of intraluminal C. botulinum may increase the amount of toxin available for absorption.(60)
Public Health Response
Because cases of foodborne botulism result from ingestion of contaminated food that still may be available to cause illness in others, a single case of foodborne botulism represents a public health emergency that might herald a larger outbreak. (1) Therefore, it is critical for clinicians who suspect botulism to discuss the case immediately with local and state public health epidemiologists. State public health officials should then immediately contact CDC. If a commercial food product is a suspected vehicle for botulism, USDA or the U.S. Food and Drug Administration also should be notified. Investigation of a suspected case of botulism includes an immediate search for other possible cases and identification of suspected food exposures, as well as confirming the diagnosis. If a number of people are affected, a rapid and detailed epidemiologic investigation is launched to assure the source is identified and controlled. Diagnostic testing of both case specimens and foods should be performed as needed.
REFERENCES
Shapiro RL, Hatheway C, Swerdlow DL. Botulism in the United States: A clinical and epidemiologic review. Ann Intern Med. 1998; 129: 221-228.
Shapiro RL, Hatheway C, Becher S, Swerdlow DL. Botulism surveillance and emergency response. A public health strategy for a global challenge. JAMA. 1997;278: 433-435.
Cato EP, George WL, Finegold SM. Genus Clostridium. In: Sneath PHA, Mair NS, Sharpe ME, et al. eds. Bergey’s Manual of Systematic Bacteriology, Vol. Baltimore: Williams & Wilkins; 1986;1141-1200.
Schiavo G, Montecucco C. The structure and mode of botulinum and tetanus toxins. In:The Clostridia. Molecular Biology and pathogenesis. Eds. Rood J, McClane BA, Songer JG, Titball RW. San Diego, California: Academic Press; 1997; 295-322.
Kim J, Foegeding PM. Principles of control. In: Hauschild AHW, Dodds KL, eds. Clostridium botulinum: Ecology and control in foods. New York: Marcel Dekker Inc.; 1992;121-176.
Ito KA, Seslar DJ, Ercern WA, et al. The thermal and chlorine resistance of Clostridium botulinum types A, B, and E spores. In: Ingram M, Roberts TA, eds. Botulism 1966. London: Chapman & Hall; 1967; 108-122.
Roberts TA, Ingram M. The resistance of spores of Clostridium botulinum type E to heat and radiation. J Appl Bacteriol 1965;28:125-137.
Zezones H, Hutchings IJ. Thermal resistance of Clostridium botulinum (62A) spores as affected by fundamental food constituents. Food Technol 1965;19:1003-1005.
State of Alaska Department of Health and Social Services. Botulism in Alaska: A guide for physicians and health care providers. Anchorage: Alaska Printing; 1998.
Meyer KF, Dubovsky BJ. The distribution of the spores of B. botulinus in the United States. J Infect Dis 1922;31:559-594.
Ward BQ, Carroll BJ, Garrett ES, et al. Survey of the U.S. Gulf Coast for the presence of Clostridium botulinum. Appl Microbiol 1967;15:629-639.
Bott TL, Deffner JS, McCoy E, et al. Clostriduim botulinum type E in fish from the Great Lakes. J Bacteriol 1966;91:919-924.
Rogers DE. Botulism, Vintage 1963. Ann Intern Med 1964;61:581-588.
Rogers DE, Koenig MG, Spickard A. Clinical and laboratory manifestations of type E botulism in man, in: Botulism. Lewis KH, Cassel K, editors. Proceedings of a symposium, (PHS Pub No. 999-FP-1), 1964;133-145.
Midura TF, Nygaard GS, Wood RM, et al. Clostridium botulinum type F: isolation from venison jerky. Appl Microbiol 1972;24:165-167.
Center for Disease Control. Botulism-California. Morb Mort Wkly Rep 1966;15:349.
Center for Disease Control. Botulism type F - California. Morb Mort Wkly Rep 1966;15:359.
Centers for Disease Control and Prevention. Foodborne botulism-Oklahoma, 1994. Morb Mort Wkly Rep 1995;44:200-202.
Benenson AS, ed. Botulism/infant botulism. In: Control of communicable diseases manual. 16th Edition. Washington, D.C.: American Public Health Association; 1995;66-69. 23
Extension Service, U.S. Department of Agriculture. Complete guide to home-canning. Washington, D.C.: U.S. Department of Agriculture, Extension Service, September 1994. (Agriculture information bulletin no.539).
Arnon SS. Infant botulism. In: Feigen R, Cherry J, eds. Textbook of Pediatric Infectious Diseases Philadelphia: W. B. Saunders; 1992; 1095-1102.
Midura TF, Arnon SS. Infant botulism: Identification of Clostridium botulinum and its toxin in faeces. Lancet 1976;2:934-936.
Pickett J, Berg B, Chaplin E, et al. Syndrome of botulism in infancy. N Engl J Med 1976;295:770-772.
Long SS, Gajewski JL, Brown LW, et al. Clinical, laboratory, and environmental features of infant botulism in southeastern Pennsylvania. Pediatrics 1985;75:935-941.
Morris JG, Snyder JD, Wilson R, et al. Infant botulism in the United States: An epidemiologic study of the cases occurring outside California. Am J Public Health 1983;73:1385-1388.
Istre GR, Compton R, Novotny T, et al. Infant botulism: three cases in a small town. Am J Dis Child 1986;140:1013-1014.
Long SS. Epidemiologic study of infant botulism in Pennsylvania. Pediatrics 1985;75:928-934.
Thompson JA, Glasgow LA, Warpinski JR. Infant botulism: Clinical spectrum and epidemiology. Pediatrics 1980;66:936-942.
Arnon SS, Midura TF, Damus K. Honey and other environmental risk factors for infant botulism. J Pediatr 1979;94:331-336.
Chin J, Arnon SS, Midura TF. Food and environmental aspects of infant botulism in California. Rev Infect Dis 1979;1:693-696.
Spika JS, Shaffer N, Hargrett-Bean N. Risk factors for infant botulism in the United States. Am J Dis Child 1989;143:828-832.
Kautter DA, Lilly T, Solomon HM, et al. Clostridium botulinum spores in infant foods: A survey. J Food Protection 1982;45:1028-1029.
Arnon SS, Damus K, Thompson B, et al. Protective role of human milk against sudden death from infant botulism. J Pediatr 1982:10:568-573.
Wilson R, Morris JG, Snyder JD, et al. Clinical characteristics of infant botulism in the United States: a study of the non-California cases. Pediatr Infect Dis 1982;1:148-150.
Wells CL, Sugiyama H, Bland SE. Resistance of mice with limited intestinal flora to enteric colonization by Clostridium botulinum. J Infect Dis 1982;146:791-796.
Weber JT, Goodpasture HC, Alexander H, et al. Wound botulism in a patient with a tooth abscess: case report and review. Clin Infect Dis 1993;16:635-639.
Hatheway CL. Botulism. In: Balows A, Hausler Jr. WJ, Ohashi M, et al., eds. Laboratory Diagnosis of Infectious Diseases: Principles and Practice, Vol. Berlin: Springer-Verlag; 1988;111-133.
Merson MH, Dowell VR. Epidemiologic, clinical, and laboratory aspects of wound botulism. N Engl J Med 1973;289:1005-1010.
MacDonald KL, Rutherford GW, Friedman SM, et al. Botulism and botulism-like illness in chronic drug abusers. Ann Intern Med 1985;102:616-618.
McCroskey LM, Hatheway CL. Laboratory findings in four cases of adult botulism 24 suggest colonization of the intestinal tract. J Clin Microbiol 1988;26:1052-1054.
Chia JK, Clark JB, Ryan CA, et al. Botulism in an adult associated with foodborne intestinal infection with Clostridium botulinum. N Engl J Med 1986;315:239-241.
Griffin PM, Hatheway CL, Rosenbaum RB, Sokolow R. Endogenous antibody production to botulinum toxin in an adult with intestinal colonization botulism and underlying Chron’s Disease. J Infect Dis 1997; 175:633-637.
Hughes JM, Blumenthal JR, Merson MH, et al. Clinical features of types A and B food-borne botulism. Ann Intern Med 1981;95:442-445.
St. Louis ME, Peck SH, Bowering D, et al. Botulism from chopped garlic: Delayed recognition of a major outbreak. Ann Intern Med 1988;108:363-368.
Woodruff BA, Griffin PM, McCroskey LM, et al. Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975-1988. J Infect Dis 1992;166:1281-1286.
L’Hommedieu C, Stough R, Brown L. Potentiation of neuromuscular weakness in infant botulism by aminogylcosides. J Pediatr 1979;95:1065-1070.
Badhey H, Cleri DJ, D’Amato RF, et al. Two fatal cases of type E adult food-borne botulism with early symptoms and terminal neurologic signs. J Clin Microbiol 1986;23:616-618.
Horwitz MA, Marr JS, Merson MH, et al. A continuing common-source outbreak of botulism in a family. Lancet 1975;1:1-6.
Koenig GM, Spickard A, Cardella MA, et al. Clinical and laboratory observations on type B botulism in man. Medicine 1964;43:517-545.
Terranova WA, Palumbo JN, Breman JG. Ocular findings in botulism type B. JAMA 1979;241:475-477.
Cherington M. Botulism: ten-year experience. Arch Neurol 1974;30:432-437.
Cherington M, Ginsberg S. Type B botulism: neurophysiologic studies. Neurology 1971;21:43-46.
Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle. Philadelphia: Davis; 1983.
Dowell VR, McCroskey LM, Hatheway CL, et al. Coproexamination for botulinal toxin and Clostridium botulinum. JAMA 1977;238:1829-1832.
Center for Disease Control. Follow-up: Botulism associated with commercial cherry peppers. Morb Mortal Wkly Rep 1976;25:148.
Terranova WA, Breman JG, Locey RP, et al. Botulism type B: epidemiologic aspects of an extensive outbreak. Am J Epidemiol 1978;108:150-156.
Tackett CO, Shandera WX, Mann JM, et al. Equine antitoxin use and other factors that predict outcome in type A foodborne botulism. Am J Med 1984;76:794-798.
Sugiyama H. Clostridium botulinum neurotoxin. Microbiol Rev 1980;44:419-448.
Hatheway CL, Snyder JD, Seals JD, et al. Antitoxin levels in botulism patients treated with trivalent equine botulism antitoxin to toxin types A, B, and E. J Infect Dis 1984;150:407-412.
Black RE, Gunn RA. Hypersensitivity reactions associated with botulinal antitoxin. Am J Med 1980;69:567-570.
American Academy of Pediatrics. Clostridium infections. In: Peter G, ed. 1997 Red Book: Report of the Committee on Infectious Diseases. 24th ed. Elk Grove Village, IL: American Academy of Pediatrics; 1997; 176.
Koenig MG, Drutz DJ, Mushlin AI, et al. Type B botulism in man. Am J Med 1967;42:208-219.
Mena E, Thompson FS, Armfield AY, et al. Evaluation of Port-A-Cul system for protection of anaerobic bacteria. J Clin Microbiol 1978;8:28-35.
Horwitz MA, Hatheway CL, Dowell VR Jr. Laboratory diagnosis of botulism complicated by pyridostigmine treatment of the patient. Am J Clin Pathol 1976;66:737-742.
Dowell VR Jr, Hawkins TM. Laboratory methods in anaerobic bacteriology. In: CDC Laboratory Manual. US Dept of HEW, PHS, Center for Disease Control, Atlanta, Georgia. DHEW Publication No (CDC) 87-8272;1987.
Johnston R, Harmon S, Kautter D. Method to facilitate the isolation of Clostridium botulinum type E. J Bacteriol 1962;88:1521-1522.
Koransky JR, Allen SD, Dowell VR Jr. Use of ethanol for selective isolation of sporeforming microorganisms. Appl Environ Microbiol 1978;35:762-765.
Lilly T Jr, Harmon SM, Kautter DA, et al. An improved medium for detection of Clostridium botulinum type E. J Milk Food Technol 1971;34:492-497. XIII.
For Further Readings
Angulo FJ & St. Louis ME. Botulism. In: Alfred S Evans, Philip Brachman, eds. Bacterial Infections of Humans; Epidemiology and Control. 3rd ed. New York: Plenum Medical Book Co.; 1998; 139-153.
Hatheway CL. Toxigenic Clostridia. Clin Microbiol Rev 1990;3:66-98. XIV. Tables and Figures
Source: Centers for Disease Control and Prevention
Sept. 2001
v Botulinum Toxin as a Biological Weapon–Medical and Public Health Management
Introduction
This is the fourth article in a series entitled Medical and Public Health Management Following the Use of a Biological Weapon: Consensus Statements of The Working Group on Civilian Biodefense . 1-3 This article is the only one in the series to feature a biological toxin rather than a replicating agent. Botulinum toxin poses a major bioweapon threat because of its extreme potency and lethality; its ease of production, transport, and misuse; and the need for prolonged intensive care among affected persons.4-5 An outbreak of botulism constitutes a medical emergency that requires prompt provision of botulinum antitoxin and, often, mechanical ventilation, and it constitutes a public health emergency that requires immediate intervention to prevent additional cases. Timely recognition of a botulism outbreak begins with an astute clinician who quickly notifies public health officials.
Botulinum toxin is the most poisonous substance known. 6-7 A single gram of crystalline toxin, evenly dispersed and inhaled, would kill more than 1 million people, although technical factors would make such dissemination difficult. The basis of the phenomenal potency of botulinum toxin is enzymatic; the toxin is a zinc proteinase that cleaves 1 or more of the fusion proteins by which neuronal vesicles release acetylcholine into the neuromuscular junction. 8
It is regrettable that botulinum toxin still needs to be considered as a bioweapon at the historic moment when it has become the first biological toxin to become licensed for treatment of human disease. In the United States, botulinum toxin is currently licensed for treatment of cervical torticollis, strabismus, and blepharospasm associated with dystonia. It is also used "off label" for a variety of more prevalent conditions that include migraine headache, chronic low back pain, stroke, traumatic brain injury, cerebral palsy, achalasia, and various dystonias. 9-13
History of Current Threat
Terrorists have already attempted to use botulinum toxin as a bioweapon. Aerosols were dispersed at multiple sites in downtown Tokyo, Japan, and at US military installations in Japan on at least 3 occasions between 1990 and 1995 by the Japanese cult Aum Shinriky. These attacks failed, apparently because of faulty microbiological technique, deficient aerosol-generating equipment, or internal sabotage. The perpetrators obtained their C botulinum from soil that they had collected in northern Japan. 14-15
Development and use of botulinum toxin as a possible bioweapon began at least 60 years ago. 16-17 The head of the Japanese biological warfare group (Unit 731) admitted to feeding cultures of C botulinum to prisoners with lethal effect during that country’s occupation of Manchuria, which began in the 1930s. 18 The US biological weapons program first produced botulinum toxin during World War II. Because of concerns that Germany had weaponized botulinum toxin, more than 1 million doses of botulinum toxoid vaccine were made for Allied troops preparing to invade Normandy on D-Day. 19-20 The US biological weapons program was ended in 1969-1970 by executive orders of Richard M. Nixon, then president. Research pertaining to biowarfare use of botulinum toxin took place in other countries as well. 21
Although the 1972 Biological and Toxin Weapons Convention prohibited offensive research and production of biological weapons, signatories Iraq and the Soviet Union subsequently produced botulinum toxin for use as a weapon.22-23 Botulinum toxin was 1 of several agents tested at the Soviet site Aralsk-7 on Vozrozhdeniye Island in the Aral Sea. 23-24 A former senior scientist of the Russian civilian bioweapons program reported that the Soviets had attempted splicing the botulinum toxin gene from C botulinum into other bacteria.25 With the economic difficulties in Russia after the demise of the Soviet Union, some of the thousands of scientists formerly employed by its bioweapons program have been recruited by nations attempting to develop biological weapons. 25-26 Four of the countries listed by the US government as "state sponsors of terrorism" (Iran, Iraq, North Korea, and Syria)27 have developed, or are believed to be developing, botulinum toxin as a weapon. 28-29
After the 1991 Persian Gulf War, Iraq admitted to the United Nations inspection team to having produced 19000 L of concentrated botulinum toxin, of which approximately 10000 L were loaded into military weapons. 22, 30 These 19000 L of concentrated toxin are not fully accounted for and constitute approximately 3 times the amount needed to kill the entire current human population by inhalation. In 1990, Iraq deployed specially designed missiles with a 600-km range; 13 of these were filled with botulinum toxin, 10 with aflatoxin, and 2 with anthrax spores. Iraq also deployed special 400-lb (180-kg) bombs for immediate use; 100 bombs contained botulinum toxin, 50 contained anthrax spores, and 7 contained aflatoxin. 22, 30 It is noteworthy that Iraq chose to weaponize more botulinum toxin than any other of its known biological agents.
Some contemporary analyses discount the potential of botulinum toxin as a bioweapon because of constraints in concentrating and stabilizing the toxin for aerosol dissemination. However, these analyses pertain to military uses of botulinum toxin to immobilize an opponent (William C. Patrick, unpublished data, 1998). In contrast, deliberate release of botulinum toxin in a civilian population would be able to cause substantial disruption and distress. For example, it is estimated that a point-source aerosol release of botulinum toxin could incapacitate or kill 10% of persons within 0.5 km downwind (William C. Patrick, unpublished data, 1998). In addition, terrorist use of botulinum toxin might be manifested as deliberate contamination of food. Misuse of toxin in this manner could produce either a large botulism outbreak from a single meal or episodic, widely separated outbreaks. 31 In the United States, the Centers for Disease Control and Prevention (CDC) maintains a well-established surveillance system for human botulism based on clinician reporting that would promptly detect such events. 32
Microbiology and Virulence Factors
| Clostridium botulinum is a spore-forming, obligate anaerobe whose natural habitat is soil, from which it can be isolated without undue difficulty. The species C botulinum consists of 4 genetically diverse groups that would not otherwise be designated as a single species except for their common characteristic of producing botulinum toxin. 33-34 Botulinum toxin exists in 7 distinct antigenic types that have been assigned the letters A through G. The toxin types are defined by their absence of cross-neutralization (e.g., anti-A antitoxin does not neutralize toxin types B-G). The toxin types also serve as convenient epidemiological markers. |
In addition to C botulinum, unique strains of Clostridium baratii and Clostridium butyricum have the capacity to produce botulinum toxin. 35-37 Botulinum toxin is a simple dichain polypeptide that consists of a 100-kd "heavy" chain joined by a single disulfide bond to a 50-kd "light" chain; its 3-dimensional structure was recently resolved to 3.3 A. 38 The toxin’s light chain is a Zn++-containing endopeptidase that blocks acetylcholine-containing vesicles from fusing with the terminal membrane of the motor neuron, resulting in flaccid muscle paralysis (Figure 1). 8
A, Release of acetylcholine at the neuromuscular junction is mediated by the assembly of a synaptic fusion complex that allows the membrane of the synaptic vesicle containing acetylcholine to fuse with the neuronal cell membrane. The synaptic fusion complex is a set of SNARE proteins, which include synaptobrevin, SNAP-25, and syntaxin. After membrane fusion, acetylcholine is released into the synaptic cleft and then bound by receptors on the muscle cell. B, Botulinum toxin binds to the neuronal cell membrane at the nerve terminus and enters the neuron by endocytosis. The light chain of botulinum toxin cleaves specific sites on the SNARE proteins, preventing complete assembly of the synaptic fusion complex and thereby blocking acetylcholine release. Botulinum toxins types B, D, F, and G cleave synaptobrevin; types A, C, and E cleave SNAP-25; and type C cleaves syntaxin. Without acetylcholine release, the muscle is unable to contract. SNARE indicates soluble NSF-attachment protein receptor; NSF, N-ethylmaleimide-sensitive fusion protein; and SNAP-25, synaptosomal-associated protein of 25 kd.
The lethal dose of botulinum toxin for humans is not known but can be estimated from primate studies. By extrapolation, the lethal amounts of crystalline type A toxin for a 70-kg human would be approximately 0.09-0.15 µg intravenously or intramuscularly, 0.70-0.90 µg inhalationally, and 70 µg orally. 10, 39-41 Therapeutic botulinum toxin represents an impractical bioterrorist weapon because a vial of the type A preparation currently licensed in the United States contains only about 0.3% of the estimated human lethal inhalational dose and 0.005% of the estimated lethal oral dose.
Pathogenesis and Clinical Manifestations
Three forms of naturally occurring human botulism exist: foodborne, wound, and intestinal (infant and adult). Fewer than 200 cases of all forms of botulism are reported annually in the United States. 42 All forms of botulism result from absorption of botulinum toxin into the circulation from either a mucosal surface (gut, lung) or a wound. Botulinum toxin does not penetrate intact skin. Wound botulism and intestinal botulism are infectious diseases that result from production of botulinum toxin by C botulinum either in devitalized (i.e., anaerobic) tissue 43 or in the intestinal lumen, 37 respectively. Neither would result from bioterrorist use of botulinum toxin.
A fourth, man-made form that results from aerosolized botulinum toxin is inhalational botulism. This mode of transmission has been demonstrated experimentally in primates, 39 has been attempted by bioterrorists, 14-15 and has been the intended outcome of at least one country’s specially designed missiles and artillery shells.22, 30 Inhalational botulism has occurred accidentally in humans. A brief report from West Germany in 1962 described three veterinary personnel who were exposed to reaerosolized botulinum toxin while disposing of rabbits and guinea pigs whose fur was coated with aerosolized type A botulinum toxin. Type A botulinum toxin was detected in serum samples from all three affected individuals. 21 Once botulinum toxin is absorbed, the bloodstream carries it to peripheral cholinergic synapses, principally, the neuromuscular junction, where it binds irreversibly. The toxin is then internalized and enzymatically blocks acetylcholine release (Figure 1). Accordingly, all forms of human botulism display virtually identical neurologic signs. However, the neurologic signs in naturally occurring foodborne botulism may be preceded by abdominal cramps, nausea, vomiting, or diarrhea. 44 These gastrointestinal symptoms are thought to be caused by other bacterial metabolites also present in the food 33 and may not occur if purified botulinum toxin is intentionally placed in foods or aerosols.
Botulism is an acute, afebrile, symmetric, descending flaccid paralysis that always begins in bulbar musculature. It is not possible to have botulism without having multiple cranial nerve palsies. Disease manifestations are similar regardless of botulinum toxin type. However, the extent and pace of paralysis may vary considerably among patients. Some patients may be mildly affected (Figure 2), while others may be so paralyzed that they appear comatose and require months of ventilatory support. The rapidity of onset and the severity of paralysis depend on the amount of toxin absorbed into the circulation. Recovery results from new motor axon twigs that sprout to reinnervate paralyzed muscle fibers, a process that, in adults, may take weeks or months to complete. 45-46
| Figure 2. Seventeen - year - old patient with mild botulism |
A, Patient at rest. Note bilateral mild ptosis, dilated pupils, disconjugate gaze, and symmetric facial muscles. B, Patient was requested to perform his maximum smile. Note absent periorbital smile creases, ptosis, disconjugate gaze, dilated pupils, and minimally asymmetric smile. As an indication of the extreme potency of botulinum toxin, the patient had 40 x 10 -12 g/mL of type A botulinum toxin in his serum (i.e, 1.25 mouse units/mL) when these photographs were taken.
Patients with botulism typically present with difficulty seeing, speaking, and/or swallowing (Table 1 and Table 2). Prominent neurologic findings in all forms of botulism include ptosis, diplopia, blurred vision, often enlarged or sluggishly reactive pupils, dysarthria, dysphonia, and dysphagia. 5, 44, 47-48 The mouth may appear dry and the pharynx injected because of peripheral parasympathetic cholinergic blockade. Sensory changes are not observed except for infrequent circumoral and peripheral paresthesias from hyperventilation as a patient becomes frightened by onset of paralysis.
As paralysis extends beyond bulbar musculature, loss of head control, hypotonia, and generalized weakness become prominent. Dysphagia and loss of the protective gag reflex may require intubation and, usually, mechanical ventilation. Deep tendon reflexes may be present initially but diminish or disappear in the ensuing days, and constipation may occur. In untreated persons, death results from airway obstruction (pharyngeal and upper airway muscle paralysis) and inadequate tidal volume (diaphragmatic and accessory respiratory muscle paralysis).
Because botulism is an intoxication, patients remain afebrile unless they also have acquired a secondary infection (e.g., aspiration pneumonia). The toxin does not penetrate brain parenchyma, so patients are not confused or obtunded. However, they often appear lethargic and have communication difficulties because of bulbar palsies (Figure 2). Botulism may be recognized by its classic triad: (1) symmetric, descending flaccid paralysis with prominent bulbar palsies in (2) an afebrile patient with (3) a clear sensorium. The prominent bulbar palsies can be summarized in part as "4 Ds": diplopia, dysarthria, dysphonia, and dysphagia.
| Table
1. Symptoms and Signs of Foodborne Botulism, Types A and B*
Cases % Symptoms Fatigue 77 Dizziness 51 Double vision 91 Blurred vision 65 Dysphagia 96 Dry mouth 93 Dysarthria 84 Sore throat 54 Dyspnea 60 Constipation 73 Nausea 64 Vomiting 59 Abdominal cramps 42 Diarrhea 19 Arm weakness 73 Leg weakness 69 Paresthesia 14 Signs Alert mental status 90 Ptosis 73 Gaze paralysis 65 Pupils dilated or fixed 44 Nystagmus 22 Facial palsy 63 Diminished gag reflex 65 Tongue weakness 58 Arm weakness 75 Leg weakness 69 Hyporeflexia or areflexia 40 Ataxia 17 *Data are from outbreaks of botulism in the Unites States in 1973-1974. The number of patients with available data varied from 35 to 55. Adapted from Hughes et al 14 with permission. |
Epidemiology
Early recognition of outbreaks of botulism, whether natural or intentional, depends on heightened clinical suspicion. Aerosol dissemination may not be difficult to recognize because a large number of cases will share a common temporal and geographical exposure and will lack a common dietary exposure. However, identification of the common exposure site initially may be difficult because of the mobility of persons exposed during the incubation period. Botulism and botulinum toxin are not contagious and cannot be transmitted from person to person. In contrast, a microbe intentionally modified to produce botulinum toxin might be contagious.
No instances of waterborne botulism have ever been reported. 42, 49-50 Although the potency of botulinum toxin has led to speculation that it might be used to contaminate a municipal water supply, this scenario is unlikely for at least 2 reasons. 51 First, botulinum toxin is rapidly inactivated by standard potable water treatments (e.g., chlorination, aeration). 52 Second, because of the slow turnover time of large-capacity reservoirs, a comparably large (and technically difficult to produce and deliver) inoculum of botulinum toxin would be needed. 53 In contrast with treated water, botulinum toxin may be stable for several days in untreated water or beverages. 52, 54 Hence, such items should be investigated in a botulism outbreak if no other vehicle for toxin can be identified.
