FIGURE 1.
| Contents | Previous | Next |
Lecturer Dr. Heddie O. Sedano, DDS, Dr. Odont
Tobacco usage either smoked (cigarette and pipe) or chewed and or dipped, has become one of the larger health problems world wide to the degree that is considered by some a world epidemic. Additional consequences to tobacco usage are, among others:
For the first time in 1997 the tobacco companies acknowledged that tobacco is addictive either smoked or chewed. Tobacco has been linked to heart disease, strokes, lung diseases, lung cancer, oral cancer, gum disease and tooth loss. It took over 500 years to definitely shift from the original assumption that tobacco was beneficial for human health to the present knowledge that tobacco is essentially a killer.
Tobacco was used by American aborigines mixed with spices and other plants, this mixture was inserted into a primitive pipe that they called tobaga and then smoked. Occasionally tobacco was also inhaled through the nose by means of a Y-shaped pipe. These aborigines used tobacco for medicinal and religious purposes. Aztecs chewed tobacco leaves to treat tooth aches and aborigines of North America mixed tobacco leaves with sea shells and lime for the same purpose. This combination induced marked teeth abrasion because of the sea shells. Poisonous snakes were numbed with tobacco smoke so that they could be manipulated during religious rites. Inhaled tobacco was also used to induce hallucinations, apparently these Indians used the tobacco species nicotiana rustica which has a high content of nicotine as well as other alkaloids as opposed to the species used presently, nicotiana tabacum, in the production of cigarettes and other forms of tobacco. According to some historians the aborigines also believed that tobacco could cure stomach ailments as well as headaches. American Indians rubbed tobacco juice on the wound produced by a snake bite in order to treat it.
Historical reports are not in agreement as to who was the first to introduce tobacco to Europe. Some maintain that Columbus brought seeds back after his first trip while others assume that Juan Ponce de León was the first to bring tobacco seeds to Europe in 1496 at his return from America on the second trip of Columbus. It is also stated that Columbus might have been the first European to oppose the use of tobacco because it is said that he admonished his sailors for engaging in the practice of inhaling tobacco smoke imitating the American Natives. Posterior explorers, especially the Portuguese, possibly recognized the potential of this "holy herb" and tobacco was fully introduced to Europe as a medicinal plant. Sir Walter Raleigh brought tobacco seeds to England after his second trip to America in 1565. The French ambassador to Lisbon, Jean Nicot de Villemain, obtained dried tobacco leaves and seeds and sent them to the queen mother in France, Catherine of Medici, exalting the medical capabilities of tobacco. Jean Nicot received the "ill honor" of having the botanical name of this plant named after him, nicotiana tabacum, as well as the main alkaloid: nicotine. The tobacco plant is a member of the nightshade family (Solanaceae family). There is no total agreement on the origin of the word tobacco, the most likely derivation is from the name of the pipe used by the Indians, either tobaga or tobaca. Some historians claim the derivation stems from the state of Tabasco in Mexico or the island of Tobago in the Caribbean, both of which are large producers of tobacco, but these are most unlikely origins of the word.
Tobacco is considered the most important factor in the etiology of the small cell carcinoma of the lungs. Additionally it has an important role in the etiology of carcinoma of the bladder and oral squamous cell carcinoma in various mucosal locations. Other pathologic entities associated with tobacco include: heart and arterial diseases, especially atherosclerotic peripheral vascular disease, esophageal and laryngeal cancer, chronic obstructive pulmonary disease, low birth weight, intrauterine growth retardation and periodontal disease. The annual prevalence of cigarette smoking among adult males is presently estimated at 28% and that for adult women at 22.5%.
Thirty percent of all cancer deaths and over 80% of lung cancer deaths are caused by tobacco. The lung cancer death rate for men was 4.9 per 100,000 in 1930 and it has increased to 75.6 per 100,000 in the decade of 1990. Ninety-two percent of oral squamous cell carcinoma (OSCC) in men and 61% of OSCC in women are attributable to tobacco usage.
The Centers for Disease Control and Prevention in 1999 reported that among high school male students smokeless tobacco was used by: 20% of white males, 6% of Hispanic males and 4% of Black males. The same report states that 48 million US adults smoke cigarettes and of those half will die of smoking related disease if they continue smoking. Hoffmann and Djordjevic in 1997 reported that the habit of chewing tobacco had declined by 30.6%, but that snuff use had increased, by almost 52%. The increase was due, primarily, to the use of oral snuff by teenage and adolescent males. Chewing of tobacco represents a risk for oral cancer. Snuff dipping is considered as one of the etiological factors of OSCC of the cheek, gingiva, and pharynx.
Presently 430,000 deaths each year are related to tobacco. Exposure to environmental tobacco smoke (ETS) is a well known fact, it has been reported that 87.9% of children and adult non-users of tobacco had detectable serum levels of the nicotine metabolite "cotinine" as a result to tobacco smoke exposure. The excess risk to develop lung cancer for women that do not smoke but which are exposed to ETS, has been estimated as 1 to 2% of that in smokers.
Moderate to severe periodontitis is found in 25.7% of cigarette smokers, 20.2% of former cigarette smokers and 13.1% of non-smokers. The difference in prevalence of periodontal disease for these three groups is statistically significant. The prevalence of moderate and severe periodontitis in current or former cigar/pipe smokers is estimated at 17.6%.
The most common precancerous lesions of the oral mucosas are leukoplakia (white plaque) and erythroplasia (red patch). It should be stated that not all white or red oral mucosal lesions are precancerous but because of the possibility of having a premalignant potential, all of them should be carefully evaluated and if deemed necessary, a biopsy should be performed. Leukoplakia has being described as a white plaque of the oral mucosas that cannot be scraped and that histologically does not resemble or represent any of the well known diseases that also manifest clinically as a white plaque such as lichen planus, candidiasis, etc. Some of these white lesions can be a consequence to trauma and histologically are diagnosed as hyperkeratosis. In such cases there is a history of trauma or a direct relationship of the white lesion with a broken denture or a broken tooth. Leukoplakia is also found in the oral mucosas of some heavy smokers. The direct relationship of leukoplakia and tobacco smoking has not been proved but it is most certainly a by-product of chewing tobacco.
|
|
|
FIGURE 1. |
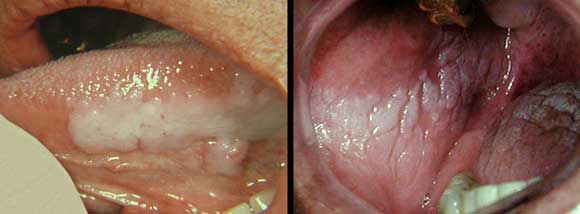
The photo to the left depicts a leukoplakia on the left lateral border of the tongue of a non-smoker patient. Note the milky-white color. The mandibular teeth on that side had rough edges and the lesion might have been the result of trauma. The photo to the right is from a 2 pack a day smoker. Areas of leukoplakia are present on the buccal mucosa, tongue and palate. Note the elevated and nodular appearance of the buccal mucosa lesion. Also note some areas of dark pigmentation on the tongue produced by the tobacco smoke.
A particular form of leukoplakia known as proliferative verrucous leukoplakia (PVL) also can be associated to tobacco usage. Around 1/3 of PLV are diagnosed in heavy smokers. PVL has a higher risk of malignant transformation than the most common non-verrucous leukoplakias.
|
|
|
FIGURE 2. |
This photo shows a proliferative verrucous leukoplakia also on the buccal mucosa of a heavy smoker. Note the proliferative appearance of the lesion (Courtesy of Dr. Sol Silverman Jr. University of California, School of Dentistry, San Francisco)
Erythroplasia is a red lesion of the oral mucosa with no apparent cause. Erythroplasia also should be carefully evaluated especially if there is history of tobacco usage. Erythroleukoplakia is a frequent combination of white and red lesions. These combined lesions have four times the potential for malignant transformation than that of a plain white or red lesion and because of this increased risk invariably they should be biopsied.
|
|
|
FIGURE 3. |
This patient had the habit of reverse smoking. He had a long history of chronic white and red lesions on the soft palate. Note the area of ulceration which is surrounded by areas of white coloration (erythroleukoplakia). Biopsy of that area demonstrated histological changes compatible with carcinoma in situ. Also note the dark pigmentation on the palate produced by the smoke.
It has been known since the 19th century that tobacco is responsible for the development of oral cancer as noted by the association of smoking and cancer of the lip. Garretson in 1869 in his book "System of Oral Surgery", discussed a case of carcinoma of the cheek and lower lip in a heavy pipe smoker. Throughout the years the role of tobacco in oral cancer etiology has been, not only studied, but well documented by means of experimental research. The factor common to all cancers is considered to be a DNA mutation, either in the germ line or, much more frequently, in somatic cells. For cells to undergo a malignant neoplastic transformation they need to be subjected to two mutations, this is in accordance to the double hit (impact) theory. Either one of the two mutations can be: genetic predisposition (oncogenes), viruses and the never ending environmental factors among which the polyaromatic hydrocarbon subfractions contained in tar from tobacco usage, are included, as well as nitrosamines such as: the tobacco-specific N-nitrosamine (TSNA), N'-nitrosonornicotine (NNN), and 4(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
A follow-up study of more than 5 years of 403 oral cancer patients at the University of California in San Francisco (UCSF), found that 72% of those patients were smokers and 50% smoked more than one pack a day. A second study of 595 patients by the same group at UCSF indicated that an average of 82% of patients with oral cancer in: tongue, oropharynx, floor of the mouth, gingiva, buccal mucosa, lips and hard palate were tobacco users. The highest prevalence being the floor of the mouth with 90% and the lowest the hard palate with 55%.
|
|
|
FIGURE 4. |
The arrow in the photo to the left points to a small lesion on the lip mucosa of a pipe smoker. Note the slight yellow color of the lesion. Biopsy of this lesion showed histological signs of premalignancy. Also note the tobacco pigmentation between the right mandibular lateral incisor and the canine. The photo to the right shows another patient with a squamous cell carcinoma of the lip. Note the enlargement of the lip which extends into the subjacent skin. The patient also smoked a pipe which was held on the right side. Note the abrasion and pigmentation of his lower incisors.
Oral squamous cell carcinoma is the most frequent form of cancer among men and the third most frequent among women in India and Pakistan. These carcinomas are associated to the habit of paan and also to reverse smoking. Paan is a quid of betel leaf containing areca nut, lime, condiments, sweeteners, and sometimes tobacco. This quid is kept in the mouth and chewed. Submucous fibrosis is another intraoral disease related to the habit of paan which also predisposes to oral carcinoma.
|
|
|
FIGURE 5. |
This was a Pakistani 32-year old male that for many years had practiced the habit of paan. He had developed submucous fibrosis. Note the marked whitening of the anterior maxillary gingiva. The patient was unable to properly open his mouth due to the extended areas of fibrosis throughout his oral mucosas. Submucous fibrosis is considered a predisposing lesion for the development of squamous cell carcinoma. Some patients seem to have a genetic predisposition to develop submucous fibrosis. Tobacco chewing and most certainly snuff dipping are also an important causative factor for cancer of the buccal mucosa and the gingiva.
|
|
|
FIGURE 6. |
The photo to the left is an example of a verrucous carcinoma occurring in a tobacco snuffer. Note the typical appearance of a snuff pouch on the left mandibular vestibule. Also note the gingival retraction and the slight tobacco pigmentation. This patient held the wad on different areas of his mouth but preferred the anterior vestibular sulcus. The photo to the right is another example of a squamous cell carcinoma developing on the mandibular right vestibular sulcus in a long standing tobacco snuffer. Note remaining pieces of tobacco and the teeth pigmentation.
|
|
|
FIGURE 7. |
Still another example of a squamous cell carcinoma developing in a tobacco pouch. Note that the lesion is large and ulcerated. Also note the marked teeth pigmentation. The patient held the tobacco only in that area.
