1.
Understanding the Immune System
|
®
Introduction ®
Self and Nonself ®
Genes and the Markers of Self ®
The Anatomy of the Immune System ®
T Cells and Lymphokines ®
The Cells and Secretions of the Immune System ®
Lymphocytes ®
B-Cells and Antibodies ®
Natural Killer Cells ®
Phagocytes, Granulocytes, and Their Relatives ®
Complement ®
Mounting an Immune Response ®
A Billion Antibodies ®
A Web of Idiotypes ®
Receptors for Recognizing Antigen ®
Immunity, Natural and Acquired ®
Vaccines Through Biotechnology ®
Disorders of the Immune Systems: Allergy ®
Autoimmune Diseases ®
Immune Complex Diseases ®
Immunodeficiency Diseases ®
Cancers of the Immune Systems ®
Bone Marrow Transplants ®
Immunology and Transplants ®
Privileged Immunity ®
Immunity and Cancer ®
The Immune System and the Nervous System ®
Frontiers in Immunology: Hybridoma Technology
|
v
Introduction
 The immune system is a complex
network of specialized cells and organs that has evolved to defend the body against attacks by
"foreign" invaders. When functioning properly it fights off infections by agents such as
bacteria, viruses, fungi, and parasites. When it malfunctions, however, it can unleash a torrent of
diseases, from allergy to arthritis to cancer to AIDS.
The immune system is a complex
network of specialized cells and organs that has evolved to defend the body against attacks by
"foreign" invaders. When functioning properly it fights off infections by agents such as
bacteria, viruses, fungi, and parasites. When it malfunctions, however, it can unleash a torrent of
diseases, from allergy to arthritis to cancer to AIDS.
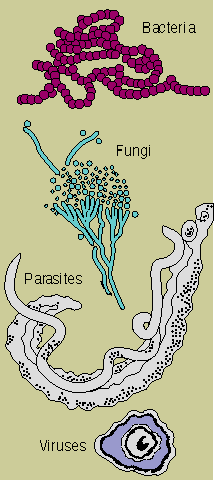 The
immune system evolved because we live in a sea of microbes. Like man, these organisms are programmed
to perpetuate themselves. The human body provides an ideal habitat for many of them and they try to
break in; because the presence of these organisms is often harmful, the body's immune system will
attempt to bar their entry or, failing that, to seek out and destroy them.
The
immune system evolved because we live in a sea of microbes. Like man, these organisms are programmed
to perpetuate themselves. The human body provides an ideal habitat for many of them and they try to
break in; because the presence of these organisms is often harmful, the body's immune system will
attempt to bar their entry or, failing that, to seek out and destroy them.
The immune system,
which equals in complexity the intricacies of the brain and nervous system, displays several
remarkable characteristics. It can distinguish between "self" and "nonself." It
is able to remember previous experiences and react accordingly; thus, once you have had chicken pox,
your immune system will prevent you from getting it again. The immune system displays both enormous
diversity and extraordinary specificity; not only is it able to recognize many millions of
distinctive nonself molecules, it can produce molecules and cells to match up with and counteract
each one of them. And it has at its command a sophisticated array of weapons.
The success of
this system in defending the body relies on an incredibly elaborate and dynamic regulatory
communications network. Millions and millions of cells, organized into sets and subsets, pass
information back and forth like clouds of bees swarming around a hive. The result is a sensitive
system of checks and balances that produces an immune response that is prompt, appropriate,
effective, and self-limiting.
v
Self and Nonself
At the heart of the immune system is the ability to distinguish between self and nonself.
Virtually every body cell carries distinctive molecules that identify it as self.
 The
body's immune defenses do not normally attack tissues that carry a self marker. Rather, immune cells
and other body cells coexist peaceably in a state known as self-tolerance. But when immune defenders
encounter cells or organisms carrying molecules that say "foreign," the immune troops move
quickly to eliminate the intruders.
The
body's immune defenses do not normally attack tissues that carry a self marker. Rather, immune cells
and other body cells coexist peaceably in a state known as self-tolerance. But when immune defenders
encounter cells or organisms carrying molecules that say "foreign," the immune troops move
quickly to eliminate the intruders.
Any substance capable of triggering an immune response is
called an antigen. An antigen can be a virus, a bacterium, a fungus, or a parasite, or even a
portion or product of one of these organisms. Tissues or cells from another individual, except an
identical twin whose cells carry identical self-markers, also act as antigens; because the immune
system recognizes transplanted tissues as foreign, it rejects them. The body will even reject
nourishing proteins unless they are first broken down by the digestive system into their primary,
non-antigenic building blocks.
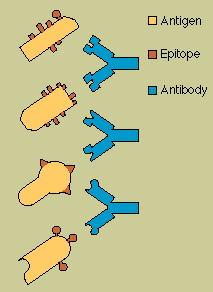
 An
antigen announces its foreignness by means of intricate and characteristic shapes called epitopes,
which protrude from its surface. Most antigens, even the simplest microbes, carry several different
kinds of epitopes on their surface; some may carry several hundred. However, some epitopes will be
more effective than others at stimulating an immune response.
An
antigen announces its foreignness by means of intricate and characteristic shapes called epitopes,
which protrude from its surface. Most antigens, even the simplest microbes, carry several different
kinds of epitopes on their surface; some may carry several hundred. However, some epitopes will be
more effective than others at stimulating an immune response.
In abnormal situations, the immune
system can wrongly identify self as nonself and execute a misdirected immune attack. The result can
be a so-called autoimmune disease such as rheumatoid arthritis or systemic lupus erythematosus.
In
some people, an apparently harmless substance such as ragweed pollen or cat hair can provoke the
immune system to set off the inappropriate and harmful response known as allergy; in these cases the
antigens are known as allergens.
v
Genes and the Markers of Self
Molecules that mark a cell as self are encoded by a group of genes that is contained in a section
of a specific chromosome known as the major histocompatibility complex (MHC). The prefix "histo"
means tissue; the MHC was discovered in the course of tissue transplantation experiments. Because
MHC genes and the molecules they encode vary widely in the details of their structure from one
individual to another (a diversity known as polymorphism), transplants are very likely to be
identified as foreign and rejected by the immune system.
Scientists eventually discovered a more
natural role for the MHC: it is essential to the immune defenses. MHC markers determine which
antigens an individual can respond to, and how strongly. Moreover, MHC markers allow immune cells
such as B cells, T cells, and macrophages to recognize and communicate with one another.
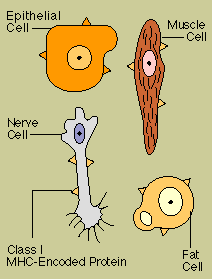
One group
of proteins encoded by the genes of the MHC are the markers of self that appear on almost all body
cells. Known as class I MHC antigens, these molecules alert killer T cells to the presence of body
cells that have been changed for the worse infected with a virus or transformed by cancer and that
need to be eliminated.
A second group of MHC proteins, class II antigens, are found on B cells,
macrophages, and other cells responsible for presenting foreign antigen to helper T cells. Class II
products combine with particles of foreign antigen in a way that showcases the antigen and captures
the attention of the helper T cell.
This focusing of T cell antigen recognition through class I
and class II molecules is known as MHC (or histocompatibility) restriction.
v
The Anatomy of the Immune System
The organs of the immune system are stationed throughout the body. They are generally referred to
as lymphoid organs because they are concerned with the growth, development, and deployment of
lymphocytes, the white cells that are the key operatives of the immune system. Lymphoid organs
include the bone marrow and the thymus, as well as lymph nodes, spleen, tonsils and adenoids, the
appendix, and clumps of lymphoid tissue in the small intestine known as Peyer's patches. The blood
and lymphatic vessels that carry lymphocytes to and from the other structures can also be considered
lymphoid organs.
Cells destined to become immune cells, like all other blood cells, are produced
in the bone marrow, the soft tissue in the hollow shafts of long bones. The descendants of some
so-called stem cells become lymphocytes, while others develop into a second major group of immune
cells typified by the large, cell and particle - devouring white cells known as phagocytes.
The
two major classes of lymphocytes are B cells and T cells. B cells complete their maturation in the
bone marrow. T cells, on the other hand, migrate to the thymus, a multilobed organ that lies high
behind the breastbone. There they multiply and mature into cells capable of producing immune
response-that is, they become immunocompetent. In a process referred to as T cell
"education," T cells in the thymus learn to distinguish self cells from nonself cells; T
cells that would react against self antigens are eliminated. Upon exiting the bone marrow and
thymus, some lymphocytes congregate in immune organs or lymph nodes. Others-both B and T
cells-travel widely and continuously throughout the body. They use the blood circulation as well as
a bodywide network of lymphatic vessels similar to blood vessels.
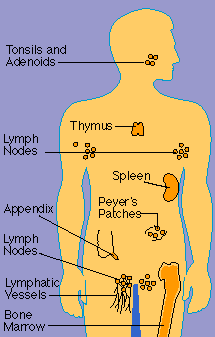
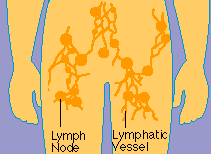
Laced
along the lymphatic routes with clusters in the neck, armpits, abdomen, and groin are small,
bean-shaped lymph nodes. Each lymph node contains specialized compartments that house platoons of B
lymphocytes, T lymphocytes, and other cells capable of enmeshing antigen and presenting it to T
cells. Thus, the lymph node brings together the several components needed to spark an immune
response.
The spleen, too, provides a meeting ground for immune defenses. A fist-sized organ at
the upper left of the abdomen, the spleen contains two main types of tissue: the red pulp that
disposes of worn-out blood cells and the white pulp that contains lymphoid tissue. Like the lymph
nodes, the spleen's lymphoid tissue is subdivided into compartments that specialize in different
kinds of immune cells. Microorganisms carried by the blood into the red pulp become trapped by the
immune cells known as macrophages. (Although people can live without a spleen, persons whose spleens
have been damaged by trauma or by disease such as sickle cell anemia, are highly susceptible to
infection; surgical removal of the spleen is especially dangerous for young children and the
immunosuppressed.)
Nonencapsulated clusters
of lymphoid tissue are found in many parts of the body. They are common around the mucous membranes
lining the respiratory and digestive tracts areas that serve as gateways to the body. They include
the tonsils and adenoids, the appendix, and Peyer's patches.
The lymphatic vessels carry lymph, a
clear fluid that bathes the body's tissues. Lymph, along with the many cells and particles it
carries notably lymphocytes, macrophages, and foreign antigens drains out of tissues and seeps
across the thin walls of tiny lymphatic vessels. The vessels transport the mix to lymph nodes, where
antigens can be filtered out and presented to immune cells. Additional lymphocytes reach the lymph
nodes (and other immune tissues) through the bloodstream. Each node is supplied by an artery and a
vein; lymphocytes enter the node by traversing the walls of the very small specialized veins.
