By Stuart C. White, DDS, PhD; Douglas C. Yoon, DDS; and Sotirios Tetradis, DDS, PhD
Abstract: Digital radiology will become an important part of dental practice. Manufacturers should develop more sophisticated tools, including software for digital subtraction; image processing routines for the diagnosis of caries, periodontitis and periapical disease; tools for three-dimensional viewing of the teeth and supporting structures; and analysis of bone trabecular pattern for early detection of systemic disease.
Hardware improvements should include increased dynamic range and sensitivity to radiation, and improved resolution. Sensors should be made the size of film, and components should be interchangeable across manufacturers. The true opportunity offered by digital imaging, computer-aided diagnosis, should continue to develop with particular attention to development of tools that add value for solving diagnostic problems and ease of use for the dentist and patient.
The 21st century will be the digital era of dental imaging much as film imaging dominated the 20th century. The early signs are clear. About 6 percent of general dentists and 30 percent of endodontists already own direct digital radiographic equipment. In 1994, the National Library of Medicine indexed two articles under the subject of "digital dental radiography"; this number rose to 50 by 1998. This trend is expected to accelerate. Immediate benefits of digital capture include time efficiency, patient education, radiation reduction and environmental compatibility.
More importantly, the future opportunities are immense. Incorporation of telediagnosis, videoconferencing, and transmission of images among and between dentists and insurance companies will be rapid. Additionally, digital imaging will become inextricably linked to electronic patient records (patient management systems) offering improved quality assurance to the dentist. l,2 A complete electronic patient record will include all visual and audio information in a seamlessly integrated, easily retrievable and user friendly format. Computer-aided diagnosis will become routine practice in clinical dentistry. The potential of digital imaging is only beginning to be explored. It is important now to identify clinical problems where this technology can best assist the dentist in providing improved diagnosis and treatment planning. Dentists should play an active role in establishing their needs and proposing solutions to help guide intelligent development of this powerful diagnostic tool. This article will discuss problems of four major areas of dental practice that should be addressed by digital imaging:
| Image display and analysis; | |
| Computer-aided diagnosis; | |
| Hardware development; and | |
| Administrative applications. |
One of the most exciting advantages of digital imaging is its inherent capability for manipulation of the display and analysis of the image. Once a digital image is acquired, whether through a charge-coupled device (CCD) sensor, storage phosphor plate, or scanner, its presentation may be readily manipulated to enhance features of diagnostic interest. Further, the image may be analyzed for patterns characteristic of disease. Various forms of analysis, measurement, feature extraction, image enhancement, and artificial intelligence techniques will be developed to improve the diagnostic acumen and work productivity of the dentist.
|
|
|
|
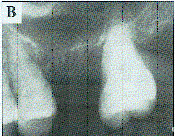
Figure 1a. In digital subtraction radiography, the differences between two radiographs are revealed. Image A was made immediately after extraction of a maxillary molar. |
Figure lb. This image was made one month later. |
|
|
Figure 1c. The subtraction of Figures 1a and b reveals areas of bone loss in black (black arrow) and bone deposition in white (white arrows). |
The basic technique of subtraction radiography is to make two radiographs of the same region of the jaws at different times. The first image may then be subtracted from the second to look for changes in the object occurring during the time interval, such as a loss of bone associated with periodontal disease or a gain in bone following successful therapy. In a controlled laboratory environment, digital subtraction allows small amounts of mineral gain or loss to be detected, much smaller amounts than can be recognized by simple visual examination of the before and after radiographs (Figure 1).
In practice, however, subtraction radiography is limited by the need to reproducibly image the patient with the same geometric relationship among the X-ray source, jaws, and image receptor, either film or digital. If the two views are made with different geometric perspectives, they cannot be properly aligned at the time of subtraction. Also, the process of image alignment or registration can be fairly tedious. In recent years, a significant body of techniques has been developed to deal with these problems. Placing the patient in a cephalostat with a fixed X-ray source and coupling the film to the teeth with impression compound help to produce images with reproducible image geometry. However, faster and less complicated methods are needed for application in dental offices. Mathematical tools are now available for correcting between changes in density and contrast due to film processing between the two images. 3 Most recently, tools are being developed that will automatically recognize anatomic features and then rotate, translate, and scale the images for automated image registration. 4 These tools will save the dentist time and improve alignment accuracy.
Digital subtraction should become increasingly useful for early detection of disease and measurement of disease progression or resolution following therapy. 3,5-11 In particular, digital subtraction has been used most frequently for evaluation of periodontal disease progression. 6, 7, l2, 22 When used skillfully, it provides a more sensitive method to detect early bone loss than conventional radiography. For similar reasons, subtraction radiography has been used for caries diagnosis. l0, 23 The tools for reproducible beam/patient/receptor alignment should be improved. Software tools for image registration, contrast correction, and subtraction radiography are available and should be built into digital systems.
Currently, images captured by digital sensors or digitized from film are displayed with minimal amounts of image processing. Most manufacturers of digital systems also provide tools for the dentist to modify sharpness, brightness, and contrast of the image. There is increasing evidence, however, that more sophisticated image processing tools may improve the image such that the dentist is better able to identify caries, marginal periodontitis, or other diseases. Techniques such as unsharp masking, nonlinear stretching, and adaptive histogram equalizations, though currently time consuming, hold promise as aids for detecting dental disease (Figure 2), especially if automated. There is already evidence that such tools may manipulate the image display so as to provide improved visualization of conditions such as caries, 24 periapical disease, and periodontal disease. 25-27 More effort should be made to develop the diagnostic potential of these techniques.
Among expected developments are "smart" tools, which segment an image into distinct anatomic regions and then apply image enhancements specific to each region or disease. For example, tools may be developed that automatically identify:
| Crowns in order to highlight occlusal and proximal surface caries; | |
| Tooth roots above bone to detect root caries; | |
| The alveolar crest to evaluate the character and location of the marginal periodontium; | |
| The periapical regions to assess the thickness of the periodontal ligament space and the integrity of the lamina aura; and | |
|
The cancellous bone and trabecular pattern to evaluate for local or systemic disease. |
For each of these regions, different image processing techniques may be used to optimize the presentation of the image to enhance diagnosis. Further, as discussed below, individualized analysis of the features of each image segment (e.g., bone, enamel, root), can be automated to screen patient images for evidence of disease.
Color in digital imaging adds value to intra- and extraoral images. Starting at the basic image acquisition level, stable and accurate color rendition will be essential for diagnosis (e.g., soft tissue lesions), treatment (e.g., prosthodontic shade selection and cosmetic tooth color matching), and longitudinal comparison of color changes. Manufacturers of both intra- and extraoral digital cameras should adhere to automatic image color calibration with monitors to ensure uniformity of image display.
|
|
|
Figure 2a through I. With image processing anatomic features can be better visualized. The three images in the left column (a, e, i) were obtained from a storage phosphor system. In the second column of images (b, f, j) the originals have been contrast stretched to use the full range from white to black. In the third column (c, g, k) unsharp masking has been added to the images in the second column to enhance edges. In the fourth column (d, h, l) the images from the third column have been inverted. Note particularly how the alveolar crest is visualized against a white background rather than a dark background. |
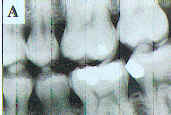
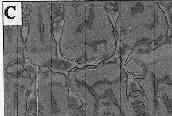
Currently, pseudocolor is being applied to digital radiographs. This technique assigns a color depending on the brightness value of a pixel. The eye is sensitive to many more colors than shades of gray; thus, the goal is to add discriminative power to the image by replacing gray level images with pseudocolor. However, the resultant image is not usually satisfactory for diagnosis because one type of structure e.g., dentin or bone may assume different colors. With image processing, structures of similar composition may be made to appear comparably in pseudocolor (Figure 3). Recently color coding has also been usefully applied to subtraction radiography so that the color represents the amount of brightness gain or loss. 28-29 Thus, there is considerable opportunity for improvement in the use of color for intraoral radiographs.
|
Figure 3a. Pseudocolor is available on most, if not all, digital radiographic software. This is a digital image made from a storage phosphor system. |

|
Figure 3b. This is the same image with color applied according to the gray scale. While cosmetically attractive, the image is less diagnostically useful than the original. |
|
Figure 3c . This image shows pseudocolor applied to the top image after image processing to make similar structures appear with comparable colors. Note how all the marrow spaces are pink and the bone trabeculae are blue. Even with this method, however, the original image, Figure 3a, remains the most diagnostic. |
The digital format will allow reconstruction of three-dimensional displays instead of the standard two-dimensional images. Reconstruction is a powerful diagnostic concept because it allows views from perspectives that are impossible to obtain by conventional means. Already, Tuned Aperture Computed Tomography (TACT) imaging allows tomographic reconstructions of thin image slices through teeth and bone. 30-32 Additionally, more advanced systems are being developed that will use multiple two-dimensional images to provide computer tomography (CT) like cross-sectional displays of teeth and bone, as well as three-dimensional surface renderings (Figure 4). Such images may be quite useful for identifying periodontal defects, 33 root fractures, the spatial relationship of impacted teeth to anatomic structures and other teeth, and potential implant sites. 34 These images will become available in dentistry within a few years at reasonable prices. It is expected that the patient doses from these examinations will be much closer to that of a conventional full-mouth X-ray than a CT examination. It is also expected that when these imaging modalities become available, static image viewing will rapidly give way to interactive 3-D-image manipulation and presentation.
|
|
|
|
Figure 4A. Multiple two-dimensional images can be combined mathematically to reconstruct a variety of views, including surface renderings, as in this figure (image courtesy of Drs. P. van der Stelt and S.M. Dunn). |
Figure 4b. Two-dimensional images were used to create this bucco-lingual section through a tooth (image courtesy of Drs. P. van der Stelt and S.M. Dunn) |
|
|
|
| Figure 4c. This figure shows an axial section of a tooth (image courtesy of Drs. P. van der Stelt and S.M. Dunn). | Figure 4d. This figure shows a mesio-distal section of a tooth (image courtesy of Drs. P. van der Stelt and S.M. Dunn). |
Artificial intelligence has been applied to many aspects of dental diagnosis. 35 Common examples include programs that evaluate a patient's signs and symptoms and generate a differential diagnosis. For example, a program called ORAD has been developed to assist the dentist in forming a differential diagnosis of a radiographic lesion. 36 It is available on the Web. 37 This program relies on a decision support system using Bayesian theory. With this method, the user enters a patient's signs and symptoms, and the program returns a list of diseases, in order of probability, that may account for the findings. ORAD considers 16 clinical and radiographic variables such as patient age; location of the lesion in the jaws; and the size, contents, and borders of the lesion. The program has a database of 140 lesions and uses Bayesian logic to evaluate these clinical features to arrive at a differential diagnosis. While most dentists recognize common diseases on radiographs, this program is often useful because it may suggest unusual lesions consistent with the clinical presentation or unusual manifestations of common lesions.
Currently, diagnosis of early disease is often difficult. Digital imaging may improve decision making by providing dentists with a wide variety of decision support (computer-assisted diagnostic) systems. 38-41 Because digital images are composed of pixel brightness values, programs have been written that measure these values and search for expected features or patterns. Automatic recognition of intrinsic disease features will provide powerful objective diagnostic tools to the dentist. Computer-assisted diagnostic programs will be helpful in several areas, including:
Caries diagnosis is difficult, especially in lesions limited to the enamel or near the dentoenamel junction. A number of investigators have developed programs for automated caries recognition. 42-50 These programs evaluate the density of the enamel and dentin, typically in vertical strips paralleling the proximal surface, and look for a reduction of density indicative of caries. Recently, a commercial product, Logicon Caries Detector by TrexTrophy, performs analysis of the density profile of proximal surfaces of teeth and identifies surfaces likely to have caries. The utility of this product is awaiting independent validation. Future development should be directed toward recurrent, root surface, and occlusal lesions.
Loss of alveolar bone is a radiographic hallmark of periodontal disease. Periodontal disease progression, measured either through loss of density or height of alveolar bone, should be developed as an automated tool for early disease identification and evaluation of treatment success. l2,l9,5l,53 Subtraction radiography should most likely be a part of this package.
Detecting periapical disease at its early stages is often difficult, particularly when associated with the buccal roots of maxillary molars. Automated morphologic analysis of the details of the apex, including width of the periodontal ligament space and integrity of the lamina aura, will assist the dentist in early detection of periapical disease. 54-56 Similarly, measurement of changes in the size and density of periapical lesions could allow early assessment of the success of endodontic treatment. For these endodontic applications, subtraction radiography will add significant analytic power.
Implants are now an established means of replacing missing teeth. A number of software programs that reconstruct CT images to allow cross-sectional viewing are available. New low-dose techniques are being developed, while tools to assess bone quality and quantity prior to implant placement should be created. In addition, means to rapidly assess the extent of osseointegration and alveolar bone loss following placement need to be established. 32, 57-59
In orthodontics, CCD and storage phosphor digital imaging receptors are being used. 60-62 There is a clear need for consistent automatic landmark identification of cephalometric images followed by craniofacial analyses of growth and development. 63-66 These and other features will save the dentist time and improve the quality and consistency of diagnosis and treatment planning.
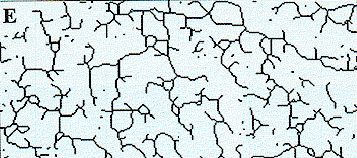
The jawbones are the most frequently imaged bones of the body. Their morphology is altered by local stimuli, systemic diseases, and metabolic disturbances. Digital radiography allows early identification of osteoporosis and other metabolic diseases of bone. 67-76 Although this field is in its infancy, analysis of morphologic features, such as the trabecular bone pattern of dental radiographs (Figure 5), will provide a valuable screening tool for patients with early abnormalities or progression of bone diseases. 77-80
|
|

|
|
Figure 5a. Image processing can be used to measure particular features of cancellous bone. This image shows a portion of a conventional radiography in the anterior maxilla. |
Figure 5b. This image is a blurred version of Figure 5a. |

|
|
|
Figure 5c . This figure shows the subtraction of Figure 5b from Figure 5a. This levels out the bright and dark areas so trabeculae show with a uniform density across the entire image. |
Figure 5d. This image is a threshold version of Figure 5c; that is, trabeculae made white and marrow made black. |
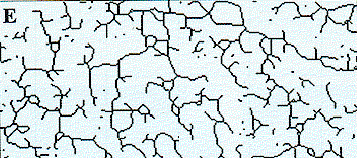
|
|
| Figure 5e. This image is a skeletonized version of Figure 5d to show the core structure of the trabeculae such as the length and branching structure. | Figure 5f. This image shows the skeletonized image in 5e superimposed on the original image (Figure 5a) to show the congruence of the two images. |
In film-based radiology, the radiographic film is both the sensor and the display. In digital radiology, the sensor and the display are separate, which allows the manufacturing of digital sensors with improved characteristics over film. However, film has many useful imaging properties that digital sensors should mimic or extend.
The dynamic range of a sensor is the range of radiation exposure that may be recorded, from the highest dose that produces a black image to the lowest dose that produces a light gray barely detected by the eye. A sensor with a wide dynamic range is highly desirable in clinical practice because it can produce a diagnostic image over a wide exposure range; it can detect small density differences of the imaged object and accurately record objects on the same image with high and low attenuation. Film has a relatively narrow dynamic range of about 1,000-to-1. 8 1 That is, the exposure that causes the film to be very black is 1,000 times higher than the exposure that causes the film to be a light gray. CCD sensors show an even shorter dynamic range than film, i.e., about 100-to-1. 81 Thus, when exposures are too high or too low, CCD sensors are more likely to have diagnostically meaningless light or dark regions on an image. The dynamic range of CCDs will have to improve to better address these important issues of diagnostic dental radiology.
The best sensor with respect to dynamic range is the photostimulable phosphor with a ratio of greater than 10,000-to-1 ratio of high vs. low detectable radiation exposure. 82 Photostimulable phosphor sensors are reported to provide diagnostic images over a wider range of exposures than film or CCDs. 83 Although this is a major advantage because it eliminates the need to repeat images due to overexposure errors, it should be addressed with caution because it hides the danger of systematically overexposing patients.
Another performance characteristic of the image sensors is the response to radiation exposure. A linear response means that the resulting image density increases or decreases in direct proportion to the amount of the X-ray exposure. The response of film to radiation is not linear. In contrast, both CCD and photostimulable phosphors have a linear response throughout their dynamic ranges of exposure. A sensor with a linear response is desirable and advantageous in clinical practice because it offers a better distribution of levels of gray at low and high densities and allows a predictable quantitation of an object's attenuation. New sensor technologies should strive for a linear response.
High sensitivity to radiation exposure is an important characteristic of the image sensor. Sensitive sensors require less radiation to produce a diagnostic image and thus reduce the radiation received by the patient. CCD and photostimulable phosphor sensors are more sensitive than film. Their use reduces patient exposure approximately in half compared with E-speed film and to one quarter compared with D-speed film. Further development toward increased sensitivity is desirable as long as image quality is maintained or improved. As faster sensors become available, X-ray generators will need improved timers for accurate control of short exposure durations.
Signal-to-noise ratio is defined as the ratio of the receptor output (film density, charge, or luminescence) that is related to diagnostic information compared to the output without diagnostic information (noise). 81 Low noise is a characteristic of a good sensor. A good sensor should be able to detect the diagnostic information in the remnant X-ray beam and separate it from the noise originating from the imaging system. An inherent and unavoidable source of noise in dental radiology comes from the statistical fluctuation of photon density in the X-ray beam. The recording medium (e.g., film or direct digital sensors) adds additional noise. Digital images add an additional source of noise from the various electronic components of the imaging system.
In general, the lower the noise, the more sensitive the sensor is to radiation. Film is considered to have a higher signal-to-noise ratio than CCDs. Manufacturers of digital sensors should continue their efforts to minimize electronic noise and thus improve the detection of diagnostic information. Commensurate with improved signal-to-noise ratio, also expected is the development of digital imaging systems with increased contrast resolution employing 10 or more bits, much like current CT displays. This will increase the number of gray levels from 256 to 1,000 or more. Such an increased range will allow windowing and leveling adjustments to gain optimal viewing of dentin, enamel, and bone.
Spatial resolution is the ability of a sensor to detect as separate images two objects that are placed close together (Figure 6). Until recently, film offered the highest resolution of all sensors.
Now several companies are offering digital sensors with resolving capability in excess of 20 line pairs per mm. 84 It is likely that more companies will soon provide comparable products. The available photostimulable phosphor sensors have a resolution of approximately 10 1p/mm. Sensors with higher resolution should be manufactured to assist clinical practitioners in everyday tasks, such as identification of root canals or root fractures. Improved resolution will allow greater magnification of images and improved diagnosis.
|
|
|
Figure 6b. This image was scanned at 5 line pairs/mm. |
| Figure 6c. This image was scanned at 8 line pairs/mm. | ||
| Figure 6d. This image is at 16 line pairs/mm. Note the progressive increase of fine detail and edges with increased resolution. For many tasks, however, high resolution is not necessary. | ||
|
Figure 6a. Image resolution is important for viewing fine detail. This is a portion of a conventional radiograph. The apical region (in white box) is shown in Figure 6a through c. |
Incorporation of density standards into receptors will allow accurate measurement of object mass leading to measurement of mineral gain or loss, such as in caries or periodontal or periapical disease. Changes in bone mass may also be useful for detecting disease progression or resolution. In this fashion, radiography will advance as a precise quantitative diagnostic tool. Trends in this direction are evident in the research in absolute calibration. 9 These technologies must still be adapted into user-friendly clinical software.
Drawing upon the successful standardized configuration of film-based imaging, manufacturers of digital systems should strive for similarly sized formats of the viewable surface area. Because of their unique sealing and packaging requirements, the proportion of viewable surface area for CCD and complimentary metal oxide semiconductor (CMOS) systems tends to be less than for film. However, the major difference between film and CCD or CMOS sensors is their thickness. Compared with film, which is about 1.6 mm thick, current CCD and CMOS sensors are about 7 to 8 mm thick, not counting the cord attachment of these devices. Storage phosphor sensors are similar in area and thickness to film and, like film, are somewhat flexible and do not have attached wires. There is a strong perception by many clinicians that decreased thickness enhances patient comfort. This remains to be verified by clinical studies.
Component Modularity and Standardization
Uniform standards of data formats and component interfacing should be adopted. The lesson learned from the development of the PC industry in the 1980s and 1990s is that closed architecture and proprietary data formats may result in short-term financial gains but that such products ultimately suffer from the inability to work with complementary technology. Medical digital imaging has shown the way by establishing the DICOM Standard to foster compatibility among competing digital systems. Thus, it is essential for the practicing clinician and the future health of the digital imaging industry that standards of interfacing be adopted.
To start with, sensors should be interchangeable. This would give the clinician the ability to easily replace broken sensors even if the original vendor has gone out of business and to easily upgrade to new sensor technology without investing in an entirely new system. It is also clear that office integration is the trend for electronic technology of the future. Based on current trends, it is reasonable to assume that, in the future, practice management software will assume a major role in office integration. To survive in this environment, producers of digital imaging technology must seamlessly integrate with practice management software. This will require adoption of standardized image file formats and I/O (input/output) protocols. For example, the DICOM Standard for medical imagery and the Twain model for I/0 interfacing are likely candidates. In this regard, the emerging dental DICOM format is a likely candidate.
The digital imaging technology of the future will need to continue to address clinical ergonomic issues. In addition to the issue of sensor size discussed previously, imaging systems of the future will need to improve chairside accessibility. Cords draping across the patient can impede typical dental procedures. Thus, there should be a move toward wireless systems or compact image storage systems (e.g., electronic or storage phosphor-based). Facilitating this process will be improvements in office networking.
Clinicians currently are concerned about already crowded conditions exacerbated by the proliferation of computer hardware in the dental operatory. Networking has provided some relief for this problem and this trend will continue. The offices of the future should have small, simple client PCs and flat-screen monitors integrated into the chair with easily accessible interface ports mounted on the tray, much the same as handpieces are mounted today. Larger, more powerful machines at the front desk can handle all sophisticated digital processing.
In the future, all patient data collection, recording, and retrieval operations should be voice-activated and integrated through a centralized patient management system. This will significantly streamline charting, history taking and radiological examinations. This hands-off approach will not only speed up operations by freeing up the dentist and assistant's hands, but will also facilitate infection control.
Advances in digital imaging technology should allow streamlining of many of the clinical administrative operations that involve visual information. These operations will benefit from the enhanced speed of transmission of visual data, encryption security and automated quality control that digital electronic technology affords. The following are examples of near-term applications of digital technology to administrative operations.
A significant near-term application of digital imaging technologies lies in teleradiology. That is, the electronic transmission of digital radiographic images for purposes of remote education and radiological consultations. Improvements in transmission band-width and the explosive growth of the Internet make remote consultations possible to any office possessing a computer, flatbed scanner, and modem. Software designers should build in the tools to give the dentist the capability of easily transmitting images to colleagues for real-time consultations.
Most major companies, including dental insurance companies, will move toward paperless operations because of potential cost savings associated with reduced paper work. The software that accompanies digital systems should provide the tools to facilitate interacting with these "paperless" companies, including the capability to electronically attach radiological and other imagery.
Many previously labor-intensive operations such as billing and scheduling have been integrated into commercial practice management software. This trend will continue with the integration of digital imagery into patient records. Sophisticated pattern-recognition technologies should be developed to enable automatic recognition of teeth and image projection. This will enable automated filing and retrieval of radiographs in the patient records according to tooth number. Pattern recognition technologies will also assist in ensuring quality control (including detecting cone cuts or excessive radiation exposure) during the radiological examination.
One of the major concerns regarding electronic data especially digital imagery has been security or data integrity. This perception stems from the ease with which images may be digitally manipulated. However, electronic conversion of image data actually affords some of the most robust forms of security based on sophisticated mathematical encryption and encoding algorithms. For example, sensitive authentication algorithms can detect the smallest change in a digital document following its creation. For instance, the DICOM Standard used in medical imaging includes Digital Signature, software to allow authentication and verification of an unaltered DICOM image. Conversely, robust watermarking algorithms can identify the original source of a digital document despite extensive manipulation of the image (e.g., Digmark Photoshop plug-in software). In conjunction with bonded agencies, these tools should be incorporated into dental imaging to provide all parties with the necessary security to prevent fraud and lack of confidence in the technology.
The future imaging world will be quite different from the current one. The integration of digital imaging into the patient record must become seamless, both in terms of use and technical support. The true opportunity offered by digital imaging– computer-aided diagnosis should continue to develop with particular attention to development of tools that add value for solving diagnostic problems. We are on the cusp of a paradigm shift in dental digital imaging that is full of opportunities for commercial and academic endeavors. The clinical dentist should be an integral part of this process.
Authors
Stuart C. White, DDS, PhD, is a professor in and chair of the Section of Oral Radiology at the University of California at Los Angeles School of Dentistry.
Douglas C. Yoon, DDS, is an adjunct associate professor in the Section of Oral Radiology at the UCLA School of Dentistry.
Sotirios Tetradis, DDS, PhD, is an assistant professor in the Section of Oral Radiology at the UCLA School of Dentistry.
Reprinted with permission.
CDA Journal. Vol. 27, No. 12, December 1999.
References
Wagner IV, The use of information technology for continuous improvement of patient care. In, Abbey LM, Zimmerman JL, eds, Dental Informatics: Integrating Technology Into the Dental Environment. Springer Verlag, New York, pp 7791, 1992.
Wagner IV, Schneider W, Compute raided quality assurance in oral health care: the image of electronic radiographs. Dentomaxillofac Radiol 21(4):1957, 1992.
Ruttimann U, Webber R, Schmidt E, A robust digital method for film contrast correction in subtraction radiography.) J Perio Res 21:48695, 1986.
Yoon DC, A new method for the automated alignment of dental radiographs for digital subtraction radiogra- phy. Dentomaxillofac Radiol, in press.
Dunn SM, van der Stelt PF, et al, A comparison of two registration techniques for digital subtraction radiography . Dentomaxillofac Radiol 22(2):7780 1993.
Christgau M, Wenzel A, et al, Quantitative digital subtraction radiography for assessment of bone density changes following periodontal guided tissue regeneration. Dentomaxillofac Radiol 25(1):2533, 1996.
Christgau M, Hiller KA, et al, Quantitative digital subtraction radiography for the determination of small changes in bone thickness: an in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85(4):46272, 1998.
Hintze H, Wenzel A, et al, Digital subtraction radiography for assessment of simulated root resorption cavities. Performance of conventional and reverse contrast modes. Endod Dent Traumatol 8(4):14954, 1992.
Jeffcoat MK, Reddy MS, et al, Efficacy of quantitative digital subtraction radiography using radiographs exposed in a multicenter trial. J Periodontal Res 31(3):15760, 1996.
Nummikoski PV, Martinez TS, et al, Digital subtraction radiography in artificial recurrent caries detection. Dentomaxillofac Radiol 21(2):5964, 1992.
Ruttimann UK, Computer based reconstruction and temporal subtraction of radiographs. Adv Dent Res 1(1):729, 1987.
Bragger U, Digital imaging in periodontal radiography. J Clin Periodontal 15:5517, 1988.
Furkart AJ, Dove SB, et al, Direct digital radiography for the detection of periodontal bone lesions. Oral Surg Oral Med Oral Pathol 74(5):65260, 1992.
Grondahl K, Computer assisted subtraction radiography in periodontal diagnosis. Swed Dentl Suppl 50:144, 1987.
Farman AG, Direct digital radiography for the detection of periodontal bone lesions. Oral Surg Oral Med Oral Pathol 74:65260, 1992.
Christgau M, Hiller KA, et al, Accuracy of quantitative digital subtraction radiography for determining changes in calcium mass in mandibular bone: an in vitro study. J Periodontal Res 33(3):13849, 1998.
Janssen PT, van Aken J, Problems around the in vitro and in vivo application of quantitative digital subtraction radiography. J Clin Periodontol 16(5):32330, 1989.
Wenzel A, Warrer K, Karring T, Digital subtraction radiography in assessing bone changes in periodontal defects following guided tissue regeneration. J Clin Periodontol 19(3):20813, 1992.
Van der Stelt PF, Modern radiographic methods in the diagnosis of periodontal disease. Adv Dent Res 7(2):15862, 1993.
Okano T, Mera T, et al, Digital subtraction of radiograph in evaluating alveolar bone changes after initial periodontal therapy. Oral Surg Oral Med Oral Pathol 69(2):25862, 1990.
Jeffcoat MK, Reddy MS, Digital subtraction radiography for longitudinal assessment of per implant bone change: method and validation. Adv Dent Res 7(2):196201, 1993.
Jeffcoat MK, Reddy MS, et al, Efficacy of quantitative digital subtraction radiography using radiographs exposed in a multicenter trial. J Periodont Res 31(3):15760, 1996.
Maggio JJ, Hausmann EM, et al, A model for dentinal caries progression by digital subtraction radiography. J Prosthet Dent 64(6):72732, 1990.
Wenzel A, Compute raided image manipulation of intraoral radiographs to enhance diagnosis in dental practice: a review. Int Dental Journal 43:99-108, 1993.
Koch S, Wagner IV, et al, Quality assurance in oral radiography: computer based methods for assessment and controlled improvement of intraoral radiographs. Med info 8 Pt 2:1707, 1995.
Koch S, Wagner IV, et al, IT based evaluation and automatic improvement of the quality of intraoral radio- graphs. Comput Methods Programs Biomed 46(1):4150, 1995.
Koch S, Wagner IV, et al, Controlled diagnosis oriented enhancement of automatically segmented radio- graphs in dentistry. Comput Methods Programs Biomed 57(12):12531, 1998.
Papika S, Ulrik Paulsen H, et al Orthodontic application of color image addition to visualize differences between sequential radiographs. Am J Orthod Dentofacial Orthop 115(5):48893, 1999.
Shi XQ, Eklund 1, et al, Comparison of observer reliability in assessing alveolar bone changes from color coded with subtraction radiographs. Dentomaxillofac Radiol 28(1):316, 1999.
Webber RL, Horton RA, et al, Tunedaperture computed tomography (TACT). Theory and application for three-dimensional demoalveolar imaging. Dentomaxillofac Radiol 26(1):5362, 1997.
Tyndall DA, Clifton TL, et al, TACT imaging of primary caries. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 84(2):21425, 1997.
Webber RL, Horton RA, et al, Comparison of film, direct digital, and tune daperture computed tomography images to identify the location of crestal defects around endosseous titanium implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81(4):48090, 1996.
Seipel S, Wagner IV, et al, Three-dimensional visualization of the mandible: a new method for presenting the periodontal status and diseases. Comput Methods Programs Biomed 46(1):517, 1995.
Seipel S, Wagner IV, et al, A virtual reality environment for enhanced oral implantology Medinfo 8 Pt 2:1710, 1995.
White SC, Decision support systems in dentistry. J Dent Educ 60(1):4763, 1996.
White SC, Compute raided differential diagnosis of oral radiographic lesions. Dentomaxillofac Radiol 18(2):539, 1989. http://www. dent.ucla. edu/cgibin/orad/orad.pl
Wagner IV, Schneider W, Computer based decision support in dentistry. J Dent Educ 55(4):2637, 1991.
Van der Stelt PF, The microcomputer in the dental office: a new diagnostic aid. Int Dent J 35(2): 103-8, 1985.
van der Stelt PF, Improved diagnosis with digital radiography. Curr Opin Dent 2: 16, 1992.
van der Stelt PF, Computer assisted interpretation in radiographic diagnosis. Dent Clin North Am 37(4):68396, 1993.
Pitts NB, Renson CE, Monitoring the behavior of posterior approximal carious lesions by image analysis of serial standardized bitewing radiographs. Br Dent J 162:1521, 1987.
Pitts NB, Detection and measurement of approximal radiolucencies by computer aided image analysis. Oral Surg Oral Med Oral Pathol 58(3):35866, 1984.
Pitts NB, Renson CE, Reproducibility of compute raided image analysis derived estimates of the depth and area of radiolucencies in approximal enamel. J Dent Res 64(10):12214, 1985.
Pitts NB, Renson CE, Further development of a compute raided image analysis method of quantifying radiolucencies in approximal enamel. Caries Res 20(4):36170, 1986.
Duncan RC, Heaven T, et al, Using computers to diagnose and plan treatment of approximal caries detected in radiographs. J Am Dent Assoc 126(7):87382, 1995.
Heaven TJ, Firestone AR, Feagin FF, Quantitative radiographic measurement of dentinal lesions. J Dent Res 69(1):514, 1990.
Heaven TJ, Firestone AR, Feagin FF, Computer based image analysis of natural approximal caries on radio- graphic films. J Dent Res 71:8469, 1992.
Heaven TJ, Weems RA, Firestone AR, The use of a computer based image analysis program for the diagnosis of approximal caries from bitewing radiographs. Caries Res 28(1):558, 1994.
van Papenrecht F, Geraets WGM, et al, Compute raided detection of approximal caries in dental radiographs. Dentomaxillofac Radiol 24:8990, 1995.
van der Stelt PF, van der Linden LW, et al, Digitized pattern recognition in the diagnosis of periodontal bone defects. J Clin Periodontol 12(10):8227, 1985.
Bragger U, Pasquali L, et al, Computer assisted densitometric image analysis in periodontal radiography. A methodological study. J Clin Periodontol 15(1):2737, 1988.
van der Stelt PF, Geraets WG, Computer-aided interpretation and quantification of angular periodontal bone defects on dental radiographs. IEEE Trans Biomed Eng 38(4):3348, 1991.
Mol A, van der Stelt PF, Application of digital image analysis in dental radiography for the description of periapical bone lesions: a preliminary study. IEEE Trans Biomed Eng 38(4):3579, 1991.
Mol A, van der Stelt PF, Locating the periapical region in dental radiographs using digital image analysis. Oral Surg Oral Med Oral Pathol 75(3):37382, 1993.
Mol A, Dunn SM, van der Stelt PF, Diagnosing periapical bone lesions on radiographs by means of texture analysis. Oral Surg Oral Med Oral Pathol 73(6):74650, 1992.
Bragger U, Burgin W, et al, Digital subtraction radiography for the assessment of changes in per implant bone density. Int J Oral Maxillofac Implants 6(2):1606, 1991.
Bragger U, Burgin W, et al, Image processing for the evaluation of dental implants. Dentomaxillofac Radiol 21(4):20812, 1992.
Reddy MS, Mayfield Donahoo TL, Jeffcoat MK, A semi automated computer assisted method for measur- ing bone loss adjacent to dental implants. Clin Oral Implants Res 3(1):2831, 1992.
Geelen W, Wenzel A, et al, Reproducibility of cephalometric landmarks on conventional film, hardcopy, and monitor displayed images obtained by the storage phosphor technique. Eur J Orthod 20(3):33140, 1998.
Naslund EB, Kruger M, et al, Analysis of low dose digital lateral cephalometric radiographs. Dentomaxillofac Radiol 27(3):1369, 1998.
Seki K, Okano T, Exposure reduction in cephalography with a digital photostimulable phosphor imaging system. Dentomaxillofac Radiol 22(3):12730, 1993.
Hing NR, The accuracy of computer generated prediction tracings. Int J Oral Maxillofac Surg 18(3):14851, 1989.
Levy Mandel AD, Venetsanopoulos AN, Tsotsos JK, Knowledge base land marking of cephalograms. Computers and Biomedical Research 19-282309, 1986.
Macri V, Wenzel A, Reliability of landmark recording on film and digital lateral cephalograms. Eur J Orthod 15(2):13748, 1993.
Pass B, Gregson PH, Automated identification of landmarks in cephalometric radiographs. J Dent Res 70:528, 1991.
Geraets WG, van der Stelt PF, et al, A new method for automatic recognition of the radiographic trabecular pattern. J Bone Miner Res 5(3):22733, 1990.
Geraets WG, van der Stelt PF, Analysis of the radiographic trabecular pattern. Patterrn Recognition Letters 12:575581, 1991.
Geraets WG, van der Stelt PF, Elders PJ, The radiographic trabecular bone pattern during menopause. Bone 14(6):85964, 1993.
Southard KA, Southard TE, Detection of simulated osteoporosis in human anterior maxillary alveolar bone with digital subtraction. Surg Oral Med Oral Pathol 78(5):65561, 1994.
Southard KA Southard TE, Detection simulated osteoporosis in dog alveolar bone with the use of digital subtraction. Oral Surg Oral Pathol 77(4):4128,1994.
Southard KA, Southard TE, Comparison of digitized radiographic alveolar features between 20 and 70-yearold women. A preliminary study. Oral Surg Oral Med Oral Pathol 74(1):1117, 1992.
Homer K, Devlin H, Clinical bone densitometric study of mandibular atrophy using dental panoramic tomography. J Dent 20(1):337, 1992.
Link TM, Majumdar S, et al, Texture analysis of direct magnification radiographs of vertebral specimens: correlation with bone mineral density and biomechanical properties. Acad Radiol 4(3):16776, 1997.
Southard TE, Southard KA, et al, Fractal dimension in radiographic analysis of alveolar process bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 82(5):56976, 1996.
Southard TE, Southard KA, Detection of simulated osteoporosis in maxillae using radiographic texture analysis. IEEE Trans Biomed 43(2):12332, 1996.
White SC, Rudolph DJ, Alterations of trabecular pattern in the jaws of patients with osteoporosis. Oral Surg Oral Med Oral Pathol Radiol Endod, 1999 in press.
White SC, Rudolph DJ, Ma L, influence of Xray beam angulation and exposure on morphologic features of trabecular bone. Int J Oral Biol 24:1723, 1999.
Kashima I, Bando S, et al, Bone trabecular pattern analysis in Down syndrome with the use of computed panoramic radiography. Part II: Visual pattern analysis with the frequency and gradational enhancement. Oral Surg Oral Med Pathol 70(3):3604, 1990.
Kumasaka S, Kashima I, Initial investigation of mathematical morphology for the digital extraction of the skeletal characteristics of trabecular bone. Dentomaxillofac Radiol 26:16111.
Versteeg CH, Sanderink GC, van der Stelt PF, Efficacy of digital intraoral radiography in clinical dentistry. J Dent 25(34):21524, 1997.
Currey TS, Dowdey JE, Murphy RC Christensen's Physics of Diagnostic Radiology. Lea & Febiger, Philadelphia, p. 134, 1990.
Borg E, Grondahl HG, On the dynamic range of different X-ray photon detectors in intraoral radiography. A comparison of image quality in film, charge coupled device and storage phosphor systems. Dentomaxillofac Radiol 25(2):82-8.
Emmott LF, Digital Radiography. Dentistry Today 18(5): 10212, 1999.