P53 is a "tumor suppressor gene" located on the short arm of chromosome 17 (17p13.1). P53 has a basic function in the cellular cycle control and subsequently in the induction of neoplastic processes. The protein p53, coded by the gene with the same name, regulates apoptosis. Apoptosis is an irreversible process that conduces to natural cell death. Mutations in p53 eventuate in cessation of apoptosis which allow for the growth and development of abnormal cells. This over expression of the mutated p53, in the oral cavity, seems to be directly related to tobacco, alcohol and arica nut (betel nut) usage. P16 is another tumor suppressor gene located on the short arm of chromosome 9 (9p21-23). Genetic mutations at the level of p16 have been reported in a subset of chewing tobacco-induced oral cancers.
A great number of papers have been published presenting solid epidemiological data demonstrating that smoking represents an increased risk for the development and continuity of periodontal disease. Furthermore, it has been shown that smoking affects tissues' vasculature and that it interferes with the normal functioning of the cellular and humoral immune reactions arresting the inflammatory process.
Various reports have documented that after a period of several years of follow-up, habitual smokers have an increased frequency of periodontal disease versus decreased frequencies in non-smokers and former smokers. These differences were statistically significant. Periodontal bone height has been shown to be significantly reduced in habitual smokers and former smokers as opposed to that of non-smokers after adjusting for oral hygiene and age. Even when plaque formation is kept to a minimum, smokers present deeper and greater number of periodontal pockets than non-smokers. The alveolar bone loss results in a two fold prevalence of furcation in smokers over that in non-smokers, as documented by radiographs. Statistical studies have shown that the severity of periodontal disease increases with the number of cigarettes smoked and the number of years that a patient has smoked.
Periodontally, smokers often have advanced attachment and bone loss associated with relatively healthy appearing gingival tissues. It is especially important to carefully probe all teeth so that these defects are identified. Serum levels of cotinine seem to correlate with the degree of attachment loss, pocket depth and alveolar bone loss. Periodontal health status remains stable in former smokers and non-smokers, thus suggesting that abandoning the habit is beneficial to the periodontium. The impact of cigarette smoking on the periodontal health has also been documented in young patients (19 to 30 years old), in that age group smokers have almost four times the chance to develop periodontitis than individuals of the same age that never had smoked.
It has been experimentally proven that skin flap survival in hamsters exposed to cigarette smoke before and after surgery had a 40% rate of success, while those exposed to smoke only before surgery had 80% success rate. The rate of success in hamsters not exposed to smoke was 100%. The three components of cigarette smoke that seem to interfere with wound healing are nicotine, carbon monoxide and hydrogen cyanide. Nicotine is absorbed in the lungs where it enters the blood circulation and is capable of producing specific effects such as diminished proliferation of erythrocytes, fibroblasts and macrophages.
Additionally nicotine induces platelet aggregation with increased blood viscosity, this phenomenon favors the formation of microclots leading to capillary embolism and ischemia of the affected tissues. Vasoconstriction is another by-product of nicotine due to the release of catecholamines. Catecholamines induce the formation of chalones which interfere with normal wound healing and epithelialization.
Carbon monoxide has a greater capability for binding to hemoglobin than that of oxygen, thus reducing the amount of oxygen binding to hemoglobin and diminishing the distribution of oxygen to tissues with consequent cellular hypoxia and interference with normal wound healing. Hydrogen cyanide also participates in the alteration of oxygen metabolism and distribution, further delaying wound healing and epithelialization. These facts can be easily extrapolated to the regenerative process and epithelial re-attachment following periodontal surgery. Therefore, it is imperative that smokers that must undergo surgical procedures be advised to stop smoking for at least 12 waking hours prior to surgery. That is the time needed for the carbon monoxide to be completely released from hemoglobin. Likewise those patients must be advised to abstain from smoking during the entire post-operative period to improve their deficient wound healing.
ANUG is an acute or subacute oral inflammation primarily involving the free gingival margin, the crest of the gingiva, and the interdental papillae. The condition may occasionally spread to other areas of the oral mucous membranes. The classic etiologic concept--that ANUG is caused only by a symbiotic relationship between Bacillus fusiformis and Borrelia vincentii (Vincent's organisms) is no longer tenable. Vibrios, hemolytic streptococci, certain viruses, poor oral hygiene and tobacco usage in combination are probably implicated, producing enzymes and toxins that cause ANUG.
Pindborg in 1947 was the first to scientifically establish a link between smoking and the development of ANUG in young Danish recruits. ANUG has its peak incidence in winter in patients in their early twenties, with a marked predilection for males. Children are rarely if ever affected. The initial lesions--swollen, red papillae--generally occur in the mandibular molar area. However, other gingival areas may be affected. The edematous gingiva rapidly undergoes ulceration, producing characteristic, punched-out erosions of the dental papillae. The free gingiva becomes covered with a yellowish-gray pseudomembrane with a red halo. Fetor ex ore (halitosis) and excessive salivation are marked, and patients frequently complain of a metallic taste. Pain, tenderness, and bleeding lead to inability to eat. Regional lymphadenitis is usually marked. Low-grade fever, headache, and malaise are common. ANUG may involve the oropharynx or other areas of the oral mucosa if the patient's resistance is low or the condition is not treated. In such patients, tachycardia, leukocytosis and gastrointestinal disturbances may occur.
|
|
|
FIGURE 8. |
This 23-year old woman with acute necrotizing ulcerative gingivitis (ANUG) was a pack a day smoker. Note the marked horizontal atrophy of the interdental papillae and the areas of necrosis.
ANUG has a rapid onset. If treated, it generally subsides within 48 hours. Spontaneous remission and healing also may occur in 1 to 3 weeks. Recurrence of the disease is observed with high frequency, generally owing to retention of micro-organisms and debris in the punched-out areas of destruction, as well as continued smoking. Occasionally, considerable tissue destruction occurs, producing marked recession of the interdental papillae and of the marginal gingiva. Bone sequestration may occur. Rarely, complications such as noma, septicemia, and even death have occurred. Healing time and the effectiveness of treatments are delayed in smokers.
NICOTINE STOMATITIS is also known as smoker's palate and it is characterized by marked hyperkeratosis of the hard palate and secondary inflammation of underlying structures. It is seen in a considerable number of heavy tobacco smokers and is independent of the type of smoking habit.
The great majority of cases are seen in males with a mean age of 50 years. Most frequently affected are pipe smokers, followed by cigarette smokers, and, far behind, cigar smokers. The initial manifestation of the condition is marked erythema of the palate. This is followed by numerous red papular elevations around the opening of the excretory ducts of the palatal minor salivary glands. These elevations, of various sizes, soon become covered with a white to gray, generally uniform layer of either ortho- or parakeratin formation. The lesion progressively extends to the rest of the hard palate. Occasionally, whitening can also be seen in the soft palate. With time, cracks and fissures appear in the palate, which then presents the late clinical picture of the condition--i.e., elevated keratinized nodules of various sizes with a central, small, red, rounded point, representing the ductal opening of a minor salivary gland, the nodules being separated by the small fissures. The entire palatal surface, then, presents a rough, irregular appearance.
|
|
|
FIGURE 9. |
These are two examples of stomatitis nicotinica (smokers palate). Both patients were heavy cigarette smokers. Note the marked whitening of the hard palate. Also note several red, rounded areas which represent the opening of minor salivary gland main excretory ducts. As a rule this lesion does not predispose to cancer.
The thickness and the general clinical appearance of the lesion vary according to amount of tobacco used and the age of the lesion.
Some authors had considered that this condition might possibly become malignant. A histologic survey, performed by the present author, in 66 cases of nicotinic palatal leukokeratosis showed that only one case presented epithelial histologic changes compatible with the diagnosis of premalignant epithelial dysplasia. The condition presents such a typical clinical appearance that differential diagnosis is essentially unnecessary. Nevertheless, hyperkeratosis of other causes could be considered. Reverse smoking, a frequent practice in some areas of India and the Philippines, also induces palatal changes but with more pronounced clinical manifestations and early malignant transformation. Biopsy should be performed in order to eliminate the possibility of malignant transformation. It is assumed that remission occurs when patients abandon the smoking habit.
SNUFF POUCH (SP) is a form of hyperkeratosis with various degrees of clinical manifestation. SP develops on those mucosal sites where the tobacco is held. The causal agents of SP are considered to be the nitrosamines and hydrocarbons contained in tobacco. Prolonged use of this habit may conduce to the development of a squamous cell carcinoma (see above) due to the carcinogenic potential of those components. SP has been classified clinically into three different degrees. Degree 1 SP has the color of normal mucosa presenting a minor degree of superficial early wrinkling, the wrinkles disappear when the lesion is stretched. Degree 2 SP is a combination of white-gray and reddish areas with moderate wrinkles, neither wrinkles nor colors disappear when the lesion is stretched.
|
|
|
FIGURE 10. |
These are two examples of snuff pouches. To the left a Degree 1 and to the right a Degree 2. Note the slight differences between the two cases as well as the teeth pigmentation.
These lesions will slowly disappear after cessation of the habit. Degree 3 SP presents a color similar to that seen in degree 2 (white-gray and reddish) but the mucosa has pronounced thick wrinkles which do not disappear when stretched. The longer the habit the higher the degree. Cessation of the habit, especially if it is not of long duration, eventuates in disappearance of the lesion.
|
|
|
FIGURE 11. |
These are two examples of snuff pouches. To the left an advance Degree 2 and to the right a Degree 3. Note the slight differences between the two cases. The patient to the right presents an area of early ulceration on the upper portion of the snuff pouch.
HAIRY TONGUE is an elongation of the filiform papillae which become pigmented to varying degrees by products of some bacteria, fungi, antibiotic therapy, medications containing bismuth or tobacco.
The dorsum of the tongue is symmetrically covered by a thick coat of enlarged hyperkeratinized filiform papillae resembling hairs. These may be black, brown or yellow. Hence, the different names used, i.e. black hairy tongue. When associated to smoking the color varies from dark brown to black. The only complication may be occasional gagging if the papillae are grossly enlarged. The pigmentation of the papillae will disappear after cessation of smoking.
|
|
|
FIGURE 12. |
This heavy smoker patient presented a pigmented hairy tongue. Note the elongated filiform papillae.
SMOKING ASSOCIATED MELANOSIS of the oral mucosas can develop especially on the gingival and palatal mucosas. Some particles in the tobacco smoke induce stimulation of the mucosal melanoblasts resulting in hyperpigmentation which imitates the physiologic gingival pigmentation. This pigmentation tends to slowly disappear over a period of several months after cessation of smoking.
Exogenous teeth pigmentation induced by smoking can be seen in patients that smoke cigarettes and/or pipe and also in tobacco chewers, snuffers and arica nut chewers. The pigmentation is produced by the deposition of tar and other substances present in the tobacco smoke and is generally seen on the cervical area of the teeth. Heavy smokers will present larger areas of pigmentation on their teeth. The color varies from dark brown to black and it is easily removed by prophylactic dental cleaning. Continued smoking will maintain and increase the pigmentation and it may even facilitate the accumulation of plaque.
|
|
|
FIGURE 13. |
This patient smoked two packs of cigarettes a day. Note the marked pigmentation on his anterior teeth. The dentin was equally pigmented. The patient was wearing a full upper denture and the attrition of the lower teeth was due to a markedly close bite against the prosthesis.
Teeth abrasion is seen in those teeth that a pipe smoker uses to support the pipe. The loss of enamel and partially of dentin, can be so severe as to produce an open bite at the level of the affected teeth. Abrasion is also seen in patients that chew tobacco and betel nut chewers.
|
|
|
FIGURE 14. |
These two photos are from the same patient. She was a 52 year-old Vietnamese that had chewed arica nut mixed with tobacco most of her life.
Note the extreme teeth pigmentation as well as the teeth abrasion produced by chewing the arica nut.
REVERSE SMOKING fortunately is a habit seldom seen in USA but is frequent in India and the Philippines especially among women which held the lit end of a cigarette inside their mouth, generally, while washing clothes by the river. The combination of heat and the carcinogenic potential of cigarette smoke induce the formation of a variety of palatal lesions including: leukoplakia, erythroleukoplakia, ulcerations and squamous cell carcinoma.
As a summary it can be said that any of the following statements have been proven to be true in relationship to tobacco usage and oral lesions, and that any one of them should be more than reason enough to stop smoking:
Albandar JM et al. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol 2000; 71:1874-81.
Anderson KE et al. Metabolites of a tobacco-specific lung carcinogen in nonsmoking women exposed to environmental tobacco smoke. J Nat Cancer Inst 2001;93:378-81.
Bergstrom J et al. A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol 2000;71:1338-47.
Campanile G et al. Cigarrete smoking, wound healing and face lift. Clinics in Dermatol 1998;16:575-8.
Cox SC. Walker DM. Oral submucous fibrosis. A review. Australian Dent J 1996;41:294-9.
Craig S, Rees TD. The effects of smoking on experimental skin flaps in hamsters. Plast Reconstr Surg 1985;75:842-6.
Greer RO Jr, Poulson TC: Oral tissue alterations associated with the use of smokeless tobacco by teenagers. I. Clinical findings. Oral Surg 1983; 56:275-284
Hart GT et al. Tobacco use and dental disease. J Tennessee Dent Ass 1995;75:25-7.
Hoffmann D; Djordjevic MV. Chemical composition and carcinogenicity of smokeless tobacco. Advances Dent Research 1997;11:322-9.
Mahale A, Saranath D. Microsatellite alterations on chromosome 9 in chewing tobacco-induced oral squamous cell carcinomas from India. Oral Oncol 2000;36:199-206.
Meraw SJ et al. Cigarrete smoking and oral lesions other than cancer. Clinics in Dermatol 1998;16:625-31.
Offenbacher S, Weathers DR: Effects of smokeless tobacco on the periodontal, mucosal and caries status of adolescent males. J Oral Pathol 1985; 14:169-181.
Pindborg JJ. Tobacco and gingivitis: I. Statistical examination of the significance of tobacco in the development of ulceromembranous gingivitis and in the formation of calculus. J Dent Res 1947;26-261-4.
Pindborg JJ. Tobacco and gingivitis: II. Correlation between consumption of tobacco, ulceromembranous gingivitis and calculus. J Dent Res 1949;28:460-3.
Position paper: tobacco use and the periodontal patient. Research, Science and Therapy Committee of the American Academy of Periodontology. J Periodontol 1999;70:1419-27.
Ralhan R et al. Induction of MDM2-P2 transcripts correlates with stabilized wild-type p53 in betel- and tobacco-related human oral cancer. Amer J Pathol 2000;157:587-96.
Scully C et al. Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control. Oral Oncol 2000;36:256-63.
Silverman S. Oral Cancer 4th Edit. Amer. Cancer Soc. B.C.Decker Inc. 1998,
Straten MV et al. Tobacco Use and Skin Disease. South Med J 2001;94:621-634
Tobacco use--United States, 1900-1999. Mmwr. Morbidity and Mortality Weekly Repor 1999;48:986-93.
Walsh PM, Epstein JB. The oral effects of smokeless tobacco. J Canadian Dent Ass 2000;66:22-5.
Retrieved from : http://www.dent.ucla.edu/pic/visitors/Tobacco/page1.html