All
lymphocytes exit lymph nodes in lymph via outgoing lymphatic vessels. Much as small creeks and
streams empty into larger rivers, the lymphatics feed into larger and larger channels. At the base
of the neck, large lymphatic vessels merge into the thoracic duct, which empties its contents into
the bloodstream.
Once in the bloodstream, the lymphocytes and other assorted immune cells are
transported to tissues throughout the body. They patrol everywhere for foreign antigens, then
gradually drift back into the lymphatic vessels, to begin the cycle all over again.
v
T Cells and Lymphokines
T cells contribute to the immune defenses in two major ways. Regulatory T cells are vital to
orchestrating the elaborate system. (B cells, for instance, cannot make antibody against most
substances without T cell help). Cytotoxic T cells, on the other hand, directly attack body cells
that are infected or malignant.
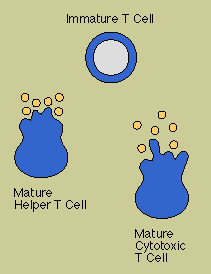
 Chief
among the regulatory T cells are "helper/inducer" cells. Typically identifiable by the T4
cell marker, helper T cells are essential for activating B cells and other T cells as well as
natural killer cells and macrophages. Another subset of T cells acts to turn off or
"suppress" these cells.
Chief
among the regulatory T cells are "helper/inducer" cells. Typically identifiable by the T4
cell marker, helper T cells are essential for activating B cells and other T cells as well as
natural killer cells and macrophages. Another subset of T cells acts to turn off or
"suppress" these cells.
Cytotoxic T cells, which usually carry the T8 marker,
are killer cells. In addition to ridding the body of cells that have been infected by viruses or
transformed by cancer, they are responsible for the rejection of tissue and organ grafts. (Although
suppressor/ cytotoxic T cells are often called T8 cells, in reality the two are not always
synonymous. The T8 molecule, like the T4 molecule, determines which MHC molecule class I or class
II-the T cell will recognize, but not how the T cell will behave.)
T cells work primarily by
secreting substances known as cytokines or, more specifically, lymphokines. Lymphokines (which are
also secreted by B cells) and their relatives, the monokines produced by monocytes and macrophages,
are diverse and potent chemical messengers. Binding to specific receptors on target cells,
lymphokines call into play many other cells and substances, including the elements of the
inflammatory response. They encourage cell growth, promote cell activation, direct cellular traffic,
destroy target cells, and incite macrophages. A single cytokine may have many functions; conversely,
several different cytokines may be able to produce the same effect.
One of the first cytokines to
be discovered was interferon. Produced by T cells and macrophages (as well as by cells outside the
immune system), interferons are a family of proteins with antiviral properties. Interferon from
immune cells, known as immune interferon or gamma interferon, activates macrophages. Two other
cytokines, closely related to one another, are lymphotoxin (from lymphocytes) and tumor necrosis
factor (from macrophages). Both kill tumor cells; tumor necrosis factor (TNF) also inhibits
parasites and viruses.
 Many
cytokines are initially given descriptive names but, as their basic structure is identified, they
are renamed as "inter-leukins"-messengers between leukocytes, or white cells.
Interleukin-1, or IL-1, is a product of macrophages (and many other cells) that helps to activate B
cells and T cells. IL-2, originally known as T cell growth factor, or TCGF, is produced by
antigen-activated T cells and promotes the rapid growth or differentiation of mature T cells and B
cells. IL-3 is a T-cell derived member of the family of protein mediators known as
colony-stimulating factors (CSF); one of its many functions is to nurture the development of
immature precursor cells into a variety of mature blood cells. IL-4, IL-5, and IL-6 help B cells
grow and differentiate; IL-4 also affects T cells, macrophages, mast cells, and granulocytes.
Many
cytokines are initially given descriptive names but, as their basic structure is identified, they
are renamed as "inter-leukins"-messengers between leukocytes, or white cells.
Interleukin-1, or IL-1, is a product of macrophages (and many other cells) that helps to activate B
cells and T cells. IL-2, originally known as T cell growth factor, or TCGF, is produced by
antigen-activated T cells and promotes the rapid growth or differentiation of mature T cells and B
cells. IL-3 is a T-cell derived member of the family of protein mediators known as
colony-stimulating factors (CSF); one of its many functions is to nurture the development of
immature precursor cells into a variety of mature blood cells. IL-4, IL-5, and IL-6 help B cells
grow and differentiate; IL-4 also affects T cells, macrophages, mast cells, and granulocytes.
 A
number of cytokines, obtained in quantity through recombinant DNA technology (genetic engineering),
are now being used-alone, in combination, linked to toxins-in clinical trials for patients with
cancers, blood disorders, and immunodeficiency diseases (including AIDS), as well as people
receiving bone marrow transplants. Their versatility, however, makes it difficult to predict the
full range of their effects.
A
number of cytokines, obtained in quantity through recombinant DNA technology (genetic engineering),
are now being used-alone, in combination, linked to toxins-in clinical trials for patients with
cancers, blood disorders, and immunodeficiency diseases (including AIDS), as well as people
receiving bone marrow transplants. Their versatility, however, makes it difficult to predict the
full range of their effects.
v
The Cells and Secretions of the Immune System
The immune system stockpiles a tremendous arsenal of cells. Some staff the general defenses,
while others are trained on highly specific targets. To work effectively, however, most immune cells
require the active cooperation of their fellows. Sometimes they communicate through direct physical
contact, sometimes by releasing versatile chemical messengers.
 In
order to have room for enough cells to match millions of possible foreign invaders, the immune
system stores just a few of each specificity. When an antigen appears, those few specifically
matched cells are stimulated to multiply into a full-scale army. Later, to prevent this army from
overexpanding wildly, like a cancer, powerful suppressor mechanisms come into play.
In
order to have room for enough cells to match millions of possible foreign invaders, the immune
system stores just a few of each specificity. When an antigen appears, those few specifically
matched cells are stimulated to multiply into a full-scale army. Later, to prevent this army from
overexpanding wildly, like a cancer, powerful suppressor mechanisms come into play.
v
Lymphocytes
 Lymphocytes are small white
blood cells that bear the major responsibility for carrying out the activities of the immune system;
they number about one trillion. The two major classes of lymphocytes are: B cells, which grow to
maturity independent of the thymus, and T cells, which are processed in the thymus. Both B cells and
T cells recognize specific antigen targets.
Lymphocytes are small white
blood cells that bear the major responsibility for carrying out the activities of the immune system;
they number about one trillion. The two major classes of lymphocytes are: B cells, which grow to
maturity independent of the thymus, and T cells, which are processed in the thymus. Both B cells and
T cells recognize specific antigen targets.
B cells work chiefly by secreting soluble substances
called antibodies into the body's fluids, or humors. (This is known as humoral immunity.) Antibodies
typically interact with circulating antigens such as bacteria and toxic molecules, but are unable to
penetrate living cells. T cells, in contrast, interact directly with their targets, attacking body
cells that have been commandeered by viruses or warped malignancy. (This is cellular immunity.)
Although
small lymphocytes look identical, even under the microscope, they can be told apart by means of
distinctive molecules they carry on their cell surface. Not only do such markers distinguish between
B cells and T cells, they distinguish among various subsets of cells that behave differently. Every
mature T cell, for instance, carries a marker known as T3 (or CD3); in addition, most helper T cells
carry a T4 (CD4) marker, a molecule that recognizes class II MHC antigens. A molecule known as T8
(CD8), which recognizes class I MHC antigens, is found on many suppressor/cytotoxic T cells. In
addition, different T cells have different kinds of antigen receptors-either alpha/beta or
gamma/delta.
v
B Cells and Antibodies
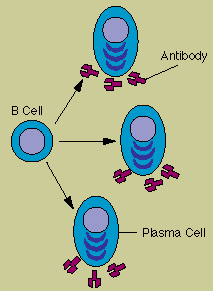 Each B cell is programmed
to make one specific antibody. For example, one B cell will make an antibody that blocks a virus
that causes the common cold, while another produces antibody that zeros in on a bacterium that
causes pneumonia.
Each B cell is programmed
to make one specific antibody. For example, one B cell will make an antibody that blocks a virus
that causes the common cold, while another produces antibody that zeros in on a bacterium that
causes pneumonia.
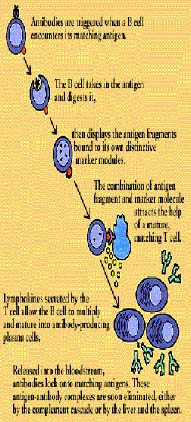
When a B cell encounters its triggering antigen (along with collaborating T
cells and accessory cells), it gives rise to many large plasma cells. Every plasma cell is
essentially a factory for producing antibody. Each of the plasma cells descended from a given B cell
(which are all members of the same family, or clone) manufactures millions of identical antibody
molecules and pours them into the bloodstream.
A given antibody matches an antigen much
as a key matches a lock. The fit varies: sometimes it is very precise, while at other times it is
little better than that of a skeleton key. To some degree, however, the antibody interlocks with the
antigen and thereby marks it for de-struction.
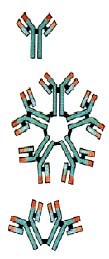
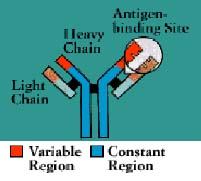
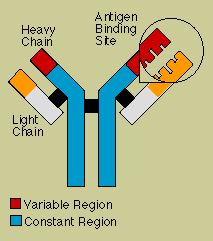 Antibodies
belong to a family of large molecules known as immunoglobulins. Immunoglobulins are proteins, made
up of chains of polypeptides, strings of the basic units known as amino acids. Each antibody has two
identical heavy polypeptide chains and two identical light chains, shaped to form a Y. The sections
that make up the tips of the Y's arms vary greatly from one antibody to another, creating a pocket
uniquely shaped to enfold a specific antigen. This is called the variable (V) region. The stem of
the Y serves to link the antibody to other participants in the immune defenses. This area is
identical in all antibodies of the same class, and is called the constant (C) region.
Antibodies
belong to a family of large molecules known as immunoglobulins. Immunoglobulins are proteins, made
up of chains of polypeptides, strings of the basic units known as amino acids. Each antibody has two
identical heavy polypeptide chains and two identical light chains, shaped to form a Y. The sections
that make up the tips of the Y's arms vary greatly from one antibody to another, creating a pocket
uniquely shaped to enfold a specific antigen. This is called the variable (V) region. The stem of
the Y serves to link the antibody to other participants in the immune defenses. This area is
identical in all antibodies of the same class, and is called the constant (C) region.
Scientists have identified nine chemically distinct classes of human immunoglobulins (Ig)-four
kinds of IgG and two kinds of IgA, plus IgM, IgE, and IgD.
Each type plays a different role in the
immune defense strategy. IgG, the major immunoglobulin in the blood, is also able to enter tissue
spaces; it works efficiently to coat microorganisms, speeding their uptake by other cells in the
immune system. IgM, which usually combines in star-shaped clusters, tends to remain in the
bloodstream, where it is very effective in killing bacteria. IgA concentrates in body fluids tears,
saliva, the secretions of the respiratory and gastrointestinal tracts guarding the entrances to the
body. IgE, which under normal circumstances occurs only in trace amounts, probably evolved as a
defense against parasites, but it is more familiar as the villain in allergic reactions. IgD is
almost exclusively found inserted into the membranes of B cells, where it somehow regulates the
cell's activation.
Antibodies can work in several ways, depending on the nature of the antigen.
Antibodies that interlock with toxins produced by certain bacteria can disable them directly (and
are known as antitoxins). Other antibodies, by coating (or opsonizing) bacteria, make the microbes
highly palatable to scavenger cells equipped to engulf and destroy them. More often an
antigen-antibody combination unleashes a group of lethal serum enzymes known as
complement. Yet other antibodies block viruses from entering into cells (a quality that is
exploited in making vaccines). And, in a phenomenon known as antibody-dependent cell-mediated
cytotoxicity (ADCC), cells coated with antibody become vulnerable to attack by several types of
white blood cells.
v
Natural Killer Cells
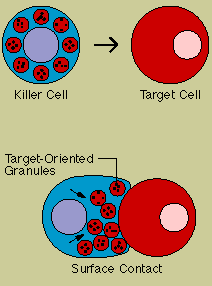 Natural killer (NK)
cells are yet another type of lethal lymphocyte. Like cytotoxic T cells, they contain granules
filled with potent chemicals. They are called "natural" killers because they, unlike
cytotoxic T cells, do not need to recognize a specific antigen before swinging into action. They
target tumor cells and protect against a wide variety of infectious microbes. In several
immunodeficiency diseases, including AIDS, natural killer cell function is abnormal. Natural killer
cells may also contribute to immuno-regulation by secreting high levels of influential lymphokines.
Natural killer (NK)
cells are yet another type of lethal lymphocyte. Like cytotoxic T cells, they contain granules
filled with potent chemicals. They are called "natural" killers because they, unlike
cytotoxic T cells, do not need to recognize a specific antigen before swinging into action. They
target tumor cells and protect against a wide variety of infectious microbes. In several
immunodeficiency diseases, including AIDS, natural killer cell function is abnormal. Natural killer
cells may also contribute to immuno-regulation by secreting high levels of influential lymphokines.
Both
cytotoxic T cells and natural killer cells kill on contact. The killer binds to its target, aims its
weapons, and then delivers a lethal burst of chemicals that produces holes in the target cell's
membrane. Fluids seep in and leak out, and the cell bursts.
v
Phagocytes, Granulocytes, and Their Relatives
Phagocytes (literally, "cell eaters") are large white cells that can engulf and digest
marauding microorganisms and other antigenic particles. Some phagocytes also have the ability to
present antigen to lymphocytes.
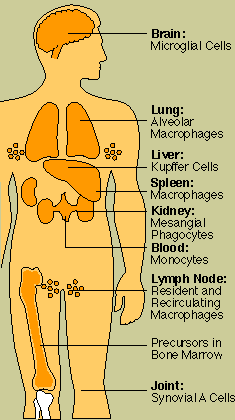
Important phagocytes are monocytes and macrophages. Monocytes
circulate in the blood, then migrate into tissues where they develop into macrophages ("big
eaters"). Macrophages are seeded throughout body tissues in a variety of guises. Specialized
macrophages include alveolar macrophages in the lungs, mesangial phagocytes in the kidneys,
microglial cells in the brain, and Kupffer cells in the liver.
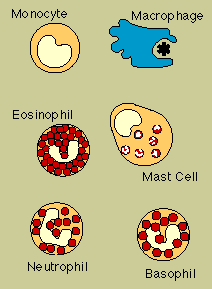 Macrophages
are versatile cells that play many roles. As scavengers, they rid the body of worn-out cells and
other debris. Foremost among the cells that "present" antigen to T cells, having first
digested and processed it, macrophages play a crucial role in initiating the immune response. As
secretory cells, monocytes and macrophages are vital to the regulation of immune responses and the
development of inflammation; they churn out an amazing array of powerful chemical substances (monokines)
including enzymes, complement proteins, and regulatory factors such as interleukin-1. At the same
time, they carry receptors for lymphokines that allow them to be "activated" into
single-minded pursuit of microbes and tumor cells.
Macrophages
are versatile cells that play many roles. As scavengers, they rid the body of worn-out cells and
other debris. Foremost among the cells that "present" antigen to T cells, having first
digested and processed it, macrophages play a crucial role in initiating the immune response. As
secretory cells, monocytes and macrophages are vital to the regulation of immune responses and the
development of inflammation; they churn out an amazing array of powerful chemical substances (monokines)
including enzymes, complement proteins, and regulatory factors such as interleukin-1. At the same
time, they carry receptors for lymphokines that allow them to be "activated" into
single-minded pursuit of microbes and tumor cells.
Macrophages are not the only cells to present
antigen to lymphocytes. Other antigen-presenting cells include B cells, as noted above, and
dendritic cells, irregularly shaped white blood cells found in the spleen and other lymphoid organs.
Dendritic cells typically have long threadlike tentacles that enmesh lymphocytes and antigens.
Langerhans cells are dendritic cells that travel about in the skin, picking up antigen and
transporting it to nearby lymph nodes. Many other types of body cells, properly stimulated, can also
be recruited to present antigens to lymphocytes.
Another critical phagocyte is the neutrophil.
Neutrophils are not only phagocytes but also granulocytes: they contain granules filled with potent
chemicals. These chemicals, in addition to destroying microorganisms, play a key role in acute
inflammatory reactions.
Also known as polymorphonuclear leukocytes or polymorphs (because their
nuclei come in "many shapes"), granulocytes include eosinophils and basophils as well as
neutrophils. (The cells are named for the way they stain in the laboratory: eosinophils, for
instance, have an affinity for acidic dyes such as eosin.) The phagocytic neutrophil uses its
prepackaged chemicals to degrade the microbes it ingests; eosinophils and basophils typically "degranulate,"
releasing their chemicals to work on cells or microbes in their surroundings.
 The mast
cell is a noncirculating counterpart of the basophil. Located in the lungs, skin, tongue, and
linings of the nose and intestinal tract, the mast cell is responsible for the symptoms of allergy.
The mast
cell is a noncirculating counterpart of the basophil. Located in the lungs, skin, tongue, and
linings of the nose and intestinal tract, the mast cell is responsible for the symptoms of allergy.
Another
related structure is the blood platelet. Platelets, too, contain granules. In addition to promoting
blood clotting and wound repair, platelets release substances that activate components of the immune
system.
v
Complement
The complement system is made up of a series of about 25 proteins that work to
"complement" the activity of antibodies in destroying bacteria, either by facilitating
phagocytosis or by puncturing the bacterial cell membrane.
Complement also helps to rid the body
of antigen-antibody complexes. In carrying out these tasks, it induces an inflammatory response.
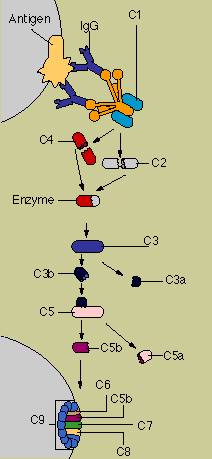 Complement
proteins circulate in the blood in an inactive form. When the first of the complement substances is
triggered-usually by antibody interlocked with an antigen-it sets in motion ripple effect. As each
component is activated in turn, it acts upon the next in a precise sequence of carefully regulated
steps known as the complement cascade."
Complement
proteins circulate in the blood in an inactive form. When the first of the complement substances is
triggered-usually by antibody interlocked with an antigen-it sets in motion ripple effect. As each
component is activated in turn, it acts upon the next in a precise sequence of carefully regulated
steps known as the complement cascade."
In the so-called "classical" pathway of
complement activation, a series of proteins gives rise to a complex enzyme capable of cleaving a key
protein, C3. In the "alternative" pathway-which can be triggered by suitable targets in
the absence of antibody-C3 interacts with a different set of factors and enzymes. But both pathways
end in creation of a unit known as the membrane attack complex.
Inserted in the wall of the target
cell, the membrane attack complex constitutes a channel that allows fluids and molecules to flow in
and out. The target cell rapidly swells and bursts.
Meanwhile, various fragments flung off during
the course of the cascade can produce other consequences. One byproduct causes mast cells and
basophils to release their contents, producing the redness, warmth, and swelling of the inflammatory
response. Another stimulates and attract neutrophils. Yet another, C3b, opsonizes or coats target
cells so as to make them more palatable to phagocytes, which carry a special receptor for C3b.
The
C3b fragment also appears to play a major role in the body's control of immune complexes. By
opsonizing antigen-antibody complexes, C3b helps prevent the formation of large and insoluble (and
thus potentially damaging) immune aggregates. Moreover, receptors for C3b are also present on red
blood cells, which appear to use the receptors to pick up complement-coated immune complexes and
deliver them to the Kupffer cells in the liver.
v
Mounting an Immune Response
Infections remain the most common cause of human disease. Produced by bacteria, viruses,
parasites and fungi, infections may range from relatively mild respiratory illnesses such as the
common cold, to debilitating conditions like chronic hepatitis, to life-threatening diseases such as
AIDS and meningitis.
To fend off the threatening horde, the body has devised astonishingly
intricate defenses. Microbes attempting to enter the body must first find a chink in the body's
external protection. The skin and the mucous membranes that line the body's portals not only pose a
physical barrier, they are also rich in scavenger cells and IgA antibodies.
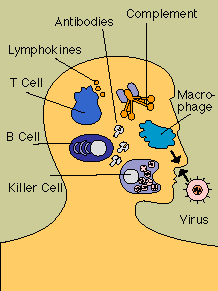
Next, invaders must elude a series of nonspecific defenses
those cells and substances equipped to tackle infectious agents without regard for their antigenic
peculiarities. Many potential infections are cut short when microbes are intercepted by patrolling
scavenger cells or disabled by complement or other enzymes or chemicals. Virus-infected cells, for
instance, secrete interferon, a chemical that rouses natural killer cells.
v
A Billion Antibodies
 Scientists were long
puzzled by the opulence of the immune system's resources. The body apparently could recognize and
mount unique responses to an endless variety of antigens-but how in the world could all that
information be crammed into a limited number of genes?
Scientists were long
puzzled by the opulence of the immune system's resources. The body apparently could recognize and
mount unique responses to an endless variety of antigens-but how in the world could all that
information be crammed into a limited number of genes?
The answer came as a surprise. A
typical gene consists of a fixed segment of DNA, which directs the manufacture of a given protein
molecule such as insulin. Antibody genes, in contrast, are assembled from bits and pieces of DNA
scattered widely throughout the genetic materials. As the B cell matures, it rearranges or shuffles
these gene components, picking and choosing among hundreds of DNA segments some for each of the
antibody's variable (V), diversity (D), joining (J), and constant (C) regions. Intervening segments
of DNA are cut out; the selected pieces are spliced together.
 The
new gene and the antibody it encodes are virtually unique. When the B cell containing this uniquely
rearranged set of gene segments proliferates, all its descendants will make this unique antibody.
Then, as the cells continue to multiply, numerous mutants arise; these allow for the natural
selection of antibodies that provide better and better "fits" for the target antigen. The
result of this entire process is that a limited number of genetically distinct B cells can respond
to a seemingly unlimited range of antigens.
The
new gene and the antibody it encodes are virtually unique. When the B cell containing this uniquely
rearranged set of gene segments proliferates, all its descendants will make this unique antibody.
Then, as the cells continue to multiply, numerous mutants arise; these allow for the natural
selection of antibodies that provide better and better "fits" for the target antigen. The
result of this entire process is that a limited number of genetically distinct B cells can respond
to a seemingly unlimited range of antigens.
A similar mechanism was found to control a
comparable structure of the T cell, the T cell's antigen receptor. The variable regions of T cell
antigen receptors, like those of antibodies, are encoded by V, D, and J segments originally far
apart, but which are brought together and fused into a single gene. With numerous candidates for
each segment, the number of possible combinations becomes astronomical. However, in contrast to
antibody genes, T cell receptor genes do not mutate as the T cells proliferate. This ensures that
the self-tolerance imposed in the thymus will not be overthrown by the inadvertent generation of
mutant T cell receptors that are anti-self.
v
A Web of Idiotypes
The unique and characteristic pocket on an antibody that recognizes a specific antigen its
variable region can itself act as an antigen. More precisely, the variable region contains a number
of antigen like segments, and these are known collectively as an idiotype. Like any other antigen,
an idiotype can trigger complementary antibody. This second-round antibody is known as an
antiidiotype. An antiidio-type, in turn, can trigger an antiantiidiotype. Like a series of mirrored
reflections, the process can go on and on.
Interactions between idiotypes and antiidio-types, it
has been proposed, constitute a mechanism whereby the immune system regulates itself. According to
the "network theory," not only antibodies but B cells and T cells carry-in their unique
antigen-receptors-idiotypes. The B cells and T cells that proliferate in response to a certain
antigen carry a complementary idiotype. Antiidiotype B cells secrete antiidiotype antibodies, which
may neutralize the original idiotypes (antibodies), or bind to idiotypes on regulatory T cells.
Alternatively, antiidiotypes may trigger antiantiidiotypes, creating a spiraling response within the
network turning on, amplifying, and shutting down immune responses.
The concept of the idiotype is
being put to practical use today in the development of experimental antigen-free vaccines.
Microbes
that breach the nonspecific barriers are confronted by specific weapons tailored to fit each one.
These may be cellular responses directed both by cells, primarily T lymphocytes and their secretions
(lymphokines), and against cells that have been infected. Or they may be humoral responses, the work
of antibodies secreted by B lymphocytes into the body's fluids or humors.
Most antigens are recognized by a limited number of specific immune cells ( and their offspring).
A few antigens, however, are capable of rousing large classes of T cells, setting off an immune
response so massive that it is harmful. Dubbed "superantigens," these substances include
bacterial toxins such as those responsible for the toxic shock syndrome.
Although immunologists
traditionally distinguished between cellular and humoral immunity, it has become increasingly clear
that the two arms of the immune response are closely intertwined. Almost all antigens evoke both a
humoral response and a cellular response and most B cell responses require T cell help. In practice,
however, one arm is usually more effective than the other, and regulatory mechanisms end up skewing
the response toward either the cellular or the humoral side.
The cell-mediated response is
initiated by a macrophage or other antigen-presenting cell. The antigen-presenting cell takes in the
antigen, digests it, and then displays antigen fragments on its own surface. Bound to the antigen
fragment is an MHC molecule. It takes both of these structures, together, to capture the T cell's
attention.
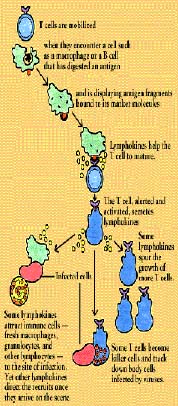
A T cell whose receptor fits this antigen-MHC complex binds to it. The binding
stimulates the antigen-presenting cell to secrete interleukins required for T cell activation and
performance.
Before activated T cells can set to work, however, they need a second go-ahead
signal. In a maneuver known as co-stimulation, the antigen-presenting cell displays a special
molecule that engages specific receptor molecules on the T cell, including one known as CD28.
Without co-stimulation, activated T cells fall into a state of unresponsiveness known as anergy.
Anergy arrests T cell
growth by blocking its ability to produce or respond to signals to proliferate.
Once up and
going, some subsets of T cells synthesize and secrete lymphokines. Interleukin-2, for instance,
spurs the growth of more T cells. Other lymphokines attract other immune cells fresh macrophages,
granulocytes, and other lymphocytes to the site of the infection. Yet others direct the cells'
activities once they arrive on the scene. Some subsets of T cells become killer (or cytotoxic)
cells, and set out to track down body cells infected by viruses. And when the infection has been
brought under control, suppressor T cells draw the immune response to a close.
v
Receptors for Recognizing Antigen
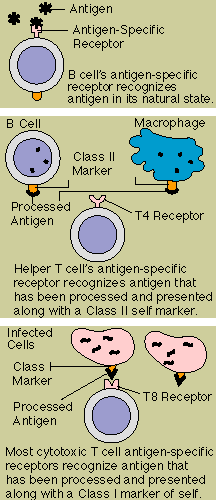 In order to recognize and
respond to the antigens that are their specific targets, both B cells and T cells carry special
receptor molecules on their surface. For the B cell, this receptor is a prototype of the antibody
the B cell is prepared to manufacture, anchored in its surface. When a B cell encounters a matching
antigen in the blood or other body fluid, this antibody-like receptor allows the B cell to interact
with it very efficiently.
In order to recognize and
respond to the antigens that are their specific targets, both B cells and T cells carry special
receptor molecules on their surface. For the B cell, this receptor is a prototype of the antibody
the B cell is prepared to manufacture, anchored in its surface. When a B cell encounters a matching
antigen in the blood or other body fluid, this antibody-like receptor allows the B cell to interact
with it very efficiently.
The T cell receptor is more complex. Structurally, it is somewhat
similar to an antibody, made of a pair of chemically linked chains with variable and constant
regions. (But to work, it needs the help of an associated set of signaling and anchoring cell
surface molecules called T3.) Unlike a B cell, however, a T cell cannot recognize antigen in its
natural state; the antigen must first be broken down, and the fragments bound to an MHC molecule, by
an antigen presenting cell.
Helper T cells (T4 cells) look for antigen bound to a class II
MHC molecule a combination displayed by macrophages and B cells. Most cytotoxic T cells (T8), on the
other hand, respond to antigen bound to MHC class I molecules, which are found on almost all body
cells.
The T cell receptor molecule thus forms a three-way complex with its specific foreign
antigen and an MHC protein. This complicated arrangement assures that T cells which affect other
cells through either direct contact or bursts of secretions act only on precise targets and at close
range.
The major antigen receptor, name alpha/beta for its two chains, is found on most T4 and T8
cells. A second, more recently discovered antigen receptor also has two chains and is known as
gamma/delta; it is found on a distinct subset of mature T cells. Like the alpha/beta receptor, the
more primitive gamma/delta receptor works in conjunction with T3. The function of T cells that carry
gamma/delta receptors is not known.
Humoral immunity chiefly involves B cells, although the
cooperation of helper T cells is almost always necessary. B cells, like macrophages, take in and
process circulating antigen. Unlike macrophages, however, a B cell can bind only that antigen that
specifically fits its antibody-like receptor.
To enlist the help of a T cell, the B cell exhibits
antigen fragments bound to its class II MHC molecules. This display attracts mature helper T cells
(which may have been already activated by macrophages presenting the same antigen). The B cell and T
cell
interact, and the helper T cell secretes several lymphokines. These lymphokines set the B cell to
multiplying, and soon there is a clone of identical B cells. The B cells differentiate into plasma
cells and begin producing vast quantities of identical antigen specific antibodies.
Released into
the bloodstream, the antibodies lock onto matching antigens. The antigen-antibody complexes trigger
the complement cascade or are removed from the circulation by clearing mechanisms in the liver and
the spleen. The infection is overcome and, in response to suppressor influences wielded by yet other
subsets of T cells, antibody production wanes.
Clinically, infections manifest themselves through
the five classic symptoms of the inflammatory response redness, warmth, swelling, pain, and loss of
function. Redness and warmth develop when, under the influence of lymphokines and complement
components, small blood vessels in the vicinity of the infection become dilated and carry more
blood. Swelling results when the vessels, made leaky by yet other immune secretions, allow fluid and
soluble immune substances to seep into the surrounding tissue, and immune cells to converge on the
site.
v
Immunity, Natural and Acquired
As long ago as the 5th century B.C., Greek physicians noted that people who had recovered from
the plague would never get it again they had acquired immunity. This is because, whenever T cells
and B cells are activated, some of the cells become "memory" cells. Then, the next time
that an individual encounters that same antigen, the immune system is primed to destroy it quickly.
The
degree and duration of immunity depend on the kind of antigen, its amount, and how it enters the
body. An immune response is also dictated by heredity; some individuals respond strongly to a given
antigen, others weakly, and some not at all.
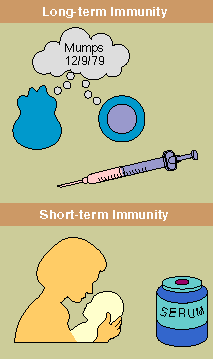 Infants
are born with relatively weak immune responses. They have, however, a natural "passive"
immunity; they are protected during the first months of life by means of antibodies they receive
from their mothers. The antibody IgG, which travels across the placenta, makes them immune to the
same microbes to which their mothers are immune. Children who are nursed also receive IgA from
breast milk; it protects the digestive tract.
Infants
are born with relatively weak immune responses. They have, however, a natural "passive"
immunity; they are protected during the first months of life by means of antibodies they receive
from their mothers. The antibody IgG, which travels across the placenta, makes them immune to the
same microbes to which their mothers are immune. Children who are nursed also receive IgA from
breast milk; it protects the digestive tract.
Passive immunity can also be conveyed by antibody-containing serum obtained from individuals who
are immune to a specific infectious agent. Immune serum globulin or "gamma globulin" is
sometimes given to protect travelers to countries where hepatitis is widespread. Passive immunity
typically lasts only a few weeks.
"Active" immunity mounting an immune response
can be triggered by both infection and vaccination. Vaccines contain microorganisms that have been
altered so they will produce an immune response but will not be able to induce full-blown disease.
