Research has revealed a great deal of valuable medical, scientific, and public health information about the human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS). The ways in which HIV can be transmitted have been clearly identified. Unfortunately, false information or statements that are not supported by scientific findings continue to be shared widely through the Internet or popular press. Therefore, the Centers for Disease Control and Prevention (CDC) has prepared this fact sheet to correct a few misperceptions about HIV.
HIV is spread by sexual contact with an infected person, by sharing needles and/or syringes (primarily for drug injection) with someone who is infected, or, less commonly (and now very rarely in countries where blood is screened for HIV antibodies), through transfusions of infected blood or blood clotting factors. Babies born to HIV-infected women may become infected before or during birth or through breast-feeding after birth.
In the healthcare setting, workers have been infected with HIV after being stuck with needles containing HIV-infected blood or, less frequently, after infected blood gets into a worker's open cut or a mucous membrane (for example, the eyes or inside of the nose). There has been only one instance of patients being infected by a healthcare worker in the United States; this involved HIV transmission from one infected dentist to six patients. Investigations have been completed involving more than 22,000 patients of 63 HIV-infected physicians, surgeons, and dentists, and no other cases of this type of transmission have been identified in the United States. Some people fear that HIV might be transmitted in other ways; however, no scientific evidence to support any of these fears has been found. If HIV were being transmitted through other routes (such as through air, water, or insects), the pattern of reported AIDS cases would be much different from what has been observed. For example, if mosquitoes could transmit HIV infection, many more young children and preadolescents would have been diagnosed with AIDS. All reported cases suggesting new or potentially unknown routes of transmission are thoroughly investigated by state and local health departments with the assistance, guidance, and laboratory support from CDC. No additional routes of transmission have been recorded, despite a national sentinel system designed to detect just such an occurrence. The following paragraphs specifically address some of the common misperceptions about HIV transmission.Scientists and medical authorities agree that HIV does not survive well in the environment, making the possibility of environmental transmission remote. HIV is found in varying concentrations or amounts in blood, semen, vaginal fluid breast milk, saliva, and tears. To obtain data on the survival of HIV, laboratory studies have required the use of artificially high concentrations of laboratory-grown virus. Although these unnatural concentrations of HIV can be kept alive for days or even weeks under precisely controlled and limited laboratory conditions, CDC studies have shown that drying of even these high concentrations of HIV reduces the amount of infectious virus by 90 to 99 percent within several hours. Since the HIV concentrations used in laboratory studies are much higher than those actually found in blood or other specimens, drying of HIV-infected human blood or other body fluids reduces the theoretical risk of environmental transmission to that which has been observed–essentially zero. Incorrect interpretation of conclusions drawn from laboratory studies have unnecessarily alarmed some people.
Results from laboratory studies should not be used to assess specific personal risk of infection because (1) the amount of virus studied is not found in human specimens or elsewhere in nature, and (2) no one has been identified as infected with HIV due to contact with an environmental surface. Additionally, HIV is unable to reproduce outside its living host (unlike many bacteria or fungi, which may do so under suitable conditions), except under laboratory conditions, therefore, it does not spread or maintain infectiousness outside its host.Although HIV has been transmitted between family members in a household setting, this type of transmission is very rare. These transmissions are believed to have resulted from contact between skin or mucous membranes and infected blood. To prevent even such rare occurrences, precautions should be taken in all settings including the home to prevent exposures to the blood of persons who are HIV infected, at risk for HIV infection, or whose infection and risk status are unknown. For example,
| Gloves should be worn during contact with blood or other body fluids that could possibly contain visible blood such as urine, feces, or vomit. | |
| Cuts, sores, or breaks on both the care giver's and patient's exposed skin should be covered with bandages. | |
| Hands and other parts of the body should be washed immediately after contact with blood or other body fluids and surfaces soiled with blood should be disinfected appropriately. | |
| Practices that increase the likelihood of blood contact, such as sharing of razors and toothbrushes, should be avoided. | |
| Needles and other sharp instruments should be used only when medically necessary and handled according to recommendations for healthcare settings. (Do not put caps back on needles by hand or remove needles from syringes. Dispose of needles in puncture-proof containers |
There is no known risk of HIV transmission to co-workers, clients, or consumers from contact in industries such as food-service establishments (see information on survival of HIV in the environment). Food-service workers known to be infected with HIV need not be restricted from work unless they have other infections or illnesses (such as diarrhea or hepatitis A) for which any food-service worker, regardless of HIV infection status, should be restricted. CDC recommends that all food-service workers follow recommended standards and practices of good personal hygiene and food sanitation.
In 1985, CDC issued routine precautions that all personal-service workers (such as hairdressers, barbers cosmetologists, and massage therapists) should follow, even though there is no evidence of transmission from a personal-service worker to a client or vice versa. Instruments that are intended to penetrate the skin (such as tattooing and acupuncture needles, ear piercing devices) should be used once and disposed of or thoroughly cleaned and sterilized. Instruments not intended to penetrate the skin but which may become contaminated with blood (for example razors) should be used for only one client and disposed of or thoroughly cleaned and disinfected after each use. Personal-service workers can use the same cleaning procedures that are recommended for healthcare institutions. CDC knows of no instances of HIV transmission through tattooing or body piercing, although hepatitis B virus has been transmitted during some of these practices. One case of HIV transmission from acupuncture has been documented. Body piercing (other than ear piercing) is relatively new in the United States, and the medical complications for body piercing appear to be greater than for tattoos. Healing of piercings generally will take weeks, and sometimes even months, and the pierced tissue could conceivably be abraded (torn or cut) or inflamed even after healing. Therefore, a theoretical HIV transmission risk does exist if the unhealed or abraded tissues come into contact with an infected person's blood or other infectious body fluid. Additionally, HIV could be transmitted if instruments contaminated with blood are not sterilized or disinfected between clients.Casual contact through closed-mouth or social kissing is not a risk for transmission of HIV. Because of the potential for contact with blood during French or open-mouth kissing, CDC recommends against engaging in this activity with a person known to be infected. However, the risk of acquiring HIV during open-mouth kissing is believed to be very low. CDC has investigated only one case of HIV infection that may be attributed to contact with blood during open-mouth kissing.
In 1997, CDC published findings from a state health department investigation of an incident that suggested blood-to-blood transmission of HIV by a human bite. There have been other reports in the medical literature in which HIV appeared to have been transmitted by a bite. Severe trauma with extensive tissue tearing and damage and presence of blood were reported in each of these instances. Biting is not a common way of transmitting HIV. In fact, there are numerous reports of bites that did not result in HIV infection.
HIV has been found in saliva and tears in very low quantities from some AIDS patients. It is important to understand that finding a small amount of HIV in a body fluid does not necessarily mean that HIV can be transmitted by that body fluid. HIV has not been recovered from the sweat of HIV-infected persons. Contact with saliva, tears, or sweat has never been shown to result in transmission of HIV.
From the onset of the HIV epidemic, there has been concern about transmission of the virus by biting and bloodsucking insects. However, studies conducted by researchers at CDC and elsewhere have shown no evidence of HIV transmission through insects—even in areas where there are many cases of AIDS and large populations of insects such as mosquitoes. Lack of such outbreaks, despite intense efforts to detect them, supports the conclusion that HIV is not transmitted by insects.
The results of experiments and observations of insect biting behavior indicate that when an insect bites a person, it does not inject its own or a previously bitten person's or animal's blood into the next person bitten. Rather, it injects saliva, which acts as a lubricant or anticoagulant so the insect can feed efficiently. Such diseases as yellow fever and malaria are transmitted through the saliva of specific species of mosquitoes. However, HIV lives for only a short time inside an insect and, unlike organisms that are transmitted via insect bites, HIV does not reproduce (and does not survive) in insects. Thus, even if the virus enters a mosquito or another sucking or biting insect, the insect does not become infected and cannot transmit HIV to the next human it feeds on or bites. HIV is not found in insect feces. There is also no reason to fear that a biting or bloodsucking insect, such as a mosquito, could transmit HIV from one person to another through HIV-infected blood left on its mouth parts. Two factors serve to explain why this is so–first infected people do not have constant, high levels of HIV in their bloodstreams and, second, insect mouth parts do not retain large amounts of blood on their surfaces. Further, scientists who study insects have determined that biting insects normally do not travel from one person to the next immediately after ingesting blood. Rather, they fly to a resting place to digest this blood meal.Condoms are classified as medical devices and are regulated by the Food and Drug Administration (FDA). Condom manufacturers in the United States test each latex condom for defects, including holes, before it is packaged. The proper and consistent use of latex or polyurethane (a type of plastic) condoms when engaging in sexual intercourse–vaginal, anal, or oral–can greatly reduce a person's risk of acquiring or transmitting sexually transmitted diseases, including HIV infection.
There are many different types and brands of condoms available–however, only latex or polyurethane condoms provide a highly effective mechanical barrier to HIV. In laboratories, viruses occasionally have been shown to pass through natural membrane (skin or lambskin) condoms, which may contain natural pores and are therefore not recommended for disease prevention (they are documented to be effective for contraception). Women may wish to consider using the female condom when a male condom cannot be used. For condoms to provide maximum protection, they must be used consistently (every time) and correctly. Several studies of correct and consistent condom use clearly show that latex condom breakage rates in this country are less than 2 percent. Even when condoms do break, one study showed that more than half of such breaks occurred prior to ejaculation. When condoms are used reliably, they have been shown to prevent pregnancy up to 98 percent of the time among couples using them as their only method of contraception. Similarly, numerous studies among sexually active people have demonstrated that a properly used latex condom provides a high degree of protection against a variety of sexually transmitted diseases, including HIV infection.In 1984, 3 years after the first reports of a disease that was to become known as AIDS, researchers discovered the primary causative viral agent, the human immunodeficiency virus type 1 (HIV-1). In 1986, a second type of HIV, called HIV-2, was isolated from AIDS patients in West Africa, where it may have been present decades earlier. Studies of the natural history of HIV-2 are limited, but to date comparisons with HIV-1 show some similarities while suggesting differences. Both HIV-1 and HIV-2 have the same modes of transmission and are associated with similar opportunistic infections and AIDS. In persons infected with HIV-2, immunodeficiency seems to develop more slowly and to be milder. Compared with persons infected with HIV-1, those with HIV-2 are less infectious early in the course of infection. As the disease advances, HIV-2 infectiousness seems to increase; however, compared with HIV-1, the duration of this increased infectiousness is shorter. HIV-1 and HIV-2 also differ in geographic patterns of infection; the United States has few reported cases.
HIV-2 infections are predominantly found in Africa. West African nations with a prevalence of HIV-2 of more than 1% in the general population are Cape Verde, Côte d'Ivoire (Ivory Coast), Gambia, Guinea-Bissau, Mali, Mauritania Nigeria, and Sierra Leone. Other West African countries reporting HIV-2 are Benin, Burkina Faso, Ghana, Guinea, Liberia Niger, São Tomé, Senegal, and Togo. Angola and Mozambique are other African nations where the prevalence of HIV-2 is more than 1%.
*Prevalence is the proportion of cases present in a population at a given point in time.The first case of HIV-2 infection in the United States was diagnosed in 1987. Since then, the Centers for Disease Control and Prevention (CDC) has worked with state and local health departments to collect demographic, clinical, and laboratory data on persons with HIV-2 infection.
Of the 79 infected persons, 66 are black and 51 are male. Fifty-two were born in West Africa, 1 in Kenya, 7 in the United States, 2 in India, and 2 in Europe. The region of origin was not known for 15 of the persons, although 4 of them had a malaria-antibody profile consistent with residence in West Africa. AIDS-defining conditions have developed in 17 and 8 have died. These case counts represent minimal estimates because completeness of reporting has not been assessed. Although AIDS is reported uniformly nationwide, the reporting of HIV infection, including HIV-2 infection, differs from state to state according to state policy.Because epidemiologic data indicate that the prevalence of HIV-2 in the United States is very low, CDC does not recommend routine HIV-2 testing at U.S. HIV counseling and test sites or in settings other than blood centers. However when HIV testing is to be performed, tests for antibodies to both HIV-1 and HIV-2 should be obtained if demographic or behavioral information suggests that HIV-2 infection might be present.
| Sex partners of a person from a country where HIV-2 is endemic (refer to countries listed earlier) | |
| Sex partners of a person known to be infected with HIV-2 | |
| People who received a blood transfusion or a nonsterile injection in a country where HIV-2 is endemic | |
| People who shared needles with a person from a country where HIV-2 is endemic or with a person known to be infected with HIV-2 | |
| Children of women who have risk factors for HIV-2 infection or are known to be infected with HIV-2 | |
| HIV-2 testing also is indicated for People with an illness that suggests HIV infection (such as an HIV-associated opportunistic infection) but whose HIV-1 test result is not positive | |
| People for whom HIV-1 Western blot exhibits the unusual indeterminate test band pattern of gag (p55, p24, or p17) plus pol (p66, p51, or p32) in the absence of env (gp160, gp120, or gp41) |
Since 1992, all U.S. blood donations have been tested with a combination HIV-1/HIV-2 enzyme immunoassay test kit that is sensitive to antibodies to both viruses. This testing has demonstrated that HIV-2 infection in blood donors is extremely rare. All donations detected with either HIV-1 or HIV-2 are excluded from any clinical use, and donors are deferred from further donations.
Little is known about the best approach to the clinical treatment and care of patients infected with HIV-2. Given the slower development of immunodeficiency and the limited clinical experience with HIV-2, it is unclear whether antiretroviral therapy significantly slows progression. Not all of the drugs used to treat HIV-1 infection are as effective against HIV-2. In vitro (laboratory) studies suggest that nucleoside analogs are active against HIV-2, though not as active as against HIV-1. Protease inhibitors should be active against HIV-2. However, non-nucleoside reverse transcriptase inhibitors (NNRTIs) are not active against HIV-2. Whether any potential benefits would outweigh the possible adverse effects of treatment is unknown.
Monitoring the treatment response of patients infected with HIV-2 is more difficult than monitoring people infected with HIV-1. No FDA-licensed HIV-2 viral load assay is available yet. Viral load assays used for HIV-1 are not reliable for monitoring HIV-2. Response to treatment for HIV-2 infection may be monitored by following CD4+ T-cell counts and other indicators of immune system deterioration, such as weight loss, oral candidiasis, unexplained fever, and the appearance of a new AIDS-defining illness. More research and clinical experience is needed to determine the most effective treatment for HIV-2. The optimal timing for antiretroviral therapy (i.e., soon after infection, when symptoms appear, or when CD4+ T cell counts fall below a certain level) remains under review by clinical experts. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents, by the Department of Health and Human Services Panel on Clinical Practices for Treatment of HIV Infection, may be helpful to the clinician who is caring for a patient infected with HIV-2; however, the recommendations on viral load monitoring and the use of NNRTIs would not apply to patients with HIV-2 infection.HIV-2 infection in children is rare. Compared with HIV-1, HIV-2 seems to be less transmissible from an infected mother to her child. However, cases of transmission from an infected woman to her fetus or newborn have been reported among women who had primary HIV-2 infection during their pregnancy.
Zidovudine therapy has been demonstrated to reduce the risk for perinatal HIV-1 transmission and also might prove effective for reducing perinatal HIV-2 transmission. Zidovudine therapy should be considered for HIV-2-infected expectant mothers and their newborns, especially for women who become infected during pregnancy.Physicians caring for patients with HIV-2 infection should decide whether to initiate antiretroviral therapy after discussing with their patients what is known, what is not known, and the possible adverse effects of treatment.
Continued surveillance is needed to monitor HIV-2 in the U.S. population because the possibility for further spread of HIV-2 exists, especially among injecting drug users and people with multiple sex partners. Programs aimed at preventing the transmission of HIV-1 also can help to prevent and control the spread of HIV-2.
The successful introduction and spread of the human immunodeficiency virus (HIV) into the global human population has occurred for many reasons. The discovery and wide spread use of penicillin and other antibiotics meant that there was treatment and cure for most sexually transmitted diseases. The existence of these new drugs changed how people perceived risks associated with sexual activity. Soldiers in World War II increasingly used prophylactics and the subsequent development of hormonal contraceptives hastened the pace of change in sexual practices, as prevention of pregnancy became a real possibility. Lifestyles also were changing: people were moving into regions that were previously uninhabited by man and long distance travel became easier and was much more common, allowing for more social migration and sexual mixing. Although the virus may have first been introduced to humans earlier in the 20th century (most likely contracted from infected animals), it was in the 1970s that wider dissemination occurred.
For industrialized countries, the first evidence of the AIDS epidemic was among groups of individuals who shared a common exposure risk. In the United States, sexually active homosexual men were among the first to present with manifestations of HIV disease, followed by recipients of blood or blood products, then injection drug users, and ultimately children of mothers at risk.
Women have represented an increasing proportion of reported AIDS cases in the United States, accounting for 23% of adult cases from July 1998-June 1999. (CDC, 1999) Eighty percent of AIDS cases in women are in African Americans and Hispanics, as compared to 61% of cases in men.
In developing countries, the AIDS epidemic manifested itself quite differently, both because the signs and symptoms were harder to identify due to other competing causes of morbidity and mortality and because the epidemic did not seem to be limited to high-risk groups and, instead, was more generalized. Worldwide, women now represent 43% of all adults living with HIV and AIDS (Table 1-1, next page) and this proportion had been steadily increasing over time. (UNAIDS, 1998) This chapter reviews the epidemiology of HIV/AIDS: beginning with how HIV is transmitted and the variables involved; the natural history of HIV infection in women, both without treatment and in the era of highly active antiretroviral therapy (HAART); and concludes with future issues regarding the HIV/AIDS epidemic.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Source: UNAIDS and WHO (2001), AIDS Epidemic Update: December 2001 (Geneva, 2001), p. 3.
Notes: * The proportion of adults (15 to 49 years of age) living with HIV/AIDS in 2000, using 2001 population number. ** Hetero (heterosexual transmission), IDU (transmission through infecting drug use), MSM (sexual transmission among men who have sex with men).
Epidemiological studies have demonstrated that HIV is transmitted by three primary routes: sexual, parenteral (blood-born), and perinatal. Virtually all cases of HIV transmission can be attributed to these exposure categories.
Transmission rates from the infected host to the uninfected recipient vary by both mode of transmission and the specific circumstances. Since HIV is a relatively large virus, has a short half-life in vitro, and can only live in primates, HIV cannot be transmitted from causal (i.e. hugging or shaking hands) or surface (i.e. toilet seats) contact or from insect bites.
Sexual transmission of HIV from an infected partner to an uninfected partner can occur through male-to-female, female-to-male, male-to-male, and female-to-female sexual contact. Worldwide, sexual transmission of HIV is the predominant mode of transmission. (Quinn, 1996) Among U.S. women with AIDS, sexual transmission constitutes 40% of reported cases as of June 1999. (CDC, 1999) This 40% is probably an underestimate when you take into consideration that a large proportion of the women with AIDS who report no identifiable risk (an additional 15% of AIDS cases in women) are actually also infected via sexual transmission. While receptive rectal and vaginal intercourse appear to present the greatest risk of infection (approximately 0.13% and 0.10.2%, respectively, per episode), insertive intercourse (both rectal and vaginal) have also been associated with HIV infection (approximately 0.06% and 0.1%, respectively, per episode). (Vittinghoff, 1999; Mastro, 1996) In addition, there have been a few case reports of male-to-male transmission from receptive oral intercourse with an HIV-infected male partner (approximately 0.04% per contact) and female-to-female transmission from oral-vaginal, oral-anal, and digital intercourse. (Marmor, 1986; Monini, 1996; Monzon, 1987; Perry, 1989; Rich, 1993; Sabatini, 1983). Parenteral transmission of HIV has occurred in recipients of blood and blood products, either through transfusion (estimated 95% risk of infection from transfusion of a single unit of HIV-infected whole blood (CDC, 1998)) or clotting factors, in intravenous or injection drug users through the sharing of needles (approximately 0.67% risk per exposure (Kaplan, 1992)), and in healthcare workers through needle sticks (approximately 0.4% risk per exposure, depending on the size and location of the inoculum (Tokar, 1993)) and less commonly mucous membrane exposure. (Hessol, 1989) Among cumulatively reported AIDS cases in U.S. women through June 1999, 42% had injection drug use as their exposure risk and 3% receipt of blood, blood products, or tissue. (CDC, 1999) Parenteral transmission patterns vary by geographic region due to social and economic factors. For instance, in regions where the prevalence of HIV infection is higher, the risk of occupational or nosocomial transmission of HIV is increased over regions where there is lower prevalence. (Consten, 1995) The transmission risk is therefore related to the prevalence of HIV in the population as well as the frequency of exposure to infected body fluids and organs and the method of exposure. (Fraser, 1995) In addition, many developing countries that have a high prevalence of HIV infection also lack the resources to implement universal precautions adequately (Gilks, 1998) and may experience a greater amount of transfusion-associated HIV transmission due to a lack of HIV antibody screening in some areas, a higher residual risk of contamination in blood supplies despite antibody screening (McFarland, 1997), and high rates of transfusion in some groups of patients.
Perinatal transmission can occur in utero, during labor and delivery, or post-partum through breast-feeding. (Gwinn, 1996) Perinatal transmission rates average 2530% (Blanche, 1989), but vary by maternal stage of disease, use of antiviral therapy, duration of ruptured membranes, practice of breast-feeding, as well as other factors. In the U.S. as of June 1999, 91% of cumulative pediatric AIDS cases were attributed to perinatal transmission.Transmission of HIV infection can be influenced by several factors, including characteristics of the HIV-infected host, the recipient, and the quantity and infectivity of the virus. A summary of factors affecting sexual transmission of HIV is presented in Table 1-2.
There is an association between the quantity of virus transmitted and the risk of HIV infection. (Roques, 1993) Several studies have found that HIV-infected persons may be more likely to transmit the infection when viral replication is high, both during the initial stage of infection (Palasanthiran, 1993) and at more advanced stages of HIV disease. (Laga 1989) People with high blood viral load are more likely to transmit HIV to recipients of blood, their sexual partners, and their offspring. (Vernazza, 1999; Quinn, 2000) HIV has been quantified in semen (Coombs, 1998; Speck, 1999; Vernazza, 1997) and detected in female genital secretions (Ghys, 1997; Mostad, 1998), and virus in these locations may facilitate transmission. However, the association between infectivity and disease stage is not absolute; HIV-infected women may transmit virus to a first-born child while not to a second-born child (deNartubim 1991), and temporal studies of semen from HIV-infected men demonstrate waxing and waning viral titers over time. (Krieger, 1991; Tindall, 1992) Factors that decrease viral titers, including anti-retroviral therapy, may decrease but not eliminate the risk of HIV transmission. (Hamed, 1993) Zidovudine has been shown to reduce vertical transmission from mothers to their fetus even when administered late in pregnancy or during labor. (CDC, 1998) (See Chapter VII on HIV and Reproduction) Individuals receiving antiretroviral therapy have also shown reduced transmission rates of HIV to their sex partners. (Musicco, 1994) Several studies have suggested that anti-retroviral treatment reduces detection of HIV in female genital secretions (Cu Uvin,1998) and the concentration of HIV in semen. (Gilliam, 1997; Gupta, 1997) Providers counseling patients on treatment should be clear that precautions to prevent transmission of the virus should be maintained since not all treatment reduce infectiousness and transmissions have been reported among individuals with undetectable HIV RNA levels. (The European Collaborative Study Group, 1999) Factors which increase the risk of exposure to blood, such as genital ulcer disease (Cameron, 1989; Plummer, 1991), trauma during sexual contact (Marmor, 1986), and menstruation of an HIV-infected woman during sexual contact (The European Collaborative Study Group, 1992; Nair, 1993; St. Louis, 1993) may all increase the risk of transmission.
Method of contraception also affects the likelihood of HIV transmission. (Daly, 1994) There is overwhelming evidence that the correct and consistent use of latex condoms protect both men and women against HIV. However, because of methodologic difficulties in studies of contraceptive use and HIV transmission, it remains unclear whether the use of hormonal contraceptives, IUDs and spermicides alter the risk of HIV transmission.
| Biologic Factor | Host-Related Infectivity Factors | |||
|---|---|---|---|---|
| HIV Concentration in Genital Secretions | Infectiousness (Transmission) | Susceptibility (Acquisition) | ||
| Mutation of chemokine-receptor gene | ? | ? | ||
| Late stage of HIV infection | Not applicable | |||
| Primary HIV infection | Not applicable | |||
| Antiretroviral therapy | ||||
| Local infection (inflammation or ulcer of reproductive tract or rectal or oral mucosa) | ||||
| Presence of cervical ectopy | ||||
| Presence of foreskin | ? | |||
| Method of contraception |
|
|
|
|
| Barrier | Not applicable |
|
|
|
|
|
Hormonal contraceptives |
|
|
|
|
|
Spermicidal agents | ? |
|
|
| Intrauterine devices | ? | ? |
|
|
| Menstruation | ? | |||
| Factors that lower cervicovaginal pH | ||||
| Immune activation | ||||
| Genital tract trauma | ||||
| Pregnancy | ||||
*The associations represented were
statistically significant in at least one study. The degrees of positivity (![]() to
to ![]()
![]()
![]() )
and negativity (
)
and negativity (![]() to
to
![]()
![]()
![]() )
of the associations are indicated with arrows, with three arrows indicating a
very strong association. The symbol
)
of the associations are indicated with arrows, with three arrows indicating a
very strong association. The symbol ![]() denotes that there is evidence in support of both a positive and a negative
association. A question mark indicates an unknown or hypothesized association
that is not currently supported by data.
denotes that there is evidence in support of both a positive and a negative
association. A question mark indicates an unknown or hypothesized association
that is not currently supported by data.
Royce RA, Sena A, Cates W Jr, Cohen MS. Current concepts: sexual transmission of HIV. N Engl J Med. 1997;336:1072-1078. Copyright 1997. Massachusetts Medical Society. All rights reserved.
Similarly, characteristics of the uninfected individual may increase the likelihood of infection for a given exposure to HIV. Specifically, inflammation or disruption of the genital or rectal mucosa (which can occur with sexually transmitted diseases and trauma), and lack of circumcision in heterosexual men may increase the risk of infection. (Cameron, 1989; Moses, 1994; Quinn, 2000) Sex during menstruation may increase womens risk of acquiring HIV infection (Lazzarin, 1991) as may bleeding during sexual intercourse. (Seidlin, 1993) In women, both uclerative and non-uclerative sexually transmitted diseases have been shown to be risk factors for getting infected with HIV. (Laga, 1993; Plummer, 1991) Cervical ectopy has been identified as a risk factor for acquisition of HIV infection in some (Nicolosi, 1994; Plourde, 1994) but not all (Mati, 1994) studies that have evaluated this condition. There is also some evidence that changes in the vaginal flora, as characterized by bacterial vaginosis, may facilitate acquisition of HIV. (Sewankambo, 1997) Non-barrier contraceptive methods have also been investigated in association with risk of HIV transmission but the results are inconclusive. The most frequently studied methods of contraception have been oral contraceptives, injectable hormones, intrauterine devices, and nonoxynol-9. (Daly, 1994; Plummer, 1998) (See Chapter III on Prevention) Traditional vaginal agents, used in African women for sexual enhancement and self-treatment of vaginal symptoms, has also been investigated as a potential cofactor for HIV transmission. (Dallabetta, 1995) For many of these studies, limitations of the study design preclude any definitive conclusions.
There is increasing evidence that host genetic or immunologic factors may protect against HIV infection. This has been investigated in cohort studies of Nairobi sex workers (Willerford, 1993) and in United States homosexual men (Dean, 1996), both of who remained uninfected despite multiple sexual exposures to HIV. Individuals who are homozygous for a null allele of CCR5 are relatively resistant to sexually transmitted infection with HIV, indicating an important, though not absolute, role for this receptor in viral transmission. However, homozygous CCR5 mutations were not found among 14 hemophiliacs who remained uninfected with HIV after being inoculated repeatedly with HIV contaminated Factor VIII concentrate from plasma during 1980-1985. (Zagury, 1998) In this study, investigators found an over production of betachemokines in most of the uninfected individuals.Several viral factors have been proposed to play a role in the transmissibility of HIV. These include phenotypic characteristics (e.g., envelope proteins required for transmission), genetic factors that control the replicative capacity and fitness of the virus, and resistance to antiviral drugs. (Vernazza, 1999) Envelope sequences can define viral quasispecies that have been phenotypically arranged according to their ability to induce syncytia formation in infected T-cells. (Paxton, 1998) It appears that the most commonly transmitted phenotype is the non-syncytia-inducing (NSI), M-tropic viral strain, which is frequently found in those who have been recently infected. During the course of HIV infection the development of a more cytopathic, syncytia-inducing (SI), T-tropic viral phenotype can be found and this is often a precursor to the development of AIDS. While some researchers have suggested that NSI isolates of HIV are preferentially transmitted (Roos, 1992), others have not been able to show preferential transmission of this isolate. (Albert, 1995) Envelope sequences can also be used to define viral subtypes, or clades, and these subtypes may also influence the transmissibility of HIV. The distribution of HIV subtypes differ according to geographic region, with A, C, D, and E predominant in sub-Saharan Africa and Asia and B predominant in the United States, the Caribbean, South America, and Western Europe. (Hu, 1996) In one study, subtype E is reported to have greater tropism for Langerhans cells than subtype B (Soto-Ramirez, 1996) and may have a greater per contact transmissibility.
Lastly, the transmission characteristics of a viral strain that is resistant to certain antiretro- viral agents may differ from transmission of wild type virus. More research is needed in this emerging field of therapy-resistant virus and its characteristics.The natural history of HIV infection in adults has been extensively documented in the medical literature. The impact of gender on the manifestations and progression of HIV-disease is still being investigated. Concerns about gender-based differences in the course of HIV infection were expressed early in the epidemic. In most industrialized countries, women tended to have lower income, be un- or under-insured for health care, know less about HIV, more likely to be Black or Hispanic, and to have a personal or partner history of injection drug or cocaine use. Women also appeared to have more rapid progression of illness than men and to present with a different constellation of opportunistic conditions than men. When sophisticated statistical methods were applied that controlled for the tendency of women to receive less care and to present with more advanced disease, gender-based differences in HIV disease course appeared to lessen. More recently, however, with better measures of viral activity and infirmity, the issue of gender-based differences in rate of disease course and virologic parameters has again been raised. These new observations have prompted active research into the impact of gender, hormones and demographic factors on the outcome of HIV infection.
HIV infects and induces cell death in a variety of human cell lines. T-helper lymphocytes (also known as CD4 cells) are a major target of viral infection, and circulating CD4-cells become steadily depleted from peripheral blood in most untreated infected persons. Thus quantification of CD4-cells in blood is a rather simple way of determining cumulative immunologic damage due to HIV. Profound CD4-cell depletion is unusual in persons who do not have HIV infection and are usually iatrogenic or associated with severe illnesses, such as chemotherapy-induced eukopenia. (Aldrich, 2000) Other immunological parameters become altered with HIV-disease progression, and though often used for research purposes, they tend to be more difficult to measure and less reliable or more costly. Untreated HIV infection is a chronic illness that progresses through characteristic clinical stages; AIDS is an endpoint of HIV infection, resulting from severe immunological damage, loss of an effective immune response to specific opportunistic pathogens and tumors. AIDS is diagnosed by the occurrence of these specific infections and cancers or by CD4-cell depletion to less than 200/mm3.HIV can cause a wide range of symptoms and clinical conditions that reflect varying level of immunological injury and different predisposing factors. Certain conditions tend to occur in association with each other and at specific CD4 cell counts. Staging systems for HIV-disease facilitate clinical evaluation and planning therapeutic interventions, help determine the individual level of infirmity, and give prognostic information. Untreated HIV infection is a chronic illness that progresses through characteristic clinical stages that can be used to describe infirmity.
Several groups have produced organized staging systems to facilitate clinical evaluation and planning therapeutic interventions. In industrialized countries, the most widely used system for classifying HIV infection and AIDS in adults and adolescents was published by the United States Centers for Disease Control in 1993. (CDC, 1992) The case definition (Table 2-3) begins first with confirmation of HIV infection either via serologic testing (combination of a screening method such as enzyme immunoassay and more specific confirmatory test such as Western blot), or direct detection of HIV in patient tissue by viral culture, antigen detection or other test such as polymerase chain reaction (PCR). The definition of each stage of illness is then based on two types of information: peripheral blood CD4 cell counts and clinical manifestations. CD4 cell counts are placed in three strata, ranging from relatively normal (gt; 500 cells/mm3) to severe CD4 depletion (lt; 200 cells/mm3).
The clinical manifestations of HIV infection are also placed in three strata, generally in accordance with the level of immunologic dysfunction associated with the various conditions. (Table 2-3) Category A includes persons who have minimal clinical findings, clinical findings that do not indicate immune injury (including absence of symptoms), generalized lymphadenopathy or resolved acute HIV infection. Category B includes conditions that indicate the presence of a defect in cell-mediated immunity or conditions that appear to be worsened by HIV infection. Category C includes conditions that are considered AIDS defining, even in the absence of a CD4 cell count less than 200 cell/mm3. (CDC, 1992) The addition of specific laboratory measures such as plasma HIV RNA level, improves prognostic value even after the occurrence of Category C conditions. (Lyles, 1999)The CDC criteria require diagnostic testing and case confirmation methods that may not be available in developing countries, so several other sets of criteria have been proposed for these regions. Since lymphocyte subset quantitation is not widely available in many countries, the Global Program on AIDS of the World Health Organization (W.H.O.) proposed a clinically based staging system that is more broadly applicable than the CDC system. (W.H.O., 1993) The system uses clinical historical data, laboratory measures (optional) and
indices of physical activity to assess level of infirmity to establish four strata that are summarized in Table 2-4. Laboratory measures include a single assessment absolute CD4 cell count, with the option of replacing this test with total lymphocyte count, each of which are placed in three strata. CD4 cell count is a better prognostic indicator than total lymphocyte count, but the two results correlate well. (Brettle, 1993)
Clinical history and functional measures are placed in four categories that range from asymptomatic to severe disease. In general, when compared with the CDC stages, the W.H.O. system requires less diagnostic test data and fewer direct observations. The definition includes broader categories for conditions that may vary by region (e.g., disseminated infections with endemic mycoses which are common in South East Asian AIDS patients but not in the United States or Europe). The inclusion of performance scale measures permits quantitative clinical assessment that is not dependent on laboratory resources.
The four clinical stages in the W.H.O. system correlated well with CD4 cell counts and HIV RNA levels in a study of 750 Ethiopians (included 336 women) by Kassa and others. (Kassa, 1999) Other studies of patient populations have also demonstrated correlation of W.H.O. clinical stage with CD4 cell count and clinical outcome. (Morgan, 1997; Morgan, 1998; Schechter, 1995) When compared with the CDC staging, the W.H.O. clinical stages demonstrated a high degree of specificity, but a lower level of sensitivity (3565%) for HIV infection. (Gallant, 1993; Gallant, 1992) In particular all of the systems for disease staging are not perfectly sensitive and specific for HIVinfection, but can be improved by the addition of HIV serologies. (Ankrah,1994; DeCock, 1991) Modifications (Table 2-4) have been proposed that improve the prognostic accuracy of the W.H.O. system. Based on observations made in a study of AIDS mortality among Rwandan women, Lifson and colleagues proposed minor modifications of clinical history definitions, replacement of body mass index (BMI) (weight(in kg) divided by height (in m2)) for weight loss and use of erythrocyte sedimentation rate (ESR) as a laboratory indicator of infirmity. (Lifson, 1995) BMI was significantly better at predicting mortality than percentage of body weight lost over two measurements taken in one year. Both ESR and hematocrit were highly predictive of mortality over a 36 month period of observation. (Lifson, 1995) Other HIV-disease classifications, such as the Caracas definition proposed by the Pan American Health Organization (Rabeneck, 1996; Weniger, 1992) have been proposed but have not been evaluated as extensively as the CDC and W.H.O. systems.
Table 2-3. 1999 Revised Classification System For HIV Infection and Expanded Surveillance Case Definition For AIDS Among Adults and Adolescents |
|||
|---|---|---|---|
| CD4+ T cell
categories (cells/cu mm) |
Clinical categories* | ||
| CD4 Cell Category | Clinical Category A | Clinical Category b | Clinical Category A |
| 500 cells/mm3 | A1 | B1 | C1 |
| 200-499 cells/mm3 | A2 | B2 | C2 |
| < 200 cells/mm3 | A3 | B3 | C3 |
| Category A Conditions | Category C Conditions | Category C Conditions | |
|
Asymptomatic HIV infection Persistent generalized lymphadenopathy Acute (primary) HIV infection with accompanying illness or history of acute HIV infection |
Bacillary angiomatosis Candidiasis, oropharyngeal (thrush) Candidiasis, vulvovaginal; persistent, frequent, or poorly responsive to therapy Cervical dysplasia (moderate or severe)/cervical carcinoma in situ Constitutional symptoms, such as fever (38.5 degrees centigrade) or diarrhea lasting greater than 1 month Hairy leukoplakia, oral Herpes zoster (shingles), involving at least two distinct episodes or more than one dermatome Idiopathic thrombocytopenic purpura Listeriosis Pelvic inflammatory disease, particularly if complicated by tubo-ovarian abscess Peripheral neuropathy |
Candidiasis of bronchi, trachea, or lungs Candidiasis, esophageal Cervical cancer, invasive Coccidioidomycosis, disseminated or extrapulmonary Cryptococcosis, extrapulmonary Cryptosporidiosis, chronic intestinal, > 1 month Cytomegalovirus disease (other than liver, spleen, or nodes) Cytomegalovirus retinitis (with loss of vision) Encephalopathy, HIV-related Herpes simplex; chronic ulcer(s) > 1 month; or bronchitis, pneumonitis, or esophagitis Histoplasmosis, disseminated or extrapulmonary Isosporiasis, chronic intestinal > 1 month Kaposi's sarcoma (KS) Lymphoma, Burkitt's (or equivalent term) Lymphoma, immunoblastic (or equivalent term) Lymphoma, primary, of brain Mycobacterium avium complex or M. kansasii, disseminated or extrapulmonary Mycobacterium tuberculosis, any site (pulmonary or extrapulmonary) Mycobacterium, other or unidentified species, disseminated or extrapulmonary Pneumocystis carinii pneumonia (PCP) Pneumonia, recurrent Progressive multifocal leukoencephalopathy (PML) Salmonella septicemia, recurrent Toxoplasmosis of brain Wasting due to HIV |
|
| * | Persons in categories A3, B3, C1, C2, and C3 have AIDS under the 1993 surveillance case definition (given below). |
| ** | PGL = persistent generalized lymphadenopathy. Clinical Category A includes acute (primary) HIV infection. |
| # | See clinical categories below. |
| ## | See list of AIDS-defining conditions for 1993 definition below. |
| Table 2-4. World Health Organization Classification System for HIV Staging System | ||||||
|
|
Laboratory Component | Clinical Group | ||||
| CD4 Cell Count | Total Lymphocyte Count | 1 | 2 | 3 | 4 | |
| A | ≥500 | ≥2000 | A1 | A2 | A3 | A4 |
| B | 200-499 | 1000-1999 | B1 | B2 | B3 | B4 |
| C | <200 | <1000 | C1 | C2 | C3 | C4 |
| Clinical Stage | Clinical History | Performance Scale Criteria | Propose Modifications | |||
|
One: Asymptomatic |
Asymptomatic infection |
Normal functional level in
performance scale |
none | |||
| Two Mild: Disease | Unintentional weight loss < 10% body weight Minor mucocutaneous manifestations (e.g., dermatitis, prurigo, fungal nail infections, angular cheilitis) Herpes zoster within previous 5 years Recurrent upper respiratory tract infections |
Performance Scale level at which symptoms, but nearly fully ambulatory | ||||
| Three: Moderate Disease | Unintentional weight loss > 10% body weight Chronic diarrhea > 1 month Prolonged fever > 1 month (constant or intermittent) Oral candidiasis Oral hairy leukoplakia Pulonary tuberculosis within the previous year Severe bacterial infections Vulvovaginal candidiasis |
Performance Scale level at which in bed more than normal but < 50% of normal daytime during the previous month | ||||
Acute HIV infection is a transient symptomatic illness that can be identified in 40-90% of cases of new HIV infection. It is characterized by a high rate of HIV replication, high titers of virus in blood and lymphoid organs (up to several million copies of HIV RNA per mm3 of plasma), and initiation of an HIV-specific immune response. The amount of virus present in blood and tissues begins to fall after appearance of cytotoxic (killer) lymphocytes that specifically react with HIV antigens; the vigor of this response varies among individuals and is associated with subsequent rate of disease progression. (Cao, 1995) A pool of persistently infected CD4 cells (latent reservoirs) emerges early in the course of HIV infection and persists indefinitely. (Chun, 1998) Symptoms have been identified from 5-30 days after a recognized exposure to HIV. (Schacker, 1998) The signs and symptoms of acute HIV infection are not specific; fever, fatigue, rash, headache, lymphadenopathy, pharyngitis, mild gastrointestinal upset, night sweats, aseptic meningitis and oral ulcerations are most frequently reported. Because the clinical signs of acute HIV infection resemble those of many acute viral illnesses, the correct diagnosis is often missed. Because early treatment at the time of acute infection may be especially beneficial (See Chapter IV on Primary Medical Care), early suspicion of and evaluation for HIV infection should be encouraged. (Kahn, 1998)
Regardless of whether the syndrome of acute HIV infection is recognized or not, after the HIV-specific immunological response begins to control the intensity of viremia, a so-called viral set-point is established, which varies by individual. With exceedingly rare exceptions, the immunological response to HIV does not eliminate infection, but rather establishes a steady state between viral replication and elimination. (Henrad, 1995) A variable level of viremia is attained, which can be measured via quantification of the number of copies of HIV RNA present in blood (viral load). Although the viral load within the first 120 days of HIV infection is not of prognostic value (Schacker, 1998), in most patients a relatively stable viral load is attained after recovery from acute infection, and this viral set point is highly predictive of the rate of future progression of illness, at least as determined in studies that were largely focused on men. In the case of a high viral load set point (i.e. values ranging up from 40,000 copies per mm3) more rapid decline in CD4 cell counts and more rapid occurrence of Clinical Class B and C conditions will occur. Some individuals have viral load set points that are low (below 500 copies per mm3), which indicates a better prognosis; no evidence of progression (CD4 cell depletion or HIV-diseases) is seen for long periods of time in a small subset of patients (see section on long-term progression, below). The viral set point is likely influenced by several factors such as presence of other infections at the time of HIV exposure, genetic characteristics (particularly the type of HIV binding receptors present on lymphocytes) viral characteristics, age and perhaps gender (see below). (Kahn, 1998) During the period of clinical stability acute illnesses and other events that can stimulate the immune system, such as influenza, herpes simplex outbreaks, and tuberculosis as well as routine vaccinations, have been demonstrated to result in 10-1000 fold increases in viral load; these increases are transient and most often resolve within two months. (Stanley, 1996; Staprans, 1995) Thus, determination of viral load for prognostic purposes should not be done during or shortly after an acute illness.
For most HIV-infected persons, viral quasispecies evolve overtime. Transition for the non-syncytia-inducing macrophage-tropic viral strains, that are commonly present after transmission to syncytia-inducing T-lymphocyte tropic strains occurs in many hosts. While variation of viral quasispecies with time is usual, the mechanism by which this process occurs has not been defined. However, transitions in viral quasispecies and cellular tropism has been observed to coincide with key clinical events such as CD4 cell depletion and development of symptomatic illness. These virologic changes may reflect evolution of a virus that is tailored to an individuals immune response or other genetic characteristics. Interventions that prevent evolution of quasispecies in a host may yield effective therapies in the future. The HIV RNA level in tissues does not correlate in a linear fashion with blood levels, so even in patients with undetectable plasma HIV RNA, intracellular and tissue HIV RNA can still be detected with more sophisticated techniques. (Hockett, 1999) Thus HIV replication continues at varying pace among infected persons, even those who can control viremia well. HIV is also frequently present in the genital tract (Fiore, 1999; Iverson, 1998), where expression of inflammatory mediators, and lymphocyte receptors differ from blood and may influence the rate of viral replication and numbers of virions present. (Anderson, 1998; Hladik, 1999) While the quantities of HIV present in cervicovaginal fluid are generally similar to blood (Hart, 1999; Shaheen, 1999), they differ in some individuals. The finding that HIV isolates from the lower genital tract can have different genotypic markers than blood isolates from a single host (DiStefano 1999; Shaheen, 1999), supports the concept that the lower genital tract sometimes functions as a separate virologic compartment.In most studies of seroconverters, (persons for whom the date of the HIV infection can be estimated), 5060% of adults will be diagnosed with an AIDS-defining condition within 10 years of infection (for the pre-HAART treatment era). Forty-eight percent of seroconverters die (due to any cause) after 10 years of infection. Increasing age is the factor most consistently associated with rate of progression and death in most groups of patients studied to date. (Alioum, 1998; UK Register of HIV Seroconverters Steering Committee, 1998; Pezzott, 1999; Prins, 1999) Date of infection also influences time from infection to an AIDS diagnosis, at least in some locations, demonstrating that even in the pre-HAART era, improvements in treatment resulted in tangible benefits. (Webber, 1998)
A large number of laboratory tests have been evaluated as prognostic indicators in HIV infection. For the most part the tests can be divided into three groups: A. measures of HIV replication, B. measures of immune function and C. measures of inflammation. Group A is specific to HIV infection, Group B, when indicating severe CD4 cell depletion is relatively specific to HIV infection and Group C are generally not specific to HIV infection. Table 2-5, on the following page, summarizes these laboratory measures, outcomes, their advantages and disadvantages. HIV RNA quantitation performed on fresh or fresh-frozen plasma or serum, is a powerful and accurate prognostic indicator in HIV infection and is uniquely useful in determining response to antiretroviral therapy. (Saag, 1996) In general the best measures of prognosis and staging include combinations of HIV RNA level, CD4 cell count and perhaps lymphocyte function (cytotoxic lymphocyte response to HIV). (Spijkerman, 1997; Vlahov, 1998)



In untreated adults the median time from HIV infection to AIDS in developed countries is 8- 10 years. However, approximately 8-15% of HIV infected persons (most studies focus on men) remain symptom free for much longer periods of time, a phenomenon that has been named long-term survival (LTS). Among these individuals who remain clinically stable without treatment for 5-8 years, two groups can be discerned, those who have stable CD4 cell counts and those who have low CD4 cell counts, but no AIDS defining conditions. (Schrager 1994) Several factors have been found to be associated with long-term survival including host characteristics such as the presence of specific anti-HIV cytotoxic lympho- cyte responses, and viral characteristics such as defective genes and gene products. (Kirchhoff, 1995) LTS patients tend to have consistently lower levels of HIV RNA after the period of acute infection suggesting better control of viral replication. (Vesanen, 1996) For example viral growth in peripheral mononuclear cells taken from LTS was markedly less than in PBMCs taken from healthy HIV-uninfected donors. (Cao, 1995)
In general the predictors of the rate of HIV-disease progression and survival among women are the same as in men. CD4 cell count depletion and higher HIV RNA level are strong predictors of progression and survival in women. (Anastos 1996b) Several recent reports, however, describe gender-based differences in HIV RNA level and in rate of CD4 cell depletion; women had HIV RNA levels that were 30-50% lower than men who had comparable CD4 cell counts. (Bush, 1996; Evans, 1997; Farzadegan, 1998) Similar results occurred when analysis was restricted to seroconverters or when HIV culture was used to quantify viremia rather than RNA assays. ()Lyles, 1998; Sterling, 1999) Intuitively, lower levels of circulating HIV RNA, which suggest lower steady state level of viremia, should be associ- ated with better outcome. However, the findings of several recent studies suggest that the lower HIV RNA level does not provide benefit to women. Women experienced more rapid CD4 cell depletion and faster progression to AIDS and death than men at similar HIV-RNA levels, even when race and age were taken into consideration. (Anastos, 1999a; Farzadegan 1998) Determination of the effect of gender on the rate of progression, time until occurrence of an AIDS defining condition and death is a complicated process. Unless the date of HIV infection can be established, duration of infection becomes a significant unknown factor in studies. In addition, particularly in developed countries, HIV-infected women and men differ by more than just their gender. Women tend to have lower income, be members of minority ethnic groups, have been born in Africa, have used injection drugs or cocaine, or to have a sexual partner who has done so, all of which are risk factors for poor health in general. In most studies women have shorter duration of infection prior to AIDS and death than men, but these differences tend to disappear when CD4 cell count and drug use are taken into consideration. (Alioum, 1998; UK Register of HIV Seronconverters Steering Committee, 1998; Pezzotti, 1999; Santoro-Lopes 1998) Several studies have reported an excess proportion of infections or deaths due to bacterial infection, often pneumonia (Feldman, 1999), among women compared with men. (Melnich, 1994; Weisser, 1998) A summary of factors that influence disease progression is shown in Table 2-6.
In countries that are able to provide highly affective antiretroviral treatments (HAART), HIV-associated morbidity and mortality have declined significantly. (Michales, 1998; Miller, 1999a; Miller 1999b; Palella, 1998; Pezotti, 1999) (See Primary Medical Care in Chapter IV for more information). These population findings, based on regional surveillance systems, were preceded by a multitude of clinical trials that demonstrated clinical and virologic benefits of HAART. (Bartlett, 1996; Collier, 1996; Deeks, 1997; Hammer, 1997) Despite the promise and documented benefits of HAART clinical progression continues to occur among recipients, particularly among persons who received antiretroviral treatment prior to initiation of HAART. (Ledergerber, 1999) Viral resistance to HAART components can occur via several mechanisms, which for the most part involve mutation of viral target proteins. (Richman, 1996; Schapiro, 1999) The emergence of antiretroviral resistance is a function of several factors: prior treatment, pre-treatment level of viremia drug levels (adherence to medication regimens, bioavailability of medications, adequate dosing) and specifics of the regimen. (Guilick, 1998; Ledergerber, 1999; Shafer, 1998) Multiple daily doses, side effects and in some cases, dietary restrictions aggravate the problem of achieving optimal drug levels since protease inhibitor agents are relatively poorly bioavailable. Suppression of viral replication and prevention of resistance are directly related to level of antiretroviral drug. Persistent viral replication provides opportunity of occurrence of resistance mutations, and selective pressure to support continued presence of such mutants. (Condra, 1998; Feinberg, 1997; Wong, 1997) Besides clinical treatment failure, emergence of antiretroviral resistance is now associated with transmission of resistant virus to previously uninfected persons, a finding that could portend significant limits to the effectiveness of these treatments in populations over long periods of time. (Boden, 1999; Brodine, 1999; Yerly, 1999)
The high cost of antiretroviral drugs and the need for clinical and laboratory services for monitoring response to and efficacy of these treatments has greatly restricted provision of HAART in the developing world. Thus the reductions in morbidity and gains in survival in HIV patients that have been demonstrated in many industrialized countries do not extend to developing countries in which the majority of HIV cases worldwide occur. A consensus statement regarding provision of these therapies has been released based on meetings held in Dakar and Abidjan during 1997. The key recommendations of conference participants include: efforts must be made to expand provision of ART, ART only makes sense in the setting of effective AIDS control programs, funding must be sustained to provide uninterrupted treatment and continuity of care, care providers must be trained in use of the treatments and basic patient rights resources for assessment of efficacy and tolerance must be available, sentinel monitoring for resistance pattern determination should be available, 3-drug combination regimens should be used when possible, treatment of pregnant women to prevent perinatal transmission must be a priority, and new drug development should focus on less costly medications. (International AIDS Society, 1999)
The HIV/AIDS epidemic continues to spread without full control in any country. Over forty million people have been infected worldwide. By the end of 1998, the United Nations Program on AIDS (UNAIDS) estimated that 33.4 million people were living with HIV, a figure which includes 13.8 million adult women (UNAIDS, 1998) (Table 1-1, page 2). In 1998 it is estimated that 5.8 million new HIV infections occurred of with 2.1 million of these occurring in women. After steady increases of the prevalence of disease among women during the 1990s, currently 43% of all persons over the age of 15 years living with HIV are women. Globally, AIDS is now the fourth leading cause of mortality; 2.5 million deaths have been attributable to AIDS, of which 900,000 occurred in women. The notable improvements in AIDS mortality reported in North America and Europe, in association with the introduction of highly active combination antiretroviral therapies, do not extend to most of the worlds cases which occur in regions in which this expensive type of treatment is not available. More than 95% of HIV-infected people live in the developing world, most in Sub-Saharan Africa. Seventy percent of infections that occurred during 1998 took place in this epicenter. The region has also experienced 83% of all AIDS deaths. Unfortunately prior projections of the epidemic course in Southern Africa underestimated the incidence of infection by half. (Balter, 1998) Improved data have revealed that the prevalence rates in southern Africa are staggering: 20-26% of adults (aged 15-49 years) are infected; in some regions 20-50% of pregnant women are infected and are likely to transmit infection to 1/3 of their offspring. The declining mortality rate, and population growth taking place in other regions cannot be extended to Sub-Saharan Africa, due to the extent of AIDS mortality. (Bongaarts 1998) AIDS has now surpassed malaria as the leading cause of death in this region. (Balter, 1999) Life expectancy will fall from 64 to 47 years by 2015. AIDS will cost, on an average, 17 years of life expectancy in the 9 Sub-Saharan countries with a > 10% prevalence of HIV infection among adults. The child mortality rates in this region are also elevated by AIDS; rates are approximately double that expected without the HIV epidemic. (UNAIDS, 1998) Within one year 2,400 Zimbabweans will succumb to AIDS per week, many in the prime of life, many leaving dependent children as orphans (up to 1 in 5 children are likely to become orphans). The United States Surgeon General, David Satcher, notes that the progress of decades of work immunizing children, controlling diseases, and improving nutrition is being negated by HIV. (Satcher, 1999) In Asia, the epidemic has a mixed pattern that includes countries with slow growth in HIV prevalence, countries with some success in control efforts and regions that appear to be experiencing explosive epidemics. Currently 7 million Asians are infected with HIV. Rapidly accelerating epidemics are possible in China, Cambodia, Vietnam and India. While urban areas were initially of greatest concern in many countries, recent information has revealed very active epidemics in specific rural areas (up to 2% of the general population), which are hosts to large proportions of the regions population.
While the outlook for AIDS in Asia is bleak, there is also cause for hope. Growth of the epidemic in the Philippines is notably slow. (Jacobs, 1999) Thailand has been successful in reducing the incidence of infection in sentinel population groups (such as members of the military and pregnant women) using a combination of good surveillance effective policy response, implementation of educational and condom promotion programs. The incidence of HIV infection among pregnant women in Thailand has dropped from a peak of 2.4% in 1995 to 1.7% in 1997. (Phoolchareon 1998) However ongoing political upheaval and cuts to the national HIV prevention budget may modify this pattern of success in the near future. In the Americas the epidemic continues to grow in specific subgroups. In the United States, as summarized earlier in this chapter, the highest incidence of infection is occurring among poor women, particularly among women of color. In Mexico the incidence of infection among men who have sex with men continues high, while in Brazil and the Caribbean heterosexual transmission is increasing. At surveillance sites in the Dominican Republic and Haiti the prevalence of HIV infection among pregnant women has reached 8%. (UNAIDS, 1998) Rapid spread of infection among injection drug users in Eastern Europe and Central Asia likely to foreshadow a large number of cases among women and increasing prevalence of perinatal transmission. The introduction of HIV into these high-risk populations has been paralleled by tremendous increases in the incidence of syphilis and other sexually transmitted diseases. (Gollub, 1999)Control of the HIV epidemic should be a worldwide health priority. Complex interactions of social, economic and cultural factors have preceded AIDS with epidemics of other sexually transmitted diseases, and now hinder control of HIV itself. Global disparities in economic status have limited efforts to control sexually transmitted diseases that are much simpler to diagnose and treat than HIV. The effect of limited monetary resources are compounded by stigmatization of HIV and sexually transmitted diseases that effect willingness to seek care, social support of afflicted individuals and health policy decision making. Traditional cultural values regarding the role of women also tends to intensify the problems. Lack of acceptance of the right of women to make decisions about child bearing and work outside the home limit options for individuals who wish to reduce risk of infection via sexual exposures. Economic independence is a crucial factor enabling women to make some decisions themselves. The options for employment outside of sex work, for divorced or widowed women, in many societies are quite restricted. These fundamental values may directly conflict with efforts to empower women to avoid risk of HIV and other sexually transmitted diseases.
To control the HIV epidemic, societies need to make deep commitments that may require an uncomfortable loss of highly valued cultural norms. Without social acceptance and encouragement, behaviorally mediated risk reduction strategies may not assume full efficacy. Vaccination is, at present, an optimal but unavailable solution. The prospects for development of an effective vaccine in the near future are not promising. Thus we have good cause to fear for the effects of HIV on women worldwide, and to increase our attention to this enormous problem as we enter the twenty-first century and the third decade of the HIV pandemic. Source: Ruth M. Greenblat, MD Nancy A. Hessel, MSPH U.S. Department of Health and Human Services Health Resources and Service Administration HIV/AIDS Bureau.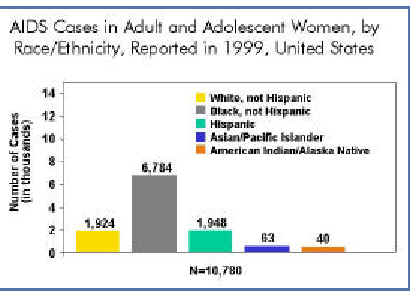
HIV infection among U.S. women has increased significantly over the last decade, especially in communities of color. CDC estimates that, in the United States, between 120,000 and 160,000 adult and adolescent females are living with HIV infection, including those with AIDS.
Between 1992 and 1998, the number of persons living with AIDS increased in all groups, as a result of the 1993 expanded AIDS case definition and, more recently, improved survival among those who have benefited from the new combination drug therapies. During that 6-year period, a growing proportion of women were living with AIDS, reflecting the ongoing shift in populations affected by the epidemic. In 1992, women accounted for 14% of persons living with AIDS–by 1998, the proportion had grown to 20%. In just over a decade, the proportion of all AIDS cases reported among adult and adolescent women more than tripled from 7% in 1985 to 23% in 1999. The epidemic has increased most dramatically among women of color. African American and Hispanic women together represent less than one-fourth of all U.S. women, yet they account for more than three-fourths (77%) of AIDS cases reported to date among women in our country. In 1999 alone (see chart above), women of color represented an even higher proportion of cases. While AIDS-related deaths among women were decreasing as of 1998, largely as a result of recent advances in HIV treatment, HIV/AIDS remains among the leading causes of death for U.S. women aged 25-44. And among African American women in this same age group, AIDS was the third leading case of death in 1998.Sex with drug users plays large role In 1999 most women(40%)reported with AIDS were infected through heterosexual exposure to HIV, injection drug use accounted for 27%. In addition to the direct risks associated with drug injection (sharing needles), drug use also is fueling the heterosexual spread of the epidemic. A large proportion of women infected heterosexually were infected through sex with an injection drug user. Reducing the toll of the epidemic among women will require efforts to combat substance abuse, in addition to reducing HIV risk behaviors.
Many HIV/AIDS cases among women in the United States are initially reported without risk information, suggesting that women may be unaware of their partners' risk factors or that healthcare providers are not documenting their risk. Historically, more than two-thirds of AIDS cases among women initially reported without identified risk were later reclassified as heterosexual transmission, and just over one-fourth were attributed to injection drug use.
| Pay attention to prevention for women. The AIDS epidemic is far from over. Scientists believe that cases of HIV infection reported among 13- to 24-year-olds are indicative of overall trends in HIV incidence (the number of new infections in a given time period, usually a year) because this age group has more recently initiated high-risk behaviors–and females made up nearly half (49%) of HIV cases in this age group reported from the 32 areas with confidential HIV | |
| reporting for adults and adolescents in 1999. Further, for all years combined, young African American and Hispanic women account for more than three-fourths of HIV infections reported among females between the ages of 13 and 24 in these areas. | |
| Implement programs that have been proven effective in changing risky behaviors among women and sustaining those changes over time, maintaining a focus on both the uninfected and infected populations of women. | |
| Increase emphasis on prevention and treatment services for young women and women of color. Knowledge about preventive behaviors and awareness of the need to practice them is critical for each and every generation of young women–prevention programs should be comprehensive and should include participation by parents as well as the educational system. Community-based programs must reach out-of-school youth in such settings as youth detention centers and shelters for runaways. | |
| Address the intersection of drug use and sexual HIV transmission. Women are at risk of acquiring HIV sexually from a partner who injects drugs and from sharing needles themselves. Additionally, women who use noninjection drugs (e.g., crack cocaine, methamphetamines) are at greater risk of acquiring HIV sexually, especially if they trade sex for drugs or money. | |
| Develop and widely disseminate effective female-controlled prevention methods. More options are urgently needed for women who are unwilling or unable to negotiate condom use with a male partner. CDC is collaborating with scientists around the world to evaluate the prevention effectiveness of the female condom and to research and develop topical microbicides that can kill HIV and the pathogens that cause STDs. | |
| Better integrate prevention and treatment services for women across the board, including the prevention and treatment of other STDs and substance abuse and access to antiretroviral therapy. |
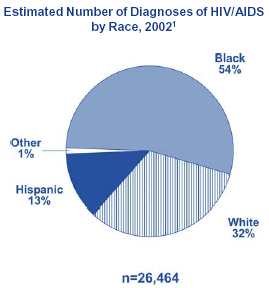 In the United States, the impact of HIV and AIDS in the African American community has been devastating. Through
December 1999, CDC had received reports of 733,374 AIDS cases - of those, 272,881 cases occurred among African
Americans. Representing only an estimated 12% of the total U.S. population, African Americans make up almost 37% of all
AIDS cases reported in this country.
In the United States, the impact of HIV and AIDS in the African American community has been devastating. Through
December 1999, CDC had received reports of 733,374 AIDS cases - of those, 272,881 cases occurred among African
Americans. Representing only an estimated 12% of the total U.S. population, African Americans make up almost 37% of all
AIDS cases reported in this country.
21,900 cases were reported among African Americans, representing nearly half (47%) of the 46,400 AIDS cases reported that year.
Almost two-thