Figure 1
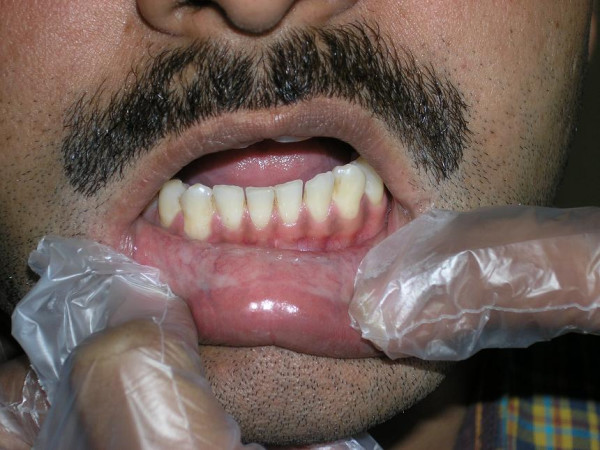
Clinical picture of a patient with oral submucous fibrosis of lower lip
| Post-Test |
Dentists now have an easy painfree way to detect oral cancer at its earliest stages. Cytobrush biopsy, is a technique which underwent clinical trials at the University of Philadelphia//School of Dental Medicine that showed it to be a significant advance over previous cytology (PAP smear type) tests.
According to Martin S. Greenberg, professor and chairman or oral medicine early detection is our most important weapon in our fight against oral cancer. The survival rate for this prevalent cancer is only 40 per cent overall, but survival rates increase to greater than 80 per cent if the cancer is found early. With the engineers and cytologists at Oral Scan Systems, a New York-based health devices company, a dentist who finds an area of concern runs a small round brush - similar to a mascara wand over the suspicious lesion.
“The bristles are like those on a toothbrush,” Greenberg said. “They can penetrate and get a better sampling of cells than the old scraping technique.” The sample is sent to a lab where it is scanned using advanced computer technology. Suspicious slides are tagged for further evaluation by a technician. The computer is so exacting, Green berg said, that the false negative rate, which was as high as 30 per cent using the scraping method, dropped to nil in the clinical trials.
The high mortality rate from oral cancer is due to several factors, but undoubtedly, the most significant is delayed diagnosis. Studies have demonstrated that the survival and cure rate dramatically increase when oral cancer is detected in its precancerous stage or as an early stage disease. For example, the 5-year survival for patients with localized disease approximates 80% compared to 20% for those with distant metastases. Unfortunately, approximately two thirds of patients at time of diagnosis are symptomatic, and over 50% display evidence of spread to regional lymph nodes and metastases. Given the significant morbidity and mortality associated with advanced oral cancer and its treatment, the need to provide clinicians with an accurate diagnostic technique that will increase the detection of early stage oral cancer has been compelling.
Advances in the early detection of oral cancer are unfolding and analogous to those made in the advances for cervical cancer. In the early 1950's, cervical cancer was the second leading cause of cancer deaths in American women. The disease was diagnosed by biopsy, which was usually triggered by advanced symptoms such as persistent spotting and bleeding. Cervical cancer, like oral cancer, is highly curable when detected at an early dysplastic stage and highly morbid when detected at a late, symptomatic stage. By the late 1960's, cervical cancer dropped to the seventh leading cause of cancer deaths in American women. The difference was due to the America female population becoming informed about cervical cancer and the opportunity for early detection, the compliance of the GYN professional community to offer screenings that were well done, and the widespread utilization of brush cytology, specifically, the cervical Papanicolau (PAP) smear. These changes have resulted in a reduction of cervical cancer deaths in the United States by 74%.
Brush cytology has the potential to assist the diagnostic portion of the "screening gap" which currently challenges the early detection of many epithelial cancers, including oral cancer. It is only one component and we will still need an informed pubic, and a compliant group of dental and medical professionals who are knowledgeable about proper screening techniques. Brush cytology can be a noninvasive means of diagnosing dysplasia and early carcinoma in those patients who are either asymptomatic or in those with minor symptoms who do not warrant immediate biopsy. The mechanism of cytology, regardless of its application to cervical, bladder or oral mucosal lining, is based upon the fact that dysplastic and cancerous cells tend to have fewer and weaker connections to each other and to their neighboring normal cells in the surrounding tissue. Dysplastic and cancerous cells therefore, tend to "slough off" or exfoliate preferentially and can easily be collected from the surface of the lesion. A sample of these cells applied to a microscope slide will often contain abnormalities if harvested from a dysplastic or cancerous lesion.
The success of the Pap smear was a primary impetus for a number of studies conducted in the mid1960's examining the sensitivity and specificity of oral exfoliative cytology. Exfoliative cytology was thought of as a technique that could facilitate and accelerate clinical and histopathologic recognition of oral cancer. The use of oral cytology for large, advanced and obviously malignant lesions is not indicated, since such growths always require a definitive biopsy-obtained diagnosis. In contrast, the value of cytology lies in the identification of early stage oral cancers and dysplasias whose clinical appearance is often innocuous or trivial. The use of oral cytology can be a means to accelerate biopsy of these clinically harmless-appearing cancers that would have otherwise been neglected.
Despite the initial high degree of interest and research, traditional, manual oral cytology currently is not utilized as a method of diagnosing oral precancers and cancers. Although many authors initially concluded from their data that oral cytology could serve as a useful adjunct to biopsy, the current general perception among both dentists and physicians is that the sensitivity of oral cytology is not sufficient to warrant its widespread use as a screening modality to triage visible lesions.
There are several reasons why traditional oral cytology has proven to be of little value in detecting oral dysplasias and cancers. When cytologic instruments are used in the mouth to diagnose precancers and cancers, their accuracy is relatively low. Specifically, exfoliative cytology performed on oral cancers has high false negative rates, which can exceed 30%. Furthermore, the effectiveness of exfoliative cytology for detecting dysplasia is even more doubtful, with false negative rates reported as high as 63%.
These poor results are due, in part, to the fact that cytology instruments do not sample the deepest layers of the oral lesion. This is essential, since unlike cervical cancer, the deepest layer of the lesion, the basal cell layer, is often the only layer that contains abnormal cells within an oral precancerous or cancerous lesion. Furthermore, since the sensitivity of cytology is often dependent on a tedious visual search for a potentially rare abnormality on the microscope slide, the precancerous or cancerous cells collected on the slide may not have been detected by the laboratory pathologist. This results from the fact that although abnormal cells may preferentially exfoliate from a dysplastic lesion, they are often vastly outnumbered on the microscope slide by the enormous numbers of normal cells that exfoliate due to constant turnover. For example, a cervical Pap smear from a patient with carcinoma-in-situ may contain 300,000 normal cells with only a dozen abnormal cells scattered among them. Several recent studies have demonstrated that women who develop advanced cervical cancer despite a history of "negative" Pap smears often experienced one or more false negative smears determined as such because they contained very few abnormal cells. In oral cytology, this false negative dilemma is exacerbated by two additional factors: 1) the number of abnormal cells available for sampling may be limited by the keratinized nature of many oral lesions and, 2) the high rate of epithelial turnover in the mouth may further numerically dilute the few abnormal cells obtained in the smear.
For cervical cancer, the false negative problem inherent in cytological screening has not deterred the use of the Pap smear, but has resulted in new recommendations from the CDC that HPV testing be done along with the pap test, as an infection with an oncogenic version of the human papilloma virus is a mandatory precursor event to development of the cervical cancer.
The oral brush biopsy was introduced to the dental profession in 1999, overcoming the limitations of traditional oral cytology. This biopsy method utilizes an improved brush to obtain a complete transepithelial biopsy specimen with cellular representation from each of the three layers of the lesion: the basal, intermediate, and superficial layers. Unlike previous cytology instruments, which collect only exfoliated superficial cells, when used properly and rubbed against a an area of suspect tissue aggressively (to the point of minor bleeding) the biopsy brush penetrates to the basement membrane, removing tissue from all three epithelial layers of the oral mucosa. The oral brush biopsy does not require topical or local anesthetic and causes minimal bleeding and pain. The brush biopsy instrument has two cutting surfaces, the flat end of the brush and the circular border of the brush. Either surface may be used to obtain the specimen. In a recent study, paired, same-site samples of tongue tissue were obtained from patients, first by brush biopsy and then by surgical punch biopsy. The study demonstrated that the brush biopsy technique, unlike cytology, sampled the full thickness of oral epithelium.
Brush biopsies are utilized routinely in the detection of precancer and cancer in other organ systems. Examples of well-known applications of brush biopsies include fiberoptic bronchoscopy (bronchial), ureteral retrograde brush biopsy (renal or ureter tissue), cholangiography (bile duct stricture), pancreatic ductal brush biopsies and others, including endometrial, nasopharynx, and GI tract applications (rectal, gastric, esophageal, colon). Their use in the oral cavity was introduced by a commercial company OralCDx in 2000.
The improved accuracy of the OralCDx brush biopsy over traditional manual cytology is due, in part, to the fact that the entire thickness of the lesion is sampled- cells from all layers are collected. Furthermore, the analysis of the specimens is aided with a highly specialized neural network-based image processing system specifically designed to detect oral precancerous and cancerous cells, detecting as few as one or two abnormal cells scattered among tens of thousands of normal cells a the company's pathology facility. Without the computer-assisted analysis, the abnormal cells are often overlooked with just manual inspection. The patented brush biopsy tool, which samples all of the layers of the lesion, together with analysis of oral brush biopsies assisted with sophisticated computers make the OralCDx test highly accurate. In two independent studies, the first published in the Journal of the American Dental Association, and the second, at a prestigious German university, OralCDx was shown to have a sensitivity of greater than 95% and a specificity over 90%.
One major problem inherent in current oral cancer screening is the US, is that by visual examination alone, precancers and early stage oral cancers are often inadequately identified, and if professionals are not actively engaged in a program of opportunistic visual and tactile screening of their patient populations, lesions may easily be overlooked and neglected. This issue is not resolved by a diagnosis tool like the CDx brush. It is well established that oral lesions which appear innocuous may occasionally harbor dysplasia or cancer and that a delay in diagnosis may limit treatment options, ultimately resulting in a poor prognosis for the patient.
The results of brush biopsy studies demonstrate that the tool can be reliably utilized on oral lesions as a method of confirming their benign nature and more importantly, revealing those that are precancerous and cancerous when they are not clinically suspected. The role for OralCDx is to help determine the true nature of lesions which would not otherwise receive any further testing, i.e. lesions which are not judged to be sufficiently suspicious on visual inspection to be referred for immediate biopsy. It is a method of identifying unsuspected oral cancers found during a visual examination, at early and curable stages, if the professional practitioner is engaged in routine opportunistic screening by means (visual and tactile) that would reveal suspect areas to be sampled.
The brush biopsy provides dentists with a diagnostic screening test similar to a Pap smear. Whereas the Pap smear is a procedure performed on all women and a brush biopsy is used only in patients with a visible mucosal spot, both tests are adjuncts to the clinical examination and are used to identify a disease at an early and curable stage, both are simple to perform, office-based, painless tests; and both procedures can be integrated into the daily routine of practice. Given the difficulty in clinically differentiating premalignant and early malignant oral lesions from those that are benign, the brush biopsy allows practitioners to test lesions that are encountered daily. When a positive result is returned by the OralCDx laboratory, conventional, gold standard incisional or punch biopsy must be performed.
Early detection of a premalignant or cancerous oral lesion promises to improve the survival and the morbidity of patients suffering from these conditions. Cytological study of oral cells is a nonaggressive technique that is well accepted by the patient, and is therefore an attractive option for the early diagnosis of oral cancer, including epithelial atypia and squamous cell carcinoma. However its usage has been limited so far due to poor sensitivity and specificity in diagnosing oral malignancies. Lately it has re-emerged due to improved methods and it's application in oral precancer and cancer as a diagnostic and predictive method as well as for monitoring patients. Newer diagnostic techniques such as "brush biopsy" and molecular studies have been developed. Recent advances in cytological techniques and novel aspects of applications of scraped or exfoliative cytology for detecting these lesions and predicting their progression or recurrence are reviewed here.
Oral cancer is the most common cancer and constitutes a major health problem in developing countries, representing the leading cause of death. Although representing 2–4% of the malignancies in the West, this carcinoma accounts for almost 40% of all cancers in the Indian subcontinent [1]. A key factor in the lack of improvement in prognosis over the years is the fact that a significant proportion of oral squamous cell carcinoma (OSCC) are not diagnosed or treated until they reach an advanced stage. This diagnostic delay may be caused by either patients (who may not report unusual oral features) or by health care workers (who may not investigate observed lesions thoroughly) and it is presumed that such delays are longer for asymptomatic lesions. The prognosis for patients with OSCC that is treated early is much better, with 5-year survival rates as high as 80%. In addition, the quality of life improves after early treatment, because cure can be achieved with less complex and less aggressive treatment than is necessary for advanced lesions. A significant proportion of oral squamous cell carcinomas (OSCC) develop from premalignant lesions such as leukoplakia and oral submucous fibrosis (Fig. 1). Adjuncts for detection of lesions and selection of biopsy sites include vital tissue staining (with Toluidine blue Fig. 2) and exfoliative cytology. Unfortunately, sensitivity of cytological diagnosis in a meta-analysis of 1306 cases from 14 studies showed an average of only 87.4% (ranging from 73.8 to 100%)[2]. Histological examination of tissue remains the gold standard for diagnosis and identification of malignant oral Application of cytology and molecular biology in diagnosing premalignant or malignant oral lesions lesions. Biopsy is an invasive technique with surgical implications, technique limitations forprofessionals and psychological implications for most patients. It also presents limitations when the lesions are large and in these cases it is important to select the most appropriate site of biopsy. Furthermore, even though the biopsy study is fundamental, it is a diagnostic method with limitedsensitivity where one of the most important features is the subjective interpretation of the examining pathologist. These issues underline the importance of discovering and developing new diagnostic methods, improving the existing ones and discovering new therapeutics targets for oral neoplastic diseases [3-6]. In recent decades, we have seen a dramatic switch from histopathological to molecular methods of disease diagnosis and exfoliative cytology has gained importance as a rapid and simple method for obtaining DNA samples. Changes occur at the molecular level before they are seen under the microscope and before clinical changes occur. Identification of high-risk oral premalignant lesions and intervention at premalignant stages could constitute one of the keys to reducing the mortality, morbidity and cost of treatment associated with OSCC. In addition, certain patients are known to be at high risk for head and neck cancer, specifically those who use tobacco or alcohol and those over 45 years of age. Such patients can be screened by physical examination, and early-stage disease, if detected, is curable. Just as visual inspection of the uterine cervix has been shown to be an unreliable means of identifying precancer and cancer, clinical inspection of the oral cavity has been shown to be equally unreliable in identifying precursor lesions and early cancers. [7,8]. In a recent study of 647 lesions interpreted by academicians to be innocuous on clinical inspection, 29 (4.5%) were confirmed to be dysplasia or carcinoma [9].
|
|
|
Figure 1 |
|
Clinical picture of a patient with oral submucous fibrosis of lower lip |
|
|
|
Figure 2 |
|
Clinical picture of a patient with dysplasia of lower lip showing positive toluidine blue staining. |
Oral cells can be obtained by different physical systems of scraping the surface of the mucosa, by rinsing the oral cavity or even by taking a sample of saliva from the patients. The reliability of the different instruments used in oral exfoliative cytology has been reviewed in different studies [10,11]. The ideal instrument used for making a good cytological smear should be easy to use in any location, cause minimum trauma and provide an adequate and representative number of epithelial cells [11]. It has been shown that a brush is an adequate instrument due to its ease in sampling and to the quality of the oral cytologic sample (Fig. 3). Brush biopsy is a simple, relatively inexpensive, high sensitive, risk-free method of screening for cancer and serves as an aid to the clinical examination (Fig. 6, 7, 8). The improved accuracy is attributed to the ease in obtaining full transepithelial cellular samples and the evaluation of smears with an image analysis system that has been adapted specifically to detect oral epithelial abnormalities by some workers [12]. Full-thickness sampling (indicated by pinpoint bleeding during procedure Fig. 4) is essential if histomorphological, evaluation of the collected cells is to yield representative findings. For example, many dysplastic lesions are first identified in the basal epithelial layers, and the diagnostic histomorphological findings may be lost as the cells mature and parakeratin and keratin are produced (Fig. 5). To the classical applications of the oral cytologic studies, such as oral candidiasis, others have been added, such as studying the epithelial infection due to Epstein-Barr virus in oral lesions of hairy leukoplakia, widening its possibilities [13]. The importance of brush biopsy has been recently emphasized in a multicenter study where nearly 5% of clinically benignappearing mucosal lesions were sampled by this technique and later confirmed by typical scalpel biopsy to represent dysplastic epithelial changes or invasive cancer. [18] Other authors have also demonstrated the ability of the brush biopsy to uncover similar type lesions that were not clinically suspicious for carcinoma or preinvasive disease [14]. There are controversies related to the real value of this technique in the early detection of OSCC. The existence of false positives has been pointed out showing high sensitivity (90%) and low specificity (3%) [15]. Nevertheless, these data have been discussed previously [16]. In a recent study by Potter et al., four false negatives of a total 115 analysed cases were found. Although the number of false positive cases is small it is important to emphasize that the mean delay time in diagnosing a carcinoma in these cases was of 117.25 days [17]. However, more independent studies analysing its true validity and reliability as well as its applicability and its comparison with other techniques are necessary. Multiple studies with different results have been carried out, analysing the application of the cytology in the detection of dysplastic lesions. In a study from Sudan, oral scrape smear cytological analysis has been proposed as a useful early diagnostic method for epithelial atypia and therefore also for malignant oral lesions [18]. Despite the improvements in the methods used for collecting oral cytological material this methodology still presents problems in diagnosing oral cancer. Problems are mainly due to the existence of false negatives obtained as a result of a non representative sample as well as the subjectivity of the cytologic evaluation [19].
|
|
|
Figure 3 |
|
Technique of brush biopsy emphasizing pin-point bleeding of the oral mucosa. |
|
|
|
Figure 4 |
|
Picture demonstrating spreading of brush biopsy sample on a slide. |
|
|
|
Figure 5 |
|
Photomicrograph of a brush biopsy specimen from oral submucousfibrosis showing Photomicrograph of a brush biopsy specimen from oral submucousfibrosis showing anucleated hyperkeratinized cells and a superficial squamous cell. (H & E × 400). |
|
|
|
Figure 6 |
|
Photomicrograph of a oral brush biopsy specimen from a patient of squamous cell carcinoma buccal mucosa showing a binucleated cell with evidence of intracellular and extracellular keratinization in a inflammatory background. (H & E × 400). (more...) |
|
|
|
Figure 7 |
|
Photomicrograph of a oral brush biopsy specimen from a patient of squamous cell carcinoma of buccal mucosa with high nucleo-cytoplasmic ratio marked atypia, and coarsely granular chromatin in a necrotic background .(Modified pap × 1000). |
|
|
|
Figure 8 |
|
Photomicrograph of a oral brush biopsy specimen from a patient of squamous cell carcinoma of buccal mucosa with high nucleo-cytoplasmic ratio coarsely granular chromatin and a multinucleated cell showing evidence of vascular invasion. (H&E × 1000). |
Since liquid-based cytology was developed in the 1990s various comparative studies have shown that it can offer significant advantages over conventional exfoliative cytology. Results obtained from uterine cervix examination, for example, have shown that the liquid-based preparations reduce the problems related to sampling error, poor transfer and fixation of the cellular sample [20-24]. In cervical uterine cancer screening, the liquid-based preparations have also demonstrated a significant reduction in false-negative rates as compared with those of conventional smears [20-23,25]. In a recent study from Brazil [26] the liquid-based preparations resulted in higher specimen resolution as well as presenting a better cytological morphology for pemphigus vulgaris, squamous cell carcinomas, HSV lesions and fungus infections. For HSV lesions, in particular, the observation of the cytopathological features indicative of viral infections (binucleation, multinucleated cells) greatly improved with the liquid-based technique [26].
Radiotherapy is frequently used as a standard treatment for locally advanced carcinoma of oral cavity. Although the response of malignant tumours and surrounding normal tissue to various doses of ionizing radiation is generally predictable, variability in the host-tumour reaction in a specific individual makes the response unpredictable. The cytological evaluation of sequential oral smears during radiation therapy presents a unique opportunity to study the radiation response of oral malignant tumours. Earlier reports have described various cytoplasmic and nuclear changes in a variety of malignant cells evaluated by cytology after radiation therapy and included cellular enlargement, vacuolization, cytoplasmic granulation, nuclear enlargement, pyknosis, karyorrhexis, karyolysis, multinucleation, micronucleation, nuclear budding and binucleation (Fig. 9, 10). Later on micronucleation was accepted as a reliable indicator for monitoring the effectiveness of chemopreventive agents against cancer and for monitoring the toxicity of chemicals. In a study by the author comparing the post-radiation changes in normal and malignant oral cells it was found that various morphological abnormalities demonstrated a consistent significant increase with radiation dose [27].
|
|
|
Figure 9 |
|
Photomicrograph of malignant cells after radiation therapy showing multinucleation and micronucleation. (H&E × 1000). |
|
|
|
Figure 10 |
|
Photomicrograph of malignant cells after radiation therapy showing multiple nuclear budding. (H&E × 1000). |
In the smears of patients treated for OSCC, the percentage of apoptotic cells has been studied [28]. This detection can also be quite useful for monitoring patients' reaction to chemotherapy.
Ogden et al. [29] suggested that quantitative techniques, based on the evaluation of parameters such as nuclear area (NA), cytoplasmic area (CA), and nucleus-to-cytoplasm area ratio (NA/CA), may increase the sensitivity of exfoliative cytology for early diagnosis of oral cancers, since these techniques are precise, objective and reproducible. Cowpe et al. [30] demonstrated that exfoliative cytology is capable of detecting malignant changes, through estimation of NA/CA using the planimeter method in Papanicolaou-stained smears. This study, published in 1985, concluded that 50 cells were sufficient to provide indication of malignant changes. Since then, a number of studies have been carried out using the technique described by these authors to evaluate the influence of diverse systemic and external factors on NA, CA and NA/CA. In these studies planimeters have been replaced by semiautomatic image analysis techniques, which are faster, more accurate and more reproducible [31,32]. Cowpe et al. [33] found that tissues undergoing malignant transformation typically show a reduction in CA before the reduction in NA. They also suggested that samples of healthy mucosa from the same patient provide the best control. Ramaesh et al. [34] used cytomorphometric techniques to assess nuclear diameter (ND) and cytoplasmic diameter (CD) in normal oral mucosa, in dysplastic lesions and in squamous cell carcinomas. They found that CD was highest in normal mucosa, lower in dysplastic lesions, and lowest in SCCs. By contrast, ND was lowest in normal mucosa, higher in dysplastic lesions, and highest in SCCs. These studies suggested that reduced nuclear size and increased cytoplasm size are useful early indicators of malignant transformation, and thus exfoliative cytology is of value for monitoring clinically suspect lesions and for early detection of malignancy.
Static cytometry permits the quantification of DNA content in cells obtained by exfoliative cytology. However, routine Haematoxylin-Eosin staining is inadequate for this purpose, and special techniques are required to ensure that staining intensity is in proportion to DNA content. The Feulgen reaction meets this criterion, since it is a stoichiometric procedure: in other words, each fixed molecule of Schiff's reagent corresponds to a constant and equivalent portion of the DNA molecule. The advantage of this procedure is that staining intensities (and thus DNA contents) can be determined automatically by spectrophotometry or densitometry as well as digital image analysis [35].
Using cytology and DNA-image cytometry, it is easy to prove that oral lesions with the diagnosis of lichen planus and other inflammatory diseases show no suspicious cells. A recent review of literature places the rate of malignant transformation of lichen planus to squamous cell carcinoma at 0.2% [36]. On the contrary, the presence of malignant cells was demonstrated in one of 21 cases with leukoplakia (4.76%), in all cases with erythroplakia and in all squamous cell carcinomas. A meta-analysis of 2236 cases of leukoplakia from five studies has revealed a range of malignant transformation of leukoplakia between 2.2 and 17.5%. Furthermore, Sciubba [37], Silverman et al. [38] and Mashberg et al. [39] emphasized the fact that erythroplakia, occurring as either an isolated lesion or as a component of leukoplakia (erythroleukoplakia) is a marker of severe epithelial dysplasia or carcinoma in situ. In fact, 90% of erythroplakia were histologically diagnosed as in situ or invasive carcinomas. In one study, it was shown that sensitivity of cytological diagnosis combined with DNA-image cytometry may reach 100%, whereas specificity was 97.4%. The authors reported a case of erythroplakia in which intraobserver variability among four pathologists led to diagnoses ranging from mild to severe dysplasia and because of the cytological and DNA cytometric diagnosis (severe dysplasia with DNA aneuploidy), this case was finally diagnosed on early cytological and DNA-cytometric diagnosis prior to the histological diagnosis [40]. Remmerbach et al have reported that sensitivity of cytological diagnosis combined with DNAimage cytometry was 98.2% and specificity 100%, when compared with the gold standard' of histology [41]. In a study, Maraki et al. analyzed 150 patients with histologically proven epithelial dysplasia of which 36 developed squamous cell carcinoma. DNA-cytometry showed DNA-diploidy in 105 patients. 20 patients had DNA-polyploidy and in 25 patients DNA-aneuploidy was found at the time of the initial diagnosis. Carcinoma developed in only three of the 105 diploid lesions when compared with 21 of the 25 aneuploid lesions. Remmerbach et al. [42] concluded in the clinical setting that DNA-aneuploidy might detect histologically obvious malignancy, 1–15 months prior to histology. Sudbo et al. analyzed archival material and reported that the nuclear DNA-content in cells of oral leukoplakia may be used to predict the risk of oral epithelial dysplasia up to 5 years before histological diagnosis [43]. Based on these observations, they proposed brush biopsies with cytological/DNA-cytometric examination for microscopic evaluation of white or red patches of the oral cavity (leukoplakia or erythroplakia). The finding of tumor cells or DNA-aneuploidy should lead to a total excision of the respective lesions and histological examination.
While the classic oral cytologic evaluation is labour intensive and requires a high degree of expertise for identifying and evaluating cells with suspicious morphology the analysis of molecular alterations is objective and tries to identify specific genetic anomalies [6]. The possibility of extracting RNA from cells obtained by scraping has recently been demonstrated emphasizing its usefulness in the early diagnosis of oral premalignant and cancerous lesions [44].
Nowadays malignancy is considered as a process caused by the accumulation of multiple genetic alterations, which affect the cell cycle as well as normal cell differentiation. These alterations are mainly acquired (somatic) although some of them may be inherited and when they activate protooncogenes, inactivate tumour suppressor genes or affect enzymes, which repair DNA, they protooncogenes, inactivate tumour suppressor genes or affect enzymes, which repair DNA, they could lead to a malignant transformation. Most of the oral cavity carcinogens are chemical (tobacco), physical (radiation) and infectious (Human papilloma virus, Candida) mutagenic agents that may cause changes in gene and chromosome structure by point mutations, deletions, insertions and rearrangements. However, some of these changes may occur spontaneously. These genetic alterations, which occur during carcinogenesis, can be used as targets for detecting tumour cells in clinical samples [4,6,45]. Molecular analysis can identify a clonal population of cancerous cells. Mutations in the tumour suppressor gene p53 are the most frequent genetic alterations in human cancer and show a variable frequency in oral cancer [46]. Several authors have studied and in some cases demonstrated the potential clinical application of oral cytology for detecting point mutations in p53 as a specific neoplastic marker in OSCC [45,47-49]. However, other authors consider that the high number of point mutations, which can be found in p53, limit its potential clinical application in cost-effective early detection of oral cancer [50].
The applicability of other molecular markers such as epigenetic alterations (hypermethylation of promoter regions) and genomic instability such as loss of hetrozygosity (LOH) and microsatellite instability (MSI) has also been studied. [50,51]. The main epigenetic modification in tumours is methylation and it seems that the changes in the methylation patterns can play an important role in tumorigenesis. These epigenetic alterations are often associated with the loss of genetic expression and their occurrence seems to be essential for the multiple necessary genetic events. So malignant progression takes place because these alterations can inactivate DNA repairing genes. Rosas et al. studied the methylation patterns of p16, MGMT and DAP-K genes in smears of patients suffering from head and neck cancer [50]. They detected abnormal hypermethylation patterns in both kinds of samples by a methylation specific Polymerase Chain Reaction (PCR). They proposed that this technique allows a sensitive and efficient detection of tumoral DNA and it is potentially useful for detecting and monitoring recurrences in these patients. Loss of heterogeneity (LOH) and other molecular changes indicative of oral carcinogenesis can be readily identified in exfoliated cells [52-54]. Huang et al. [55] used PCR techniques to amplify DNA from exfoliated cytology samples from oral carcinomas, for analysis of Restriction-Fragment Length Polymorphisms (RFLPs). They found that 66% of the tumours studied showed LOH at one position in the p53 sequence, while 55% showed LOH at some other location. PCR and RFLP analysis have also been used for the detection of microsatellite markers, i.e. short repetitive DNA sequences. Microsatellite mutations, LOH or instability (MI) are all characteristic of the squamous cell carcinomas of head and neck, and can thus be used as molecular markers of malignancy. Microsatellite regions are distributed along the genome and have been widely and satisfactorily used as molecular markers for carcinogenesis. Alterations in these regions have been used as clonal markers and for detecting tumoral cells among normal cells [56,57]. Several studies have demonstrated these by using microsatellite markers that alterations in certain regions of chromosomes 3p, 9p, 17p and 18q are associated with the development of head and neck squamous cell carcinomas [58,59]. Nunes et al.[60] performed a microsatellite analysis of cells sampled from the oral cavity of oral and oropharyngeal cancer patients by exfoliative cytology and by mouthwash, finding LOH in 84% of samples, though with differences depending on tumour stage. These authors suggested that techniques of this type might be useful for early diagnosis and for patient monitoring. In another study, Spafford et al. identified genetic alterations (LOH or MI) in all of the malignant lesions of the oral cavity included in their sample. [6] Conversely, none of their healthy patients showed such alterations, indicating the very high specificity of these methods.
Archival cytology slides can also be used for HPV DNA detection with ISH. The diagnosis of metastatic lesions usually is determined by fine-needle aspiration. Human papillomavirus (HPV) is now being considered as a causative agent in a subset of HNSCC (FF). Presence of HPV DNA by in situ hybridization (ISH) in metastatic lesions from HNSCC using alcohol-fixed, archival,cytopathological material; was studied and the cytologic features of HPV-positive metastatic lesions of HNSCC were characterized; and HPV DNA and the origin of metastatic lesions was correlated [61]
Ki 67 has been studied in oral cytological smears using Immunocytochemistry to evaluate the nature of lesion and response to treatment. Sharma et al, evaluated Ki-67 expression in cytologic scrapes from oral squamous cell carcinoma before and after 24 Gray radiotherapy in 43 patients. Ki-67 expression was seen in an extremely small number of cells. Only 10 tumours showed positive cells, and the labeling index in them varied from 0.1 % to 0.01 %. After 24 Gray irradiation, no case showed Ki-67 positive cells[62]. The validity of oral cytology for analyzing the number of keratinised cells and the nucleolar activity (AgNORs) in smoking patients has recently been demonstrated [63]. Remmerbach reported on AgNOR analysis in oral cytology and concluded that this may be used as a routine method for diagnosing oral cancer [64].
The identification of tumoral markers, notably cytokeratins in smears from the oral cavity has attracted considerable interest. Cytokeratin expression profile provides useful information on cell differentiation status [64] but its potential for early diagnosis of oral cancer is limited [66]. However, certain cytokeratins, such as K8 and K19 are useful if not definitive indicators of malignancy, particularly if their presence is interpreted in conjunction with other information, such as DNA profile [67,68].
Oral cytology is becoming increasingly important in the early diagnosis of oral cancers, as a procedure for obtaining cell samples that can then be analysed by sophisticated diagnostic techniques such as cytomorphometry, DNA cytometry, and molecular analyses. The advent of techniques like Toluidine blue staining, brush biopsy and application of sophisticated computer programs has changed the scenario and made the interpretation of findings far more reliable. than earlier. The cytological study of oral cavity cells is simple and rapid, non-aggressive and relatively painless: it is thus well accepted by patients and suitable for routine application in population screening programmes, for early analysis of suspect lesions, and for pre-and post-treatment monitoring of confirmed malignant lesions
Courtesy of the Oral Cancer Foundation