Some vaccines are made from microbes that have been killed. Others use microbes that have been
changed slightly so they can no longer produce infection. They may, for instance, be unable to
multiply. Some vaccines are made from a live virus that has been weakened, or attenuated, by growing
it for many cycles in animals or cell cultures.
Recent research, benefiting from the biotechnology
revolution, has focused on developing vaccines that use only part of the infectious agent. Such
subunit vaccines, which are now available for meningitis, pneumonia, and hepatitis B, produce the
desired immunity without stirring up separate and potentially harmful immune reactions to the many
antigens carried, for instance, on a single bacterium.
v
Vaccines Through Biotechnology
Through genetic engineering, scientists can isolate specific genes and insert them into DNA of
certain microbes or mammalian cells; the microbes or cells become living factories, mass producing
the desired antigen. Then, using another product of biotechnology, a monoclonal antibody that
recognizes the antigen, the scientists can separate the antigen from all the other material produced
by the microbe or cell. This technique has been used to produce immunogenic but safe segments of the
hepatitis B virus and the malaria parasite.
In another approach, scientists have inserted genes
for desired antigens into the DNA of the vaccinia virus, the large cowpox virus familiar for its
role in smallpox immunization. When the reengineered vaccinia virus is inoculated, it stimulates an
immune reaction to both the vaccinia and the products of its passenger genes. These have included,
in animal experiments, genes from the viruses that cause hepatitis B, influenza, rabies, and AIDS.
Instead
of adding a gene, some scientists have snipped a key gene out of an infectious organism. Thus
crippled, the microbe can produce immunity but not disease. This technique has been tried with a
bacterium that causes the severe diarrheal disease cholera; such a vaccine is commercially available
against a virus disease of pigs.
A totally different approach to vaccine development lies in
chemical synthesis. Once scientists have isolated the gene that encodes an antigen, they are able to
determine the precise sequence of amino acids that make up the antigen. They then pinpoint small key
areas on the large protein molecule, and assemble it chemical by chemical. Wholly synthetic vaccines
are being explored for malaria and for the major diarrheal diseases that are so devastating
in developing countries.
Another pioneering vaccine strategy exploits antiidiotype antibodies.
The original antibody (or idiotype) provokes an antiantibody (or antiidiotype) that resembles the
original antigen on the disease causing organism. The antiidiotype will not itself cause disease,
but it can serve as a mock antigen, inducing the formation of antibodies that recognize and block
the original antigen. To make such a vaccine, scientists inject animals with a monoclonal antibody (idiotype)
against a disease-causing microorganism, then harvest the antiidiotypes produced in response.
v
Disorders of the Immune System: Allergy
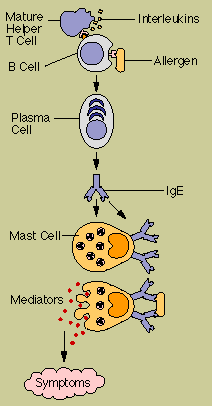 The
most common types of allergic reactions hay fever, some kinds of asthma, and hives are produced when
the immune system responds to a false alarm. In a susceptible person, a normally harmless substance
grass pollen or house dust, for example is perceived as a threat and is attacked.
The
most common types of allergic reactions hay fever, some kinds of asthma, and hives are produced when
the immune system responds to a false alarm. In a susceptible person, a normally harmless substance
grass pollen or house dust, for example is perceived as a threat and is attacked.
Such allergic
reactions are related to the antibody known as immunoglobulin E. Like other antibodies, each IgE
antibody is specific; one reacts against oak pollen, another against ragweed. The role of IgE in the
natural order is not known, although some scientists suspect that it developed as a defense against
infection by parasitic worms.
The first time an allergy-prone person is exposed to an allergen, he
or she makes large amounts of the corresponding IgE antibody.
These IgE molecules attach to the
surfaces of mast cells (in tissue) or basophils (in the circulation). Mast cells are plentiful in
the lungs, skin, tongue, and linings of the nose and intestinal tract.
When an IgE antibody siting
on a mast cell or basophil encounters its specific allergen, the IgE antibody signals the mast cell
or basophil to release the powerful chemicals stored within its granules. These chemicals include
histamine, heparin, and substances that activate blood platelets and attract secondary cells such as
eosinophils and neutrophils. The activated mast cell or basophil also synthesizes new mediators,
including prostaglandins and leukotrienes, on the spot.
It is such chemical mediators that cause
the symptoms of allergy, including wheezing, sneezing, runny eyes and itching. They can also produce
anaphylactic shock, a life-threatening allergic reaction characterized by swelling of body tissues,
including the throat, and a sudden fall in blood pressure.
v
Autoimmune Diseases
Sometimes the immune system's recognition apparatus breaks down, and the body begins to
manufacture antibodies and T cells directed against the body's own constituents cells, cell
components, or specific organs. Such antibodies are known as autoantibodies, and the diseases they
produce are called autoimmune diseases. (Not all autoantibodies are harmful; some types appear to be
integral to the immune system's regulatory scheme.)
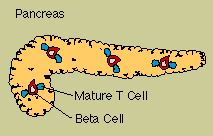 Autoimmune
reactions contribute to many enigmatic diseases. For instance, autoantibodies to red blood cells can
cause anemia, autoantibodies to pancreas cells contribute to juvenile diabetes, and autoantibodies
to nerve and muscle cells are found in patients with the chronic muscle weakness known as myasthenia
gravis. Autoantibody known as rheumatoid factor is common in persons with rheumatoid arthritis.
Autoimmune
reactions contribute to many enigmatic diseases. For instance, autoantibodies to red blood cells can
cause anemia, autoantibodies to pancreas cells contribute to juvenile diabetes, and autoantibodies
to nerve and muscle cells are found in patients with the chronic muscle weakness known as myasthenia
gravis. Autoantibody known as rheumatoid factor is common in persons with rheumatoid arthritis.
Persons
with systemic lupus erythematosus (SLE), whose symptoms encompass many systems, have antibodies to
many types of cells and cellular components. These include antibodies directed against substances
found in the cell's nucleus-DNA, RNA, or proteins-which are known as antinuclear antibodies, or ANAs.
These antibodies can cause serious damage when they link up with self antigens to form circulating
immune complexes, which become lodged in body tissue and set off inflammatory reactions.
Autoimmune
diseases affect the immune system at several levels. In patients with SLE, for instance, B cells are
hyperactive while suppressor cells are underactive; it is not clear which defect comes first.
Moreover, production of IL-2 is low, while levels of gamma interferon are high. Patients with
rheumatoid arthritis, who have a defective suppressor T cell system, continue to make antibodies to
a common virus, whereas the response normally shuts down after about a dozen days.
No one knows
just what causes an autoimmune disease, but several factors are likely to be involved. These may
include viruses and environmental factors such as exposure to sunlight, certain chemicals, and some
drugs, all of which may damage or alter body cells so that they are no longer recognizable as self.
Sex hormones may be important, too, since most autoimmune diseases are far more common in women than
in men.
Heredity also appears to play a role. Autoimmune reactions, like many other immune
responses, are influenced by the genes of the MHC. A high proportion of human patients with
autoimmune disease have particular histocompatibility types. For example, many persons with
rheumatoid arthritis display the self marker known as HLA-DR4.
Many types of therapies are being
used to combat autoimmune diseases. These include corticosteroids, immunosuppressive drugs developed
as anticancer agents, radiation of the lymph nodes, and plasmapheresis, a sort of "blood
washing" that removes diseased cells and harmful molecules from the circulation.
v
Immune Complex Diseases
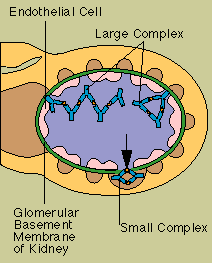 Immune complexes are
clusters of interlocking antigens and antibodies. Under normal conditions immune complexes are
rapidly removed from the bloodstream by macrophages in the spleen and Kupffer cells in the liver. In
some circumstances, however, immune complexes continue to circulate. Eventually they become trapped
in the tissues of the kidneys, lung, skin, joints, or blood vessels. Just where they end up probably
depends on the nature of the antigen, the class of antibodY-IgG, for instance, instead of IgM-and
the size of the complex. There they set off reactions that lead to inflammation and tissue damage.
Immune complexes are
clusters of interlocking antigens and antibodies. Under normal conditions immune complexes are
rapidly removed from the bloodstream by macrophages in the spleen and Kupffer cells in the liver. In
some circumstances, however, immune complexes continue to circulate. Eventually they become trapped
in the tissues of the kidneys, lung, skin, joints, or blood vessels. Just where they end up probably
depends on the nature of the antigen, the class of antibodY-IgG, for instance, instead of IgM-and
the size of the complex. There they set off reactions that lead to inflammation and tissue damage.
Immune
complexes work their damage in many diseases. Sometimes, as is the case with malaria and viral
hepatitis, they reflect persistent low-grade infections. Sometimes they arise in response to
environmental antigens such as the moldy hay that causes the disease known as farmer's lung.
Frequently, immune complexes develop in autoimmune disease, where the continuous production of
autoantibodies overloads the immune complex removal system.
v
Immunodeficiency Diseases
Lack of one or more components of the immune system results in immunodeficiency disorders. These
can be inherited, acquired through infection or other illness, or produced as an inadvertent side
effect of certain drug treatments.
People with advanced cancer may experience immune deficiencies
as a result of the disease process or from extensive anticancer therapy. Transient immune
deficiencies can develop in the wake of common viral infections, including influenza, infectious
mononucleosis, and measles. Immune responsiveness can also be depressed by blood transfusions,
surgery malnutrition, and stress.
Some children are born with defects in their immune systems.
Those with flaws in the B cell components are unable to produce antibodies (immunoglobulins). These
conditions, known as agammaglobulinemias or hypogammaglobulinemias, leave the children vulnerable to
infectious organisms; such disorders can be combated with injections of immunoglobulins.
Other
children, whose thymus is either missing or small and abnormal, lack T cells. The resultant
disorders have been treated with thymic transplants.
Very rarely, infants are born lacking all the
major immune defenses; this is known as severe combined immunodeficiency disease (SCID). Some
children with SCID have lived for years in germ-free rooms and "bubbles." A few SCID
patients have been successfully treated with transplants of bone marrow.
The devastating
immunodeficiency disorder known as the acquired immunodeficiency syndrome (AIDS) was first
recognized in 1981.
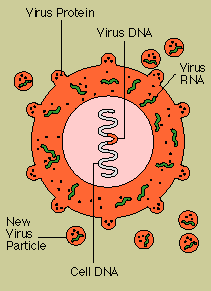 Caused
by a virus (the human immunodeficiency virus, or HIV) that destroys T4 cells and that is harbored in
macrophages as well as T4 cells, AIDS is characterized by a variety of unusual infections and
otherwise rare cancers. The AIDS virus also damages tissue of the brain and spinal cord, producing
progressive dementia.
Caused
by a virus (the human immunodeficiency virus, or HIV) that destroys T4 cells and that is harbored in
macrophages as well as T4 cells, AIDS is characterized by a variety of unusual infections and
otherwise rare cancers. The AIDS virus also damages tissue of the brain and spinal cord, producing
progressive dementia.
AIDS infections are known as "opportunistic" because they are
produced by commonplace organisms that do not trouble people whose immune systems are healthy, but
which take advantage of the "opportunity" provided by an immune defense in disarray. The
most common infection is an unusual and life-threatening form of pneumonia caused by a one-celled
organism (a Protozoa) called Pneumocystis carinii. AIDS patients are also susceptible to unusual
lymphomas and Kaposi's sarcoma, a rare cancer that results from the abnormal proliferation of
endothelial cells in the blood vessels.
Some persons infected with the AIDS virus develop a
condition known as AIDS-related complex, or ARC, characterized by fatigue, fever, weight loss,
diarrhea, and swollen lymph glands. Yet other persons who are infected with the AIDS virus
apparently remain well; however, even though they develop no symptoms, they can transmit the virus
to others.
AIDS is a contagious disease, spread by intimate sexual contact, by direct inoculation
of the virus into the bloodstream, or from mother to child during pregnancy. Most of the AIDS cases
in the United States have been found among homosexual and bisexual men with multiple sex partners,
and among intravenous drug abusers. Others have involved men who received untreated blood products
for hemophilia; persons who received transfusions of inadvertently contaminated blood primarily
before the AIDS virus was discovered and virtually eliminated from the nation's blood supply with a
screening test; the heterosexual partners of persons with AIDS; and children born to infected
mothers.
There is presently no cure for AIDS, although the antiviral agent zidovuzine (AZT)
appears to hold the virus in check, at least for a time. Many other antiretroviral drugs are being
tested, as are agents to bolster the immune system and agents to prevent or treat opportunistic
infections. Research on vaccines to prevent the spread of AIDS is also under way.
v
Cancers of the Immune System
Cells of the immune system, like those of other body systems, can proliferate uncontrollably; the
result is cancer. Leukemias are caused by the proliferation of white blood cells, or leukocytes. The
uncontrolled growth of antibody-producing (plasma) cells can lead to multiple myeloma. Cancers of
the lymphoid organs, known as lymphomas, include Hodgkin's disease. These disorders can be
treated-some of them very successfully-by drugs and/or irradiation.
v
Bone Marrow Transplants
When the immune response is severely depressed as the result of inherited defects, cancer
therapy, or AIDS one possible remedy is a transfer of healthy bone marrow. Bone marrow transplants
are also used to treat patients with cancers of the blood, the blood forming organs, and the
lymphoid system the leukemias and lymphomas.
Once in the circulation, transplanted bone marrow
cells travel to the bones where the immature cells grow into functioning B and T cells. Like other
transplanted tissue, however, bone marrow from a donor must carry self markers that closely match
those of the person intended to receive it. This match is essential not only to prevent the
transplant from being rejected, but also to fend off a life-threatening situation known as
graft-versus-host disease. In graft-versus-host disease, mature T cells from the donor attack and
destroy the tissues of the recipient.
To prevent graft-versus-host disease, scientists have
developed techniques to "cleanse" the donor marrow of potentially dangerous mature cells.
These include chemicals and, more recently, a monoclonal antibody (OKT3) that specifically
recognizes and eliminates mature T cells.
For cancer patients who face immunosuppressive therapy
but who have no readily matched donor, doctors have used "autologous" transplants: the
person's bone marrow is removed, frozen, and stored until therapy is complete; then the cells are
thawed and reinfused.
v
Immunology and Transplants
Since organ transplantation was introduced over a quarter of a century ago, it has become a
widespread remedy for life-threatening disease. Several thousand kidney transplants are performed
each year in the United States alone. In addition, physicians have succeeded in transplanting the
heart, lungs, liver and pancreas.
The success of a transplant whether it is accepted or rejected
depends on the stubbornness of the immune system. For a transplant to "take," the body of
the recipient must be made to suppress its natural tendency to get rid of foreign tissue.
Scientists
have tackled this problem in two ways. The first is to make sure that the tissue of the donor and
the recipient are as similar as possible. Tissue typing, or histocompatibility testing, involves
matching the markers of self on body tissues; because the typing is usually done on white blood
cells, or leukocytes, the markers are referred to as human leukocyte antigens (HLA). Each cell has a
double set of six major antigens, designated HLA-A, B, C, and three types of HLA-D-DR, DP, and DQ. (HLA-A,
B, and C are the same as the class I antigens encoded by the genes of the major histocompatibility
complex; HLA-D region molecules are the class II MHC antigens.)
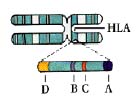 Each
of the HLA antigens exist-in different individuals- in as many as 20 varieties, so that the number
of possible HLA types reaches about 10,000. Histocompatibility testing relies on antibodies to
determine if a potential organ donor and recipient share two or more HLA anti gens, and thus are
likely to make a good "match." The best matches are identical twins; next best are close
relatives, especially brothers and sisters.
Each
of the HLA antigens exist-in different individuals- in as many as 20 varieties, so that the number
of possible HLA types reaches about 10,000. Histocompatibility testing relies on antibodies to
determine if a potential organ donor and recipient share two or more HLA anti gens, and thus are
likely to make a good "match." The best matches are identical twins; next best are close
relatives, especially brothers and sisters.
The second approach to taming rejection is to lull
the recipient's immune system. This can be achieved through a variety of powerful immunosuppressive
drugs. Steroids suppress lymphocyte function; the drug cyclosporine holds down the production of the
lymphokine interleukin-2, which is necessary for T cell growth. When such measures fail, the graft
may yet be saved with a new treatment: OKT3 is a monoclonal antibody that seeks out the T3 marker
carried on all mature T cells. By either destroying T cells or incapacitating them, OKT3 can bring
an acute rejection crisis to a halt.
Not surprisingly, any such all-out assault on the immune
system leaves a transplant recipient susceptible to both opportunistic infections and lymphomas.
Although such patients need careful medical followup, many of them are able to lead active and
essentially normal lives.
v
Privileged Immunity
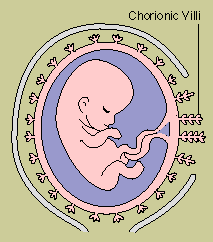 A child developing in the
womb carries foreign antigens from its father as well as immunologically compatible self antigens
from its mother, and might be expected to trigger a graft rejection. But the uterus is an "immunologically
privileged" site where immune responses are subdued. One source of protection appears to be a
substance produced by the child, perhaps in response to antibodies from the mother. The substance
promotes the development of special white blood cells in the uterus, and these cells release a
factor that blocks the actions of IL-2. Another substance, produced by the uterus, helps disguise
antigens on the fetal surface of the placenta, shielding them from the mother's immune defenses.
A child developing in the
womb carries foreign antigens from its father as well as immunologically compatible self antigens
from its mother, and might be expected to trigger a graft rejection. But the uterus is an "immunologically
privileged" site where immune responses are subdued. One source of protection appears to be a
substance produced by the child, perhaps in response to antibodies from the mother. The substance
promotes the development of special white blood cells in the uterus, and these cells release a
factor that blocks the actions of IL-2. Another substance, produced by the uterus, helps disguise
antigens on the fetal surface of the placenta, shielding them from the mother's immune defenses.
v
Immunity and Cancer
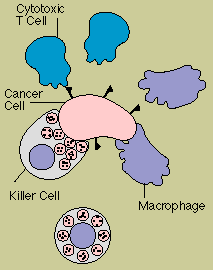 The immune system provides
one of the body's main defenses against cancer. When normal cells turn into cancer cells, some of
the antigens on their surface change. These new or altered antigens flag immune defenders, including
cytotoxic T cells, natural killer cells, and macrophages.
The immune system provides
one of the body's main defenses against cancer. When normal cells turn into cancer cells, some of
the antigens on their surface change. These new or altered antigens flag immune defenders, including
cytotoxic T cells, natural killer cells, and macrophages.
According to one theory, patrolling cells of the immune system provide continuing bodywide
surveillance, spying out and eliminating cells that undergo malignant transformation. Tumors develop
when the surveillance system breaks down or is overwhelmed. Some tumors may elude the immune
defenses by hiding or disguising their tumor antigens. Alternatively, tumors may survive by
encouraging the production of suppressor T cells; these T cells act as the tumor's allies, blocking
cytotoxic T cells that would normally attack it.
Blood tests show that people can develop
antibodies to many types of tumor antigens (although the antibodies may not actually be effective in
fighting the tumor). Skin testing (similar to skin testing for tuberculosis) has demonstrated that
tumors provoke cellular immunity as well. Furthermore, studies indicated that cancer patients have a
better prognosis when their tumors are infiltrated with many immune cells. Immune responses may
underlie the spontaneous disappearance of some cancers.
Tests using antibodies derived from
batches of human serum can detect various tumor-associated antigens including carcinoembryonic
antigen (CEA) and alphafetoprotein (AFP)-in blood samples. Because such antigens develop not only in
cancer but in other diseases as well, the antibody tests are not useful for cancer screening in the
general population. They are however, valuable in monitoring the course of disease and the
effectiveness of treatment in patients known to have cancer.
Scientists have developed monoclonal
antibodies (Hybridoma Technology) that are targeted specifically at tumor antigens. Linked to
radioactive substances, these antibodies can be used to track down and reveal hidden cancer
metastases within the body. Monoclonal antitumor antibodies are also being used experimentally to
treat cancer-either in their native form or as immunotoxins, linked to natural toxins, anticancer
drugs, or radioactive substances.
Other efforts to attack cancer through the immune system center
on stimulating or replenishing the patient's immune responses with substances known as biological
response modifiers. Among these are interferons (now obtained through genetic engineering) and
interleukins. In some cases biological response modifiers are injected directly into the patient; in
other cases they are used in the laboratory to transform some of the patient's own lymphocytes into
tumor-hungry cells known as lymphokine-activated killer (LAK) cells and tumor-infiltrating
lymphocytes (TILS), which are then injected back into the patient. Researchers are even using
structures from the tumor cells themselves to construct custom-made anticancer "vaccines."
v
The Immune System and the Nervous System
A new field of research, known as psychoneuroimmunology, is exploring how the immune system and
the brain may interact to influence health. For years stress has been suspected of increasing
susceptibility to various infectious diseases or cancer. Now evidence is mounting that the immune
system and the nervous system may be inextricably interconnected.
Research has shown that a wide
range of stresses, from losing a spouse to facing a tough examination, can deplete immune resources,
causing levels of B and T cells to drop, natural killer cells to become less responsive, and fewer
IgA antibodies to be secreted in the saliva.
Biological links between the immune system and the
central nervous system exist at several levels. One well-known pathway involves the adrenal glands,
which, in response to stress messages from the brain, release corticosteroid hormones into the
blood. In addition to helping a person respond to emergencies by mobilizing the body's energy
reserves, these "stress hormones" decrease antibodies and reduce lymphocytes in both
number and strength.
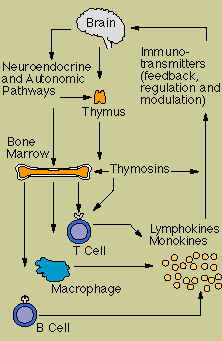 More
recently, it has become apparent that hormones and neuropeptides (hormone-like chemicals released by
nerve cells), which convey messages to other cells of the nervous system and organs throughout the
body, also "speak" to cells of the immune system.
More
recently, it has become apparent that hormones and neuropeptides (hormone-like chemicals released by
nerve cells), which convey messages to other cells of the nervous system and organs throughout the
body, also "speak" to cells of the immune system.
Macrophages and T cells carry
receptors for certain neuropeptides; natural killer cells, too, respond to them. Even more
surprising, some macrophages and activated lymphocytes actually manufacture typical neuropeptides.
At the same time, some lymphokines, secreted by activated lymphocytes such as interferon and the
interleukins, can transmit information to the nervous system. Hormones produced by the thymus, too,
act on cells in the brain.
In addition, the brain may directly influence the immune system
by sending messages down nerve cells. Networks of nerve fibers have been found that connect to the
thymus gland, spleen, lymph nodes, and bone marrow. Moreover, experiments show that immune function
can be altered by actions that destroy specific brain areas.
The image that is emerging is of closely interlocked systems facilitating a two-way flow of
information, primarily through the language of hormones. Immune cells, it has been suggested, may
function in a sensory capacity, detecting the arrival of foreign invaders and relaying chemical
signals to alert the brain. The brain, for its part, may send signals that guide the traffic of
cells through the lymphoid organs.
v
Frontiers in Immunology: Hybridoma Technology
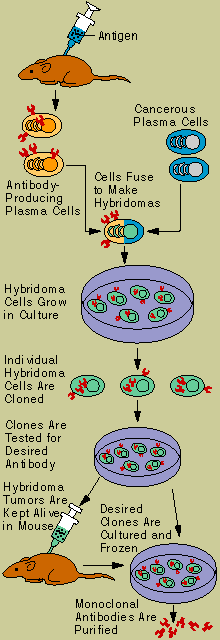 Through a stratagem
known as hybridoma technology, scientists are now able to obtain, in quantity, substances secreted
by cells of the immune system-both antibodies and lymphokines. The ready supply of these materials
has not only revolutionized immunology but has also created a resounding impact throughout medicine
and industry.
Through a stratagem
known as hybridoma technology, scientists are now able to obtain, in quantity, substances secreted
by cells of the immune system-both antibodies and lymphokines. The ready supply of these materials
has not only revolutionized immunology but has also created a resounding impact throughout medicine
and industry.
A hybridoma is created by fusing two cells, a secreting cell from the immune system
and a long-lived cancerous immune cell, within a single membrane. The resulting hybrid cell can be
cloned, producing many identical offspring. Each of these daughter clones will secrete, over a long
period of time, the immune cell product. A B-cell hybridoma secretes a single specific antibody.
Such
monoclonal antibodies, as they are known, have opened remarkable new approaches to preventing,
diagnosing, and treating disease. Monoclonal antibodies are used, for instance, to distinguish
subsets of B cells and T cells. This knowledge is helpful not
only for basic research but also for identifying different types of leukemias and lymphomas and
allowing physicians to tailor treatment accordingly. Quantitating the number of B cells and helper T
cells is all-important in immune disorders such as AIDS. Monoclonal antibodies are being used to
track cancer antigens and, alone or linked to anticancer agents, to attack cancer metastases. The
monoclonal antibody known as OKT3 is saving organ transplants threatened with rejection, and
preventing bone marrow transplants from setting off graft-versus-host disease.
Monoclonal
antibodies are essential to the manufacture of genetically engineered proteins; they single out the
desired protein product so it can be separated from the jumble of molecules surrounding it.
Monoclonal antibodies are also the key to developing new types of vaccines.
With growing
experience, scientists have devised several sophisticated variants on the monoclonal antibody. For
instance, they have created some monoclonal antibodies of human rather than mouse origin; human
monoclonal antibodies can be used for therapy without risking an immune reaction to mouse proteins.
They have also succeeded in "humanizing" mouse antibodies by splicing the mouse genes for
the highly specific antigen-recognizing portion of the antibody into the human genes that encode the
rest of the antibody molecule.
Other monoclonal antibodies have been designed to behave like
enzymes; these so-called catalytic antibodies or abzymes speed up, or catalyze, selected chemical
reactions by binding to a chemical reactant and holding it in a highly unstable "transition
state." By, in fact, cutting the proteins to which they bind, such antibodies may be useful for
such things as dissolving blood clots or destroying tumor cells. Yet other researchers, by fusing
two hybridoma cells that produce two different antibodies, have created hybrid hybridomas that
secrete artificial antibodies made up of two nonidentical halves. While one arm of the bispecific
antibody binds to one antigen, the second arm binds to another. One may bind to a marker molecule,
for instance, and the second to a target cell, creating an entirely new way to stain cells. Or, one
arm of a chimeric antibody may bind to a killer cell while the other locks to a tumor cell, creating
a lethal bridge between the two.
Source:
National Cancer Institute
National Institute of Allergy and Infectious Diseases
National
Institutes of Health
 The
immune system evolved because we live in a sea of microbes. Like man, these organisms are programmed
to perpetuate themselves. The human body provides an ideal habitat for many of them and they try to
break in; because the presence of these organisms is often harmful, the body's immune system will
attempt to bar their entry or, failing that, to seek out and destroy them.
The
immune system evolved because we live in a sea of microbes. Like man, these organisms are programmed
to perpetuate themselves. The human body provides an ideal habitat for many of them and they try to
break in; because the presence of these organisms is often harmful, the body's immune system will
attempt to bar their entry or, failing that, to seek out and destroy them. The immune system is a complex
network of specialized cells and organs that has evolved to defend the body against attacks by
"foreign" invaders. When functioning properly it fights off infections by agents such as
bacteria, viruses, fungi, and parasites. When it malfunctions, however, it can unleash a torrent of
diseases, from allergy to arthritis to cancer to AIDS.
The immune system is a complex
network of specialized cells and organs that has evolved to defend the body against attacks by
"foreign" invaders. When functioning properly it fights off infections by agents such as
bacteria, viruses, fungi, and parasites. When it malfunctions, however, it can unleash a torrent of
diseases, from allergy to arthritis to cancer to AIDS.  The
body's immune defenses do not normally attack tissues that carry a self marker. Rather, immune cells
and other body cells coexist peaceably in a state known as self-tolerance. But when immune defenders
encounter cells or organisms carrying molecules that say "foreign," the immune troops move
quickly to eliminate the intruders.
The
body's immune defenses do not normally attack tissues that carry a self marker. Rather, immune cells
and other body cells coexist peaceably in a state known as self-tolerance. But when immune defenders
encounter cells or organisms carrying molecules that say "foreign," the immune troops move
quickly to eliminate the intruders. An
antigen announces its foreignness by means of intricate and characteristic shapes called epitopes,
which protrude from its surface. Most antigens, even the simplest microbes, carry several different
kinds of epitopes on their surface; some may carry several hundred. However, some epitopes will be
more effective than others at stimulating an immune response.
An
antigen announces its foreignness by means of intricate and characteristic shapes called epitopes,
which protrude from its surface. Most antigens, even the simplest microbes, carry several different
kinds of epitopes on their surface; some may carry several hundred. However, some epitopes will be
more effective than others at stimulating an immune response.

 Many
cytokines are initially given descriptive names but, as their basic structure is identified, they
are renamed as "inter-leukins"-messengers between leukocytes, or white cells.
Interleukin-1, or IL-1, is a product of macrophages (and many other cells) that helps to activate B
cells and T cells. IL-2, originally known as T cell growth factor, or TCGF, is produced by
antigen-activated T cells and promotes the rapid growth or differentiation of mature T cells and B
cells. IL-3 is a T-cell derived member of the family of protein mediators known as
colony-stimulating factors (CSF); one of its many functions is to nurture the development of
immature precursor cells into a variety of mature blood cells. IL-4, IL-5, and IL-6 help B cells
grow and differentiate; IL-4 also affects T cells, macrophages, mast cells, and granulocytes.
Many
cytokines are initially given descriptive names but, as their basic structure is identified, they
are renamed as "inter-leukins"-messengers between leukocytes, or white cells.
Interleukin-1, or IL-1, is a product of macrophages (and many other cells) that helps to activate B
cells and T cells. IL-2, originally known as T cell growth factor, or TCGF, is produced by
antigen-activated T cells and promotes the rapid growth or differentiation of mature T cells and B
cells. IL-3 is a T-cell derived member of the family of protein mediators known as
colony-stimulating factors (CSF); one of its many functions is to nurture the development of
immature precursor cells into a variety of mature blood cells. IL-4, IL-5, and IL-6 help B cells
grow and differentiate; IL-4 also affects T cells, macrophages, mast cells, and granulocytes. A
number of cytokines, obtained in quantity through recombinant DNA technology (genetic engineering),
are now being used-alone, in combination, linked to toxins-in clinical trials for patients with
cancers, blood disorders, and immunodeficiency diseases (including AIDS), as well as people
receiving bone marrow transplants. Their versatility, however, makes it difficult to predict the
full range of their effects.
A
number of cytokines, obtained in quantity through recombinant DNA technology (genetic engineering),
are now being used-alone, in combination, linked to toxins-in clinical trials for patients with
cancers, blood disorders, and immunodeficiency diseases (including AIDS), as well as people
receiving bone marrow transplants. Their versatility, however, makes it difficult to predict the
full range of their effects. In
order to have room for enough cells to match millions of possible foreign invaders, the immune
system stores just a few of each specificity. When an antigen appears, those few specifically
matched cells are stimulated to multiply into a full-scale army. Later, to prevent this army from
overexpanding wildly, like a cancer, powerful suppressor mechanisms come into play.
In
order to have room for enough cells to match millions of possible foreign invaders, the immune
system stores just a few of each specificity. When an antigen appears, those few specifically
matched cells are stimulated to multiply into a full-scale army. Later, to prevent this army from
overexpanding wildly, like a cancer, powerful suppressor mechanisms come into play. Each B cell is programmed
to make one specific antibody. For example, one B cell will make an antibody that blocks a virus
that causes the common cold, while another produces antibody that zeros in on a bacterium that
causes pneumonia.
Each B cell is programmed
to make one specific antibody. For example, one B cell will make an antibody that blocks a virus
that causes the common cold, while another produces antibody that zeros in on a bacterium that
causes pneumonia. Antibodies
belong to a family of large molecules known as immunoglobulins. Immunoglobulins are proteins, made
up of chains of polypeptides, strings of the basic units known as amino acids. Each antibody has two
identical heavy polypeptide chains and two identical light chains, shaped to form a Y. The sections
that make up the tips of the Y's arms vary greatly from one antibody to another, creating a pocket
uniquely shaped to enfold a specific antigen. This is called the variable (V) region. The stem of
the Y serves to link the antibody to other participants in the immune defenses. This area is
identical in all antibodies of the same class, and is called the constant (C) region.
Antibodies
belong to a family of large molecules known as immunoglobulins. Immunoglobulins are proteins, made
up of chains of polypeptides, strings of the basic units known as amino acids. Each antibody has two
identical heavy polypeptide chains and two identical light chains, shaped to form a Y. The sections
that make up the tips of the Y's arms vary greatly from one antibody to another, creating a pocket
uniquely shaped to enfold a specific antigen. This is called the variable (V) region. The stem of
the Y serves to link the antibody to other participants in the immune defenses. This area is
identical in all antibodies of the same class, and is called the constant (C) region.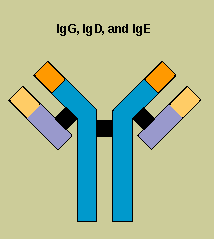
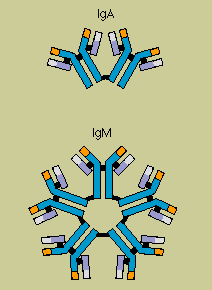
 Natural killer (NK)
cells are yet another type of lethal lymphocyte. Like cytotoxic T cells, they contain granules
filled with potent chemicals. They are called "natural" killers because they, unlike
cytotoxic T cells, do not need to recognize a specific antigen before swinging into action. They
target tumor cells and protect against a wide variety of infectious microbes. In several
immunodeficiency diseases, including AIDS, natural killer cell function is abnormal. Natural killer
cells may also contribute to immuno-regulation by secreting high levels of influential lymphokines.
Natural killer (NK)
cells are yet another type of lethal lymphocyte. Like cytotoxic T cells, they contain granules
filled with potent chemicals. They are called "natural" killers because they, unlike
cytotoxic T cells, do not need to recognize a specific antigen before swinging into action. They
target tumor cells and protect against a wide variety of infectious microbes. In several
immunodeficiency diseases, including AIDS, natural killer cell function is abnormal. Natural killer
cells may also contribute to immuno-regulation by secreting high levels of influential lymphokines. Macrophages
are versatile cells that play many roles. As scavengers, they rid the body of worn-out cells and
other debris. Foremost among the cells that "present" antigen to T cells, having first
digested and processed it, macrophages play a crucial role in initiating the immune response. As
secretory cells, monocytes and macrophages are vital to the regulation of immune responses and the
development of inflammation; they churn out an amazing array of powerful chemical substances (monokines)
including enzymes, complement proteins, and regulatory factors such as interleukin-1. At the same
time, they carry receptors for lymphokines that allow them to be "activated" into
single-minded pursuit of microbes and tumor cells.
Macrophages
are versatile cells that play many roles. As scavengers, they rid the body of worn-out cells and
other debris. Foremost among the cells that "present" antigen to T cells, having first
digested and processed it, macrophages play a crucial role in initiating the immune response. As
secretory cells, monocytes and macrophages are vital to the regulation of immune responses and the
development of inflammation; they churn out an amazing array of powerful chemical substances (monokines)
including enzymes, complement proteins, and regulatory factors such as interleukin-1. At the same
time, they carry receptors for lymphokines that allow them to be "activated" into
single-minded pursuit of microbes and tumor cells. The mast
cell is a noncirculating counterpart of the basophil. Located in the lungs, skin, tongue, and
linings of the nose and intestinal tract, the mast cell is responsible for the symptoms of allergy.
The mast
cell is a noncirculating counterpart of the basophil. Located in the lungs, skin, tongue, and
linings of the nose and intestinal tract, the mast cell is responsible for the symptoms of allergy. Complement
proteins circulate in the blood in an inactive form. When the first of the complement substances is
triggered-usually by antibody interlocked with an antigen-it sets in motion ripple effect. As each
component is activated in turn, it acts upon the next in a precise sequence of carefully regulated
steps known as the complement cascade."
Complement
proteins circulate in the blood in an inactive form. When the first of the complement substances is
triggered-usually by antibody interlocked with an antigen-it sets in motion ripple effect. As each
component is activated in turn, it acts upon the next in a precise sequence of carefully regulated
steps known as the complement cascade."
 In order to recognize and
respond to the antigens that are their specific targets, both B cells and T cells carry special
receptor molecules on their surface. For the B cell, this receptor is a prototype of the antibody
the B cell is prepared to manufacture, anchored in its surface. When a B cell encounters a matching
antigen in the blood or other body fluid, this antibody-like receptor allows the B cell to interact
with it very efficiently.
In order to recognize and
respond to the antigens that are their specific targets, both B cells and T cells carry special
receptor molecules on their surface. For the B cell, this receptor is a prototype of the antibody
the B cell is prepared to manufacture, anchored in its surface. When a B cell encounters a matching
antigen in the blood or other body fluid, this antibody-like receptor allows the B cell to interact
with it very efficiently. Infants
are born with relatively weak immune responses. They have, however, a natural "passive"
immunity; they are protected during the first months of life by means of antibodies they receive
from their mothers. The antibody IgG, which travels across the placenta, makes them immune to the
same microbes to which their mothers are immune. Children who are nursed also receive IgA from
breast milk; it protects the digestive tract.
Infants
are born with relatively weak immune responses. They have, however, a natural "passive"
immunity; they are protected during the first months of life by means of antibodies they receive
from their mothers. The antibody IgG, which travels across the placenta, makes them immune to the
same microbes to which their mothers are immune. Children who are nursed also receive IgA from
breast milk; it protects the digestive tract. The
most common types of allergic reactions hay fever, some kinds of asthma, and hives are produced when
the immune system responds to a false alarm. In a susceptible person, a normally harmless substance
grass pollen or house dust, for example is perceived as a threat and is attacked.
The
most common types of allergic reactions hay fever, some kinds of asthma, and hives are produced when
the immune system responds to a false alarm. In a susceptible person, a normally harmless substance
grass pollen or house dust, for example is perceived as a threat and is attacked. Autoimmune
reactions contribute to many enigmatic diseases. For instance, autoantibodies to red blood cells can
cause anemia, autoantibodies to pancreas cells contribute to juvenile diabetes, and autoantibodies
to nerve and muscle cells are found in patients with the chronic muscle weakness known as myasthenia
gravis. Autoantibody known as rheumatoid factor is common in persons with rheumatoid arthritis.
Autoimmune
reactions contribute to many enigmatic diseases. For instance, autoantibodies to red blood cells can
cause anemia, autoantibodies to pancreas cells contribute to juvenile diabetes, and autoantibodies
to nerve and muscle cells are found in patients with the chronic muscle weakness known as myasthenia
gravis. Autoantibody known as rheumatoid factor is common in persons with rheumatoid arthritis. Immune complexes are
clusters of interlocking antigens and antibodies. Under normal conditions immune complexes are
rapidly removed from the bloodstream by macrophages in the spleen and Kupffer cells in the liver. In
some circumstances, however, immune complexes continue to circulate. Eventually they become trapped
in the tissues of the kidneys, lung, skin, joints, or blood vessels. Just where they end up probably
depends on the nature of the antigen, the class of antibodY-IgG, for instance, instead of IgM-and
the size of the complex. There they set off reactions that lead to inflammation and tissue damage.
Immune complexes are
clusters of interlocking antigens and antibodies. Under normal conditions immune complexes are
rapidly removed from the bloodstream by macrophages in the spleen and Kupffer cells in the liver. In
some circumstances, however, immune complexes continue to circulate. Eventually they become trapped
in the tissues of the kidneys, lung, skin, joints, or blood vessels. Just where they end up probably
depends on the nature of the antigen, the class of antibodY-IgG, for instance, instead of IgM-and
the size of the complex. There they set off reactions that lead to inflammation and tissue damage. Caused
by a virus (the human immunodeficiency virus, or HIV) that destroys T4 cells and that is harbored in
macrophages as well as T4 cells, AIDS is characterized by a variety of unusual infections and
otherwise rare cancers. The AIDS virus also damages tissue of the brain and spinal cord, producing
progressive dementia.
Caused
by a virus (the human immunodeficiency virus, or HIV) that destroys T4 cells and that is harbored in
macrophages as well as T4 cells, AIDS is characterized by a variety of unusual infections and
otherwise rare cancers. The AIDS virus also damages tissue of the brain and spinal cord, producing
progressive dementia. A child developing in the
womb carries foreign antigens from its father as well as immunologically compatible self antigens
from its mother, and might be expected to trigger a graft rejection. But the uterus is an "immunologically
privileged" site where immune responses are subdued. One source of protection appears to be a
substance produced by the child, perhaps in response to antibodies from the mother. The substance
promotes the development of special white blood cells in the uterus, and these cells release a
factor that blocks the actions of IL-2. Another substance, produced by the uterus, helps disguise
antigens on the fetal surface of the placenta, shielding them from the mother's immune defenses.
A child developing in the
womb carries foreign antigens from its father as well as immunologically compatible self antigens
from its mother, and might be expected to trigger a graft rejection. But the uterus is an "immunologically
privileged" site where immune responses are subdued. One source of protection appears to be a
substance produced by the child, perhaps in response to antibodies from the mother. The substance
promotes the development of special white blood cells in the uterus, and these cells release a
factor that blocks the actions of IL-2. Another substance, produced by the uterus, helps disguise
antigens on the fetal surface of the placenta, shielding them from the mother's immune defenses.
 The immune system provides
one of the body's main defenses against cancer. When normal cells turn into cancer cells, some of
the antigens on their surface change. These new or altered antigens flag immune defenders, including
cytotoxic T cells, natural killer cells, and macrophages.
The immune system provides
one of the body's main defenses against cancer. When normal cells turn into cancer cells, some of
the antigens on their surface change. These new or altered antigens flag immune defenders, including
cytotoxic T cells, natural killer cells, and macrophages.
 More
recently, it has become apparent that hormones and neuropeptides (hormone-like chemicals released by
nerve cells), which convey messages to other cells of the nervous system and organs throughout the
body, also "speak" to cells of the immune system.
More
recently, it has become apparent that hormones and neuropeptides (hormone-like chemicals released by
nerve cells), which convey messages to other cells of the nervous system and organs throughout the
body, also "speak" to cells of the immune system. Through a stratagem
known as hybridoma technology, scientists are now able to obtain, in quantity, substances secreted
by cells of the immune system-both antibodies and lymphokines. The ready supply of these materials
has not only revolutionized immunology but has also created a resounding impact throughout medicine
and industry.
Through a stratagem
known as hybridoma technology, scientists are now able to obtain, in quantity, substances secreted
by cells of the immune system-both antibodies and lymphokines. The ready supply of these materials
has not only revolutionized immunology but has also created a resounding impact throughout medicine
and industry.