If food were deliberately used as a vehicle for the toxin, the outbreak would need to be distinguished from naturally occurring foodborne botulism. During the past 20 years, the epidemiology of foodborne botulism has expanded beyond its traditional association with home-preserved foods and now includes nonpreserved foods and public eating places,47 features that could make terrorist use of botulinum toxin more difficult to detect. Characteristics of outbreaks of botulism include:
| Table 2. Symptoms and Signs of Inhalational Botulism in Order of Onset |
| Humans (n=3)21 Monkeys (n=9) 30 |
| Third day after exposure 12-18 hours after exposure |
| Mucus in throat Mild muscular weakness |
| Difficulty swallowing solid food Intermittent ptosis |
| Dizziness Disconjugate gaze |
| Fourthday after exposure Followed by |
| Difficulty moving eyes Severe weakness of postural neck muscles |
| Mild pupillary dilation and nystagmus Occassional mouth breathing |
| Indistinct speech Serous nasal discharge |
| Unsteady gait Salivation, dysphagia |
| Extreme weakness Mouth breathing |
|
Rates Anorexia Several generalized weakness Lateral recumbency Second to fourth day after exposure Death in some animals |
| *After exposure to 4 to 7 monkey median lethal doses of botulinum toxin. The time and pace of paralysis were dose-dependent. Adapted from Middlebrook and Franz with permission. |
Incubation Period
The rapidity of onset and severity of botulism depend on the rate and amount of toxin absorption. Symptoms of foodborne botulism may begin as early as 2 hours or as long as 8 days after ingestion of toxin. 55-56 Typically, cases present 12 to 72 hours after the implicated meal. In one large foodborne outbreak, new cases presented during the ensuing three days at a fairly even rate before decreasing (Figure 3).57 The time to onset of inhalational botulism cannot be stated with certainty because so few cases are known. Monkeys showed signs of botulism 12 to 80 hours after aerosol exposure to 4 to 7 multiples of the monkey median lethal dose. 39 The three known human cases of inhalational botulism had onset of symptoms approximately 72 hours after exposure to an unknown but probably small amount of reaerosolized toxin .21
Figure 3. Fifty-nine cases of botulism, by interval between eating at a restaurant and onset of first neurologic symptom— Michigan, 1977
Reproduced from Terranova et al57 with permission of Oxford University Press.
Age and Sex
Persons of all ages are potentially susceptible to botulism. There are no sex differences in susceptibility.
Agent and Vehicles
Botulinum toxin in solution is colorless, odorless, and, as far as is known, tasteless. The toxin is readily inactivated by heat (85°C for 5 minutes). 33-34,52 Thus, foodborne botulism is always transmitted by foods that are not heated, or not heated thoroughly, before eating. Almost every type of food has been associated with outbreaks of botulism, but the most commonly implicated foods in the United States are vegetables, particularly "low-acid" (i.e, higher pH) vegetables such as beans, peppers, carrots, and corn.42, 50, 58
A novel epidemiological development is the occurrence of foodborne botulism after eating various nonpreserved foods in restaurants or delicatessens. Foil-wrapped baked potatoes are now known to be capable of causing restaurant-associated foodborne botulism 59 when held at room temperature after baking and then served plain,60 as potato salad,61-62 or as a Mediterranean-style dip.59 Other outbreaks that originated in restaurants resulted from contaminated condiments such as sautéed onions,63 garlic in oil,64 and commercial cheese sauce.65 Additional examples of notable commercial foods that have caused botulism outbreaks include inadequately eviscerated fish, 66 yogurt,67 cream cheese,68 and jarred peanuts.69
Incidence and Outbreak Size
Naturally occurring foodborne botulism is a rare disease. Approximately 9 outbreaks of foodborne botulism and a median of 24 cases occur annually in the United States. 42, 47 The mean outbreak size has remained constant over the years at approximately 2.5 cases per outbreak. The largest outbreak of foodborne botulism in the United States in the last 100 years occurred in Michigan in 1977; 59 cases resulted from eating home-preserved jalapeño peppers at a restaurant. 57 However, only 45 of the 59 patients had clinically evident weakness and hypotonia.
Toxin Types
Of the 135 foodborne outbreaks in the 16 years from 1980 to 1996 in the United States, the toxin types represented were: type A, 54.1%; type B, 14.8%; type E, 26.7%; type F, 1.5%; and unknown, 3.0%.42 Type F foodborne outbreaks are rare in the United States; a 1962 outbreak resulted from homemade venison jerky,70 while other type F cases actually may have had intestinal botulism. 71 Toxin types C and D cause botulism in wildlife and domestic animals but have not caused human foodborne disease. However, humans are thought to be susceptible to these toxin types because they have caused botulism in primates when ingested.72-74 Toxin type G is produced by a bacteria species discovered in South American soil in 1969 that has never caused recognized foodborne botulism.75 Aerosol challenge studies in monkeys have established the susceptibility of primates to inhaled botulinum toxin types C, D, and G. 48
Distribution
Although outbreaks of foodborne botulism have occurred in almost all states, more than half (53.8%) of the US outbreaks have occurred in just 5 western states (California, Washington, Oregon, Colorado, and Alaska). East of the Mississippi River, 60% of the foodborne outbreaks have resulted from type B toxin, while west of the Mississippi River, 85% have resulted from type A toxin. In the 46 years between 1950 and 1996, 20 states, mainly in the eastern United States, did not report any type A botulism outbreaks, while 24 states, mostly in the western United States, did not report any type B outbreaks. 42 In Canada and Alaska, most foodborne outbreaks resulted from type E toxin associated with native Inuit and Eskimo foods. 50, 76
Bioterrorism Considerations
Any outbreak of botulism should bring to mind the possibility of bioterrorism, but certain features would be particularly suggestive (Box 1). The availability and speed of air transportation mandate that a careful travel and activity history, as well as a careful dietary history, be taken. Patients should also be asked whether they know of other persons with similar symptoms. Absence of a common dietary exposure among temporally clustered patients should suggest the possibility of inhalational botulism.
|
Box 1. Features of an Outbreak That Would Suggest a Deliberate Release of Botulinum Toxin
Note: A careful travel and activity history, as well as dietary history, should be taken in any suspected botulism outbreak. Patients should also be asked if they know of other persons with similar symptoms. |
Diagnosis and Differential Diagnosis
Clinical diagnosis of botulism is confirmed by specialized laboratory testing that often requires days to complete. Routine laboratory test results are usually unremarkable. Therefore, clinical diagnosis is the foundation for early recognition of and response to a bioterrorist attack with botulinum toxin.
Any case of suspected botulism represents a potential public health emergency because of the possibility that a contaminated food remains available to others or that botulinum toxin has been deliberately released. In these settings, prompt intervention by civil authorities is needed to prevent additional cases. Consequently, clinicians caring for patients with suspected botulism should notify their local public health department and hospital epidemiologist immediately to coordinate shipment of therapeutic antitoxin, laboratory diagnostic testing, and epidemiological investigation (Box 2). In most jurisdictions of the United States, botulism suspected on clinical grounds alone by law must be reported immediately by telephone to local public health authorities. The attending clinician needs to be both prompt and persistent in accomplishing this notification.
|
Box 2. Clinicians Caring for Patients With Suspected Botulism Should Immediately Contact Their: (1) Hospital epidemiologist or infection control practitioner; and (2) Local and state health departments. Consult your local telephone operator; the telephone directory under "government listings," or the Internet at: http://www.cdc.gov/other.htm#states or http://www.astho.org/state.html If the local and state health departments are unavailable, contact the Centers for Disease Control and Prevention: (404) 639-2206; (404) 639-2888 [after hours]. |
Differential Diagnosis
Botulism is frequently misdiagnosed, most often as a polyradiculoneuropathy (Guillain-Barré or Miller-Fisher syndrome), myasthenia gravis, or a disease of the central nervous system (Table 3). In the United States, botulism is more likely than Guillain-Barré syndrome, intoxication, or poliomyelitis to cause a cluster of cases of acute flaccid paralysis. Botulism differs from other flaccid paralyses in its prominent cranial nerve palsies disproportionate to milder weakness and hypotonia below the neck, in its symmetry, and in its absence of sensory nerve damage.
A large, unintentional outbreak of foodborne botulism caused by a restaurant condiment in Canada provides a cautionary lesson about the potential difficulties in recognizing a covert, intentional contamination of food. 64 During a 6-week period in which the condiment was served, 28 persons in 2 countries became ill, but all were misdiagnosed (Table 3). The 28 were identified retrospectively only after correct diagnoses in a mother and her two daughters who had returned to their home more than 2000 miles away from the restaurant. Four (14%) of the cases had been misdiagnosed as having psychiatric disease, including "factitious" symptoms. It is possible that hysterical paralysis might occur as a conversion reaction in the anxiety that would follow a deliberate release of botulinum toxin.
Diagnostic Testing
At present, laboratory diagnostic testing for botulism in the United States is available only at the CDC and approximately 20 state and municipal public health laboratories. 42 The laboratory should be consulted prospectively about specimen collection and processing. Samples used in diagnosis of botulism include serum (30 mL of blood in "tiger"- or red- tubes from adults, less from children), stool, gastric aspirate, and, if available, vomitus and suspect foods. Serum samples must be obtained before therapy with antitoxin, which nullifies the diagnostic mouse bioassay. An enema may be required to obtain an adequate fecal sample if the patient is constipated. Sterile water should be used for this procedure because saline enema solution can confound the mouse bioassay. Gastric aspirates and, perhaps, stool may be useful for detecting inhaled aerosolized botulinum toxin released in a bioterrorist attack. 77 A list of the patient’s medications should accompany the diagnostic samples because anticholinesterases, such as pyridostigmine bromide, and other medicines that are toxic to mice can be dialyzed from samples before testing. All samples should be kept refrigerated after collection.
The standard laboratory diagnostic test for clinical specimens and foods is the mouse bioassay,42 in which type-specific antitoxin protects mice against any botulinum toxin present in the sample. The mouse bioassay can detect as little as 0.03 ng of botulinum toxin10 and usually yields results in 1 to 2 days (range, 6-96 hours). Fecal and gastric specimens also are cultured anaerobically, with results typically available in 7 to 10 days (range, 5-21 days). Toxin production by culture isolates is confirmed by the mouse bioassay.
An electromyogram with repetitive nerve stimulation at 20 to 50 Hz can sometimes distinguish between causes of acute flaccid paralysis. 78-79 The characteristic electromyographic findings of botulism include normal nerve conduction velocity, normal sensory nerve function, a pattern of brief, small-amplitude motor potentials, and, most distinctively, an incremental response (facilitation) to repetitive stimulation often seen only at 50 Hz. Immediate access to electrophysiological studies may be difficult to obtain in an outbreak of botulism.
Additional diagnostic procedures may be useful in rapidly excluding botulism as the cause of paralysis (Table 3). Cerebrospinal fluid (CSF) is unchanged in botulism but is abnormal in many central nervous system diseases. Although the CSF protein level eventually is elevated in Guillain-Barré syndrome, it may be normal early in illness. Imaging of the brain, spine, and chest may reveal hemorrhage, inflammation, or neoplasm. A test dose of edrophonium chloride briefly reverses paralytic symptoms in many patients with myasthenia gravis and, reportedly, in some with botulism. 64 A close inspection of the skin, especially the scalp, may reveal an attached tick that is causing paralysis. 80 Other tests that require days for results include stool culture for Campylobacter jejuni as a precipitant of Guillain-Barré syndrome and assays for the autoantibodies that cause myasthenia gravis, Lambert-Eaton syndrome, and Guillain-Barré syndrome.
Foods suspected of being contaminated should be refrigerated until retrieval by public health personnel. The US Food and Drug Administration and the US Department of Agriculture can assist other public health laboratories with testing of suspect foods by using methods similar to those applied to clinical samples.
| Table 3. Selected Mimics
and Misdiagnoses of Botulism
Feature That Distinguish Condition Condition From Botulism |
|
Common Misdiagnoses |
|
Guillain-Barré syndrome† and its variants, History of antecedent infection; paresthesias; especially Miller-Fisher syndrome often ascending paralysis; early areflexia; eventual CSF protein increase; EMG findings |
|
Myasthenia gravis† Recurrent paralysis; EMG findings; sustained response to anticholinesterase therapy |
|
Stroke† Paralysis often asymmetric; abnormal CNS image |
|
Intoxication with depressants (e.g., acute History of exposure; excessive drug levels ethanol intoxication), organophosphates, directed in body fluids carbon monoxide, or nerve gas |
|
Lambert-Eaton syndrome Increased strength with sustained contraction; evidence of lung carcinoma; EMG findings similar to botulism |
|
Tick paralysis Paresthesias; ascending paralysis; tick attached to skin |
|
Other Misdiagnoses |
|
Poliomyelitis Antecedent febrile illness; asymmetric paralysis; CSF pleocytosis |
|
CNS infections, especially of the brainstem Mental status changes; CSF and EEG abnormalities |
|
CNS tumor Paralysis often asymmetric; abnormal CNS image |
|
Streptococcal pharyngitis (pharyn- Absence of bulbar palsies; positive rapid geal erythema can occur in botulism) antigen test result or throat culture |
| Psychiatric illness† Normal EMG in conversion paralysis |
|
Viral syndrome Absence of bulbar palsies and flaccid paralysis |
| Inflammatory myopathy† Elevated creatine kinase levels |
| Psychiatric illness† Normal EMG in conversion paralysis |
|
Viral syndrome Absence of bulbar palsies and flaccid paralysis |
| Inflammatory myopathy† Elevated creatine kinase levels |
|
Diabetic complications† Sensory neuropathy; few cranial nerve palsies |
|
Hyperemesis gravidarum† Absence of bulbar palsies and acute flaccid paralysis |
| Hypothyroidism† Abnormal thyroid function test results |
|
Laryngeal trauma† Abnormal of flaccid paralysis; dysphonia without bulbar palsies |
|
Overexertion† Absence of bulbar palsies and acute flaccid paralysis |
| *CSF indicates cerebrospinal fluid; EMG, electromyogram; CNS, central nervous system; and EEG, electroencephalogram. †Misdiagnoses made in a large outbreak of botulism. |
Therapy
The mortality and sequelae associated with botulism have diminished with contemporary therapy. In the United States, the percentage of persons who died of foodborne botulism decreased from 25% during 1950-1959 to 6% during 1990-1996, with a similar reduction for each botulinum toxin type. 42 Despite this increase in survival, the paralysis of botulism can persist for weeks to months with concurrent requirements for fluid and nutritional support, assisted ventilation, and treatment of complications.
Therapy for botulism consists of supportive care and passive immunization with equine antitoxin. Optimal use of botulinum antitoxin requires early suspicion of botulism. Timely administration of passive neutralizing antibody will minimize subsequent nerve damage and severity of disease but will not reverse existent paralysis.81-82 Antitoxin should be given to patients with neurologic signs of botulism as soon as possible after clinical diagnosis.47 Treatment should not be delayed for microbiological testing. Antitoxin may be withheld at the time of diagnosis if it is certain that the patient is improving from maximal paralysis.
In the United States, botulinum antitoxin is available from the CDC via state and local health departments (Box 2). The licensed trivalent antitoxin contains neutralizing antibodies against botulinum toxin types A, B, and E, the most common causes of human botulism. If another toxin type was intentionally disseminated, patients could potentially be treated with an investigational heptavalent (ABCDEFG) antitoxin held by the US Army. 83 However, the time required for correct toxin typing and subsequent administration of heptavalent antitoxin would decrease the utility of this product in an outbreak.
The dose and safety precautions for equine botulinum antitoxin have changed over time. Clinicians should review the package insert with public health authorities before using antitoxin. At present, the dose of licensed botulinum antitoxin is a single 10-mL vial per patient, diluted 1:10 in 0.9% saline solution, administered by slow intravenous infusion. One vial provides between 5500 and 8500 IU of each type-specific antitoxin. The amount of neutralizing antibody in both the licensed and the investigational equine antitoxins far exceeds the highest serum toxin levels found in foodborne botulism patients, and additional doses are usually not required. If a patient has been exposed to an unnaturally large amount of botulinum toxin as a biological weapon, the adequacy of neutralization by antitoxin can be confirmed by retesting serum for toxin after treatment.
There are few published data on the safety of botulinum antitoxins. From 1967 to 1977, when the recommended dose was larger than today, approximately 9% of recipients of equine botulinum antitoxin in the United States displayed urticaria, serum sickness, or other reactions suggestive of hypersensitivity. 84 Anaphylaxis occurred within 10 minutes of receiving antitoxin in 2% of recipients. When the US Army’s investigational heptavalent antitoxin was given to 50 individuals in a large Egyptian outbreak of type E foodborne botulism in 1991, one recipient (2%) displayed serum sickness, and nine (18%) had mild reactions.83 To screen for hypersensitivity, patients are given small challenge doses of equine antitoxin before receiving a full dose. Patients responding to challenge with a substantial wheal and flare may be desensitized over 3 to 4 hours before additional antitoxin is given. During the infusion of antitoxin, diphenhydramine and epinephrine should be on hand for rapid administration in case of adverse reaction. Although both equine antitoxins have been partially despeciated by enzymatic cleavage of the allogenic F c region, each contains a small residual of intact antibody that may sensitize recipients to additional doses.
Botulism patients require supportive care that often includes feeding by enteral tube or parenteral nutrition, intensive care, mechanical ventilation, and treatment of secondary infections. Patients with suspected botulism should be closely monitored for impending respiratory failure. In nonventilated infants with botulism, a reverse Trendelenburg positioning with cervical vertebral support has been helpful, but applicability of this positioning to adults with botulism remains untested. This tilted, flat-body positioning with neck support may improve ventilation by reducing entry of oral secretions into the airway and by suspending more of the weight of the abdominal viscera from the diaphragm, thereby improving respiratory excursion (Figure 4). In contrast, placing a botulism patient in a supine or semirecumbent position (trunk flexed 45° at the waist) may impede respiratory excursion and airway clearance, especially if the patient is obese. The desired angle of the reverse Trendelenburg position is 20° to 25°.
Figure 4. Preferred Positioning of Nonventilated Botulism Patients
Note flat, rigid mattress tilted at 20°, tightly rolled cloth to support cervical vertebrae, and bumpers to prevent downward sliding. Use of this position may postpone or avoid the need for mechanical ventilation in mildly affected patients because of improved respiratory mechanics and airway protection.
Botulism patients should be assessed for adequacy of gag and cough reflexes, control of oropharyngeal secretions, oxygen saturation, vital capacity, and inspiratory force. Airway obstruction or aspiration usually precedes hypoventilation in botulism. When respiratory function deteriorates, controlled, anticipatory intubation is indicated. The proportion of patients with botulism who require mechanical ventilation has varied from 20% in a foodborne outbreak 64 to more than 60% in infant botulism. 85 In a large outbreak of botulism, the need for mechanical ventilators, critical care beds, and skilled personnel might quickly exceed local capacity and persist for weeks or months. Development of a reserve stockpile of mechanical ventilators in the United States is under way86 and will require a complement of staff trained in their use.
Antibiotics have no known direct effect on botulinum toxin. However, secondary infections acquired during botulism often require antibiotic therapy. Aminoglycoside antibiotics and clindamycin are contraindicated because of their ability to exacerbate neuromuscular blockade.87-88 Standard treatments for detoxification, such as activated charcoal, 89 may be given before antitoxin becomes available, but there are no data regarding their effectiveness in human botulism.
Special Populations
Based on limited information, there is no indication that treatment of children, pregnant women, and immunocompromised persons with botulism should differ from standard therapy. Despite the risks of immediate hypersensitivity and sensitization to equine proteins, both children 43, 90 and pregnant women 91-92 have received equine antitoxin without apparent short-term adverse effects. The risks to fetuses of exposure to equine antitoxin are unknown. Treatment with human-derived neutralizing antibody would decrease the risk of allergic reactions posed by equine botulinum antitoxin, but use of the investigational product, Botulism Immune Globulin Intravenous (Human) (California Department of Health Services, Berkeley), is limited to suspected cases of infant botulism. 82, 93
Prophylaxis
Botulism can be prevented by the presence of neutralizing antibody in the bloodstream. Passive immunity can be provided by equine botulinum antitoxin or by specific human hyperimmune globulin, while endogenous immunity can be induced by immunization with botulinum toxoid.
Use of antitoxin for postexposure prophylaxis is limited by its scarcity and its reactogenicity. Because of the risks of equine antitoxin therapy, it is less certain how best to care for persons who may have been exposed to botulinum toxin but who are not yet ill. In a small study of primates exposed to aerosolized toxin in which supportive care was not provided, all 7 monkeys given antitoxin after exposure but before the appearance of neurologic signs survived, while 2 of 4 monkeys treated with antitoxin only after the appearance of neurologic signs died.39 Moreover, all monkeys infused with neutralizing antibody before exposure to toxin displayed no signs of botulism. In a balance between avoiding the potential adverse effects of equine antitoxin and needing to rapidly neutralize toxin, it is current practice in foodborne botulism outbreaks to closely monitor persons who may have been exposed to botulinum toxin and to treat them promptly with antitoxin at the first signs of illness. 47 To facilitate distribution of scarce antitoxin following the intentional use of botulinum toxin, asymptomatic persons who are believed to have been exposed should remain under close medical observation and, if feasible, near critical care services.
In the United States, an investigational pentavalent (ABCDE) botulinum toxoid is distributed by the CDC for laboratory workers at high risk of exposure to botulinum toxin and by the military for protection of troops against attack. 94 A recombinant vaccine is also in development. 95 The pentavalent toxoid has been used for more than 30 years to immunize more than 3000 laboratory workers in many countries. Immunization of the population with botulinum toxoid could in theory eliminate the hazard posed by botulinum toxins A through E. However, mass immunization is neither feasible nor desirable for reasons that include scarcity of the toxoid, rarity of natural disease, and elimination of the potential therapeutic benefits of medicinal botulinum toxin. Accordingly, preexposure immunization currently is neither recommended for nor available to the general population. Botulinum toxoid induces immunity over several months and, so, is ineffective as postexposure prophylaxis.
Decontamination
Despite its extreme potency, botulinum toxin is easily destroyed. Heating to an internal temperature of 85°C for at least 5 minutes will detoxify contaminated food or drink.52 All foods suspected of contamination should be promptly removed from potential consumers and submitted to public health authorities for testing.
Persistence of aerosolized botulinum toxin at a site of deliberate release is determined by atmospheric conditions and the particle size of the aerosol. Extremes of temperature and humidity will degrade the toxin, while fine aerosols will eventually dissipate into the atmosphere. Depending on the weather, aerosolized toxin has been estimated to decay at between less than 1% to 4% per minute.96 At a decay rate of 1% per minute, substantial inactivation (13 logs) of toxin occurs by 2 days after aerosolization.
Recognition of a covert release of finely aerosolized botulinum toxin would probably occur too late to prevent additional exposures. When exposure is anticipated, some protection may be conferred by covering the mouth and nose with clothing such as an undershirt, shirt, scarf, or handkerchief. 97 In contrast with mucosal surfaces, intact skin is impermeable to botulinum toxin.
After exposure to botulinum toxin, clothing and skin should be washed with soap and water. 98 Contaminated objects or surfaces should be cleaned with 0.1% hypochlorite bleach solution if they cannot be avoided for the hours to days required for natural degradation. 33, 52, 98
Infection Control
Medical personnel caring for patients with suspected botulism should use standard precautions. Patients with suspected botulism do not need to be isolated, but those with flaccid paralysis from suspected meningitis require droplet precautions.
Research Needs
Additional research in diagnosis and treatment of botulism is required to minimize its threat as a weapon. Rapid diagnostic and toxin typing techniques currently under development would be useful for recognizing and responding to a bioterrorist attack. Although polymerase chain reaction assays can detect the botulinum toxin gene,99 they are unable, as yet, to determine whether the toxin gene is expressed and whether the expressed protein is indeed toxic. Assays that exploit the enzymatic activity of botulinum toxin have the potential to supplant the mouse bioassay as the standard for diagnosis.100 Detection of botulinum toxin in aerosols by enzyme-linked immunosorbent assay101 is a component of the US military’s Biological Integrated Detection System for rapid recognition of biological agents in the battlefield.17
The distribution of botulinum antitoxin to local hospitals from regional depots takes several hours. In contrast, standard detoxification techniques can be applied immediately. Studies are needed to assess whether activated charcoal and osmotic catharsis can prevent gastrointestinal tract absorption or reduce circulating levels of botulinum toxin. Enteral detoxification may be less useful in inhalational botulism than in foodborne disease.
The competing needs for immunity to weaponized botulinum toxin and for susceptibility to medicinal botulinum toxin could be reconciled by supplying human antibody that neutralizes toxin. With a half-life of approximately 1 month, 102 human antibody would provide immunity for long periods and avoid the reactogenicity of equine products. Existing in vitro technologies could produce the stockpiles of fully human antibody necessary both to deter terrorist attacks and to avoid the rationing of antitoxin that currently would be required in a large outbreak of botulism. 103-106 A single small injection of oligoclonal human antibodies could, in theory, provide protection against toxins A through G for many months. Until such a product becomes available, the possibilities for reducing the population’s vulnerability to the intentional misuse of botulinum toxin remain limited.
AUTHOR INFORMATION
Ex Officio Participants in the Working Group on Civilian Biodefense:
George Counts, MD, National Institutes of Health; Margaret Hamburg, MD, and Stuart Nightingale, MD, Office of Assistant Secretary for Planning and Evaluation; Robert Knouss, MD, Office of Emergency Preparedness; and Brian Malkin, US Food and Drug Administration.
Funding/Support: Funding for this study primarily was provided by each participant’s institution or agency. The Johns Hopkins Center for Civilian Biodefense Studies provided travel funds for 6 members of the group.
Disclaimers: In some instances, the indications, dosages, and other information in this article are not consistent with current approved labeling by the US Food and Drug Administration (FDA). The recommendations on use of drugs and vaccine for uses not approved by the FDA do not represent the official views of the FDA nor of any of the federal agencies whose scientists participated in these discussions. Unlabeled uses of the products recommended are noted in the sections of this article in which these products are discussed. Where unlabeled uses are indicated, information used as the basis for the recommendation is discussed.
REFERENCES
Inglesby TV, Henderson DA, Bartlett JG, et al for the Working Group on Civilian Biodefense. Anthrax as a biological weapon: medical and public health management. JAMA. 1999;281:1735-1745.
Henderson DA, Inglesby TV, Bartlett JG, et al for the Working Group on Civilian Biodefense. Smallpox as a biological weapon: medical and public health management. JAMA. 1999;281:2127-2137.
Inglesby TV, Henderson DA, Bartlett JG, et al for the Working Group on Civilian Biodefense. Plague as a biological weapon: medical and public health management. JAMA. 2000;283:2281-2290.
Biological and chemical terrorism: strategic plan for preparedness and response: recommendations of the CDC Strategic Planning Workgroup. MMWR Morb Mortal Wkly Rep 2000;49(RR-4):1-14.
Franz DR, Jahrling PB, Friedlander AM, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA. 1997;278:399-411.
Gill MD. Bacterial toxins: a table of lethal amounts. Microbiol Rev. 1982;46:86-94.
National Institute of Occupational Safety and Health. Registry of Toxic Effects of Chemical Substances (R-TECS). Cincinnati, Ohio: National Institute of Occupational Safety and Health; 1996.
Montecucco C, ed. Clostridial neurotoxins: the molecular pathogenesis of tetanus and botulism. Curr Microbiol Immunol. 1995;195:1-278.
Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. J Pediatr Ophthalmol Strabismus. 1980;17:21-25.
Schantz EJ, Johnson EA. Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol Rev. 1992;56:80-99.
Jankovic J, ed, Hallet M, ed. Therapy With Botulinum Toxin. New York, NY: Marcel Dekker Inc; 1994.
Silberstein S, Mathew N, Saper J, Jenkins S for the Botox Migraine Clinical Research Group. Botulinum toxin type A as a migraine preventive treatment. Headache. 2000;40:445-450.
Foster L, Clapp L, Erickson M, Jabbari B. Botulinum toxin A and mechanical low back pain [abstract]. Neurology. 2000;54(suppl 3):A178.
Tucker JB, ed. Toxic Terror: Assessing the Terrorist Use of Chemical and Biological Weapons. Cambridge, Mass: MIT Press; 2000.
WuDunn S, Miller J, Broad WJ. How Japan germ terror alerted world. New York Times. May 26, 1998:A1, A10.
Geissler E, ed, Moon JE, ed. Biological and Toxin Weapons: Research, Development and Use From the Middle Ages to 1945. New York, NY: Oxford University Press; 1999. Sipri Chemical & Biological Warfare Studies No. 18.
Smart JK. History of chemical and biological warfare: an American perspective. In: Sidell FR, Takafuji ET, Franz DR, eds. Medical Aspects of Chemical and Biological Warfare. Washington, DC: Office of the Surgeon General; 1997:9-86. Textbook of Military Medicine; part I, vol 3.
Hill EV. Botulism. In: Summary Report on B. W. Investigations. Memorandum to Alden C. Waitt, Chief Chemical Corps, United States Army, December 12, 1947; tab D. Archived at the US Library of Congress.
Cochrane RC. History of the Chemical Warfare Service in World War II (1 July 1940–15 August 1945). Historical Section, Plans, Training and Intelligence Division, Office of Chief, Chemical Corps, United States Army; November 1947. Biological Warfare Research in the United States; vol II. Archived at the US Army Medical Research Institute of Infectious Diseases, Ft Detrick, Md.
Bryden J. Deadly Allies: Canada’s Secret War, 1937-1947. Toronto, Ontario: McClelland & Stewart; 1989.
Holzer VE. Botulism from inhalation [in German]. Med Klin. 1962;57:1735-1738.
United Nations Security Council. Tenth Report of the Executive Chairman of the Special Commission Established by the Secretary-General Pursuant to Paragraph 9(b)(I) of Security Council Resolution 687 (1991), and Paragraph 3 of Resolution 699 (1991) on the Activities of the Special Commission. New York, NY: United Nations Security Council; 1995. S/1995/1038.
Bozheyeva G, Kunakbayev Y, Yeleukenov D. Former Soviet Biological Weapons Facilities in Kazakhstan: Past, Present and Future. Monterey, Calif: Center for Nonproliferation Studies, Monterey Institute of International Studies; June 1999:1-20. Occasional paper No. 1.
Miller J. At bleak Asian site, killer germs survive. New York Times . June 2, 1999:A1, A10.
Alibek K, Handleman S. Biohazard. New York, NY: Random House; 1999.
Smithson AE. Toxic Archipelago: Preventing Proliferation From the Former Soviet Chemical and Biological Weapons Complexes. Washington, DC: The Henry L. Stimson Center; December 1999:7-21. Report No. 32. Available at: http://www.stimson.org/cwc/toxic.htm. Accessed January 16, 2001.
United States Department of State. Patterns of Global Terrorism 1999. Washington, DC: US Dept of State; April 2000. Department of State publication 10687. Available at: http://www.state.gov/www/global/terrorism/1999report/1999index.html. Accessed February 1, 2001.
Cordesman AH. Weapons of Mass Destruction in the Gulf and Greater Middle East: Force Trends, Strategy, Tactics and Damage Effects. Washington, DC: Center for Strategic and International Studies; November 9, 1998:18-52.
Bermudez JS. The Armed Forces of North Korea. London, England: IB Tauris; 2001.
Zilinskas RA. Iraq’s biological weapons: the past as future? JAMA. 1997;278:418-424.
Hooper RR. The covert use of chemical and biological warfare against United States strategic forces. Mil Med. 1983;148:901-902.
Shapiro RL, Hatheway C, Becher J, Swerdlow DL. Botulism surveillance and emergency response: a public health strategy for a global challenge. JAMA. 1997;278:433-435.
Smith LDS. Botulism: The Organism, Its Toxins, the Disease. Springfield, Ill: Charles C. Thomas Publisher; 1977.
Hatheway CL, Johnson EA. Clostridium: the spore-bearing anaerobes. In: Collier L, Balows A, Sussman M, eds. ley & Wilson’s Microbiology and Microbial Infections. 9th ed. New York, NY: Oxford University Press; 1998:731-782.
Hall JD, McCroskey LM, Pincomb BJ, Hatheway CL. Isolation of an organism resembling Clostridium baratii which produces type F botulinal toxin from an infant with botulism. J Clin Microbiol. 1985;21:654-655.
Aureli P, Fenicia L, Pasolini B, Gianfranceschi M, McCroskey LM, Hatheway CL. Two cases of type E infant botulism caused by neurotoxigenic Clostridium butyricum in Italy. J Infect Dis. 1986;154:207-211.
Arnon SS. Botulism as an intestinal toxemia. In: Blaser MJ, Smith PD, Ravdin JI, Greenberg HB, Guerrant RL, eds. Infections of the Gastrointestinal Tract. New York, NY: Raven Press; 1995:257-271.
Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat Struct Biol. 1998;5:898-902.
Franz DR, Pitt LM, Clayton MA, Hanes MA, Rose KJ. Efficacy of prophylactic and therapeutic administration of antitoxin for inhalation botulism. In: DasGupta BR, ed. Botulinum and Tetanus Neurotoxins: Neurotransmission and Biomedical Aspects. New York, NY: Plenum Press; 1993:473-476.
Herrero BA, Ecklung AE, Streett CS, Ford DF, King JK. Experimental botulism in monkeys: a clinical pathological study. Exp Mol Pathol. 1967;6:84-95.
Scott AB, Suzuki D. Systemic toxicity of botulinum toxin by intramuscular injection in the monkey. Mov Disord. 1988;3:333-335.
Centers for Disease Control and Prevention. Botulism in the United States 1899-1996: Handbook for Epidemiologists, Clinicians, and Laboratory Workers. Atlanta, Ga: Centers for Disease Control and Prevention; 1998. Available at: http://www.cdc.gov/ncidod/dbmd/diseaseinfo/botulism.pdf. Accessed January 16, 2001.
Weber JT, Goodpasture HC, Alexander H, Werner SB, Hatheway CL, Tauxe RV. Wound botulism in a patient with a tooth abscess: case report and literature review. Clin Infect Dis. 1993;16:635-639.
Hughes JM, Blumenthal JR, Merson MH, Lombard GL, Dowell VR Jr, Gangarosa EJ. Clinical features of types A and B food-borne botulism. Ann Intern Med. 1981;95:442-445.
Duchen LW. Motor nerve growth induced by botulinum toxin as a regenerative phenomenon. Proc R Soc Med. 1972;65:196-197.
Mann JM, Martin S, Hoffman R, Marrazzo S. Patient recovery from type A botulism: morbidity assessment following a large outbreak. Am J Public Health. 1981;71:266-269.
Shapiro RL, Hatheway C, Swerdlow DL. Botulism in the United States: a clinical and epidemiologic review. Ann Intern Med. 1998;129:221-228.
Middlebrook JL, Franz DR. Botulinum toxins. In: Sidell FR, Takafuji ET, Franz DR, eds. Medical Aspects of Chemical and Biological Warfare. Washington, DC: Office of the Surgeon General; 1997:643-654. Textbook of Military Medicine; part I, vol 3.
Gangarosa EJ, Donadio JA, Armstrong RW, Meyer KF, Brachman PH, Dowell VR. Botulism in the United States, 1899-1969. Am J Epidemiol. 1971;93:93-101.
Hauschild AH. Epidemiology of human foodborne botulism. In: Hauschild AH , Dodds KL, eds. Clostridium botulinum: Ecology and Control in Foods. New York, NY: Marcel Dekker Inc; 1993:69-104.
Wannemacher RW Jr, Dinterman RE, Thompson WL, Schmidt MO, Burrows WD. Treatment for removal of biotoxins from drinking water. Frederick, Md: US Army Biomedical Research and Development Command; September 1993. Technical Report 9120.
Siegel LS. Destruction of botulinum toxin in food and water. In: Hauschild AH, Dodds KL, eds. Clostridium botulinum: Ecology and Control in Foods. New York, NY: Marcel Dekker Inc; 1993:323-341.
Burrows WD, Renner SE. Biological warfare agents as threats to potable water. Environ Health Perspect. 1999;107:975-984.
Kazdobina IS. Stability of botulin toxins in solutions and beverages [in Russian with English abstract]. Gig Sanit. January-February 1995:9-12.
Koenig MG, Drutz D, Mushlin AI, Schaffer W, Rogers DE. Type B botulism in man. Am J Med. 1967;42:208-219.
Geiger JC, Dickson EC, Meyer KF. The Epidemiology of Botulism. Washington, DC: US Government Printing Office; 1922. Public Health Bulletin 127.
Terranova W, Breman JG, Locey RP, Speck S. Botulism type B: epidemiological aspects of an extensive outbreak. Am J Epidemiol. 1978;108:150-156.
Meyer KF, Eddie B. Sixty-Five Years of Human Botulism in the United States and Canada: Epidemiology and Tabulations of Reported Cases 1899 Through 1964. San Francisco, Calif: G. W. Hooper Foundation and University of California San Francisco; 1965.
Angulo FJ, Getz J, Taylor JP, et al. A large outbreak of botulism: the hazardous baked potato. J Infect Dis. 1998;178:172-177.
MacDonald KL, Cohen ML, Blake PA. The changing epidemiology of adult botulism in the United States. Am J Epidemiol. 1986;124:794-799.
Mann JM, Hatheway CL, Gardiner TM. Laboratory diagnosis in a large outbreak of type A botulism: confirmation of the value of coproexamination. Am J Epidemiol. 1982;115:598-695.
Seals JE, Snyder JD, Kedell TA, et al. Restaurant-associated type A botulism: transmission by potato salad. Am J Epidemiol. 1981;113:436-444.
MacDonald KL, Spengler RF, Hatheway CL, Hargrett NT, Cohen ML. Type A botulism from sauteed onions: clinical and epidemiological observations. JAMA. 1985;253:1275-1278.
St Louis ME, Peck SH, Bowering D, et al. Botulism from chopped garlic: delayed recognition of a major outbreak. Ann Intern Med. 1988;108:363-368.
Townes JM, Cieslak PR, Hatheway CL, et al. An outbreak of type A botulism associated with a commercial cheese sauce. Ann Intern Med. 1996;125:558-563.
Telzak EE, Bell EP, Kautter DA, et al. An international outbreak of type E botulism due to uneviscerated fish. J Infect Dis. 1990;161:340-342.
O’Mahony M, Mitchell E, Gilbert RJ, et al. An outbreak of foodborne botulism associated with contaminated hazelnut yoghurt. Epidemiol Infect. 1990;104:389-395.
Aureli P, Franciosa G, Pourshaban M. Foodborne botulism in Italy. Lancet. 1996;348:1594.
Chou JH, Hwant PH, Malison MD. An outbreak of type A foodborne botulism in Taiwan due to commercially preserved peanuts. Int J Epidemiol. 1988;17:899-902.
Midura TF, Nygaard GS, Wood RM, Bodily HL. Clostridium botulinum type F: isolation from venison jerky. Appl Microbiol. 1972;24:165-167.
McCroskey LM, Hatheway CL, Woodruff BA, Greenberg JA, Jurgenson P. Type F botulism due to neurotoxigenic Clostridium baratii from an unknown source in an adult. J Clin Microbiol. 1991;29:2618-2620.
Gunnison JB, Meyer KF. Susceptibility of monkeys, goats and small animals to oral administration of botulinum toxin types B, C and D. J Infect Dis. 1930;46:335-340.
Dolman CE, Murakami L. Clostridium botulinum type F with recent observations on other types. J Infect Dis. 1961;109:107-128. ISI
Smart JL, Roberts TA, McCullagh KG, Lucke VM, Pearson H. An outbreak of type C botulism in captive monkeys. Vet Rec. 1980;107:445-446.
Giménez DF, Ciccarelli AS. Another type of Clostridium botulinum . Zentralbl Bakteriol [Orig]. 1970;215:221-224.
Beller M, Gessner B, Wainwright R, Barrett DH. Botulism in Alaska: A Guide for Physicians and Health Care Providers. Anchorage: State of Alaska, Dept of Health and Social Services, Division of Public Health, Section of Epidemiology; 1993.
Woodruff BA, Griffin PM, McCroskey LM, et al. Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975-1988. J Infect Dis. 1992;166:1281-1286.
Maselli RA, Bakshi N. American Association of Electrodiagnostic Medicine case report 16: botulism. Muscle Nerve. 2000;23:1137-1144.
Cherington M. Clinical spectrum of botulism. Muscle Nerve. 1998;21:701-710.
Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
Tacket CO, Shandera WX, Mann JM, Hargrett NT, Blake PA. Equine antitoxin use and other factors that predict outcome in type A foodborne botulism. Am J Med. 1984;76:794-798.
Arnon SS. Infant botulism. In: Feigin RD, Cherry JD, eds. Textbook of Pediatric Infectious Diseases. 4th ed. Philadelphia, Pa: WB Saunders Co; 1998:1570-1577.
Hibbs RG, Weber JT, Corwin A, et al. Experience with the use of an investigational F(ab’)2 heptavalent botulism immune globulin of equine origin during an outbreak of type E botulism in Egypt. Clin Infect Dis. 1996;23:337-340.
Black RE, Gunn RA. Hypersensitivity reactions associated with botulinal antitoxin. Am J Med. 1980;69:567-570.
Schreiner MS, Field E, Ruddy R. Infant botulism: a review of 12 years’ experience at the Children’s Hospital of Philadelphia. Pediatrics. 1991;87:159-165.
Kahn AS, Morse S, Lillibridge S. Public-health preparedness for biological terrorism in the USA. Lancet. 2000;356:1179-1182.
Santos JI, Swensen P, Glasgow LA. Potentiation of Clostridium botulinum toxin by aminoglycoside antibiotics: clinical and laboratory observations. Pediatrics. 1981;68:50-54.
Schulze J, Toepfer M, Schroff KC, et al. Clindamycin and nicotinic neuromuscular transmission. Lancet. 1999;354:1792-1793.
Olson KR, ed. Poisoning and Drug Overdose. 3rd ed. Stamford, Conn: Appleton & Lange; 1999.
Keller MA, Miller VH, Berkowitz CD, Yoshimori RN. Wound botulism in pediatrics. Am J Dis Child. 1982;136:320-322.
Robin L, Herman D, Redett R. Botulism in a pregnant woman. N Engl J Med. 1996;335:823-824.
St Clair EH, DiLiberti JH, O’Brien ML. Observations of an infant born to a mother with botulism. J Pediatr. 1975;87:658.
Arnon SS. Clinical trial of human botulism immune globulin. In: DasGupta BR, ed. Botulinum and Tetanus Neurotoxins: Neurotransmission and Biomedical Aspects. New York, NY: Plenum Press; 1993:477-482.
Siegel LS. Human immune response to botulinum pentavalent (ABCDE) toxoid determined by a neutralization test and by an enzyme-linked immunosorbent assay. J Clin Microbiol. 1988;26:2351-2356.
Byrne MP, Smith LA. Development of vaccines for prevention of botulism. Biochimie. 2000;82:955-966.
Dorsey EL, Beebe JM, Johns EE. Responses of air-borne Clostridium botulinum toxin to certain atmospheric stresses. Frederick, Md: US Army Biological Laboratories; October 1964. Technical Memorandum 62.
Wiener SL. Strategies for the prevention of a successful biological warfare aerosol attack. Mil Med. 1996;161:251-256.
Franz DR. Defense Against Toxin Weapons. Ft Detrick, Md: US Army Medical Research Institute of Infectious Diseases; 1997.
Franciosa G, Ferreira JL, Hatheway CL. Detection of type A, B, and E botulism neurotoxin genes in Clostridium botulinum and other Clostridium species by PCR: evidence of unexpressed type B toxin genes in type A toxigenic organisms. J Clin Microbiol. 1994;32:1911-1917.
Wictome M, Newton K, Jameson K, et al. Development of an in vitro bioassay for Clostridium botulinum type B neurotoxin in foods that is more sensitive than the mouse bioassay. Appl Environ Microbiol. 1999;65:3787-3792.
Dezfulian M, Bartlett JG. Detection of Clostridium botulinum type A toxin by enzyme-linked immunosorbent assay with antibodies produced in immunologically tolerant animals. J Clin Microbiol. 1984;19:645-648.
Sarvas H, Seppala I, Kurikka S, Siegberg R, Makela O. Half-life of the maternal IgG1 allotype in infants. J Clin Immunol. 1993;13:145-151.
Amersdorfer P, Marks JD. Phage libraries for generation of anti-botulinum scFv antibodies. Methods Mol Biol. 2000;145:219-240.
Green LL, Hardy MC, Maynard-Currie CE, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet. 1994;7:13-21.
Bavari S, Pless DD, Torres ER, Lebeda FJ, Olson MA. Identifying the principal protective antigenic determinants of type A botulinum neurotoxin. Vaccine. 1998;16:1850-1856.
Marks C, Marks JD. Phage libraries: a new route to clinically useful antibodies. N Engl J Med. 1996;335:730-733.
Reprinted with permission.
The Journal of American Medical Association.
Vol. 285, No. 8, Feb. 28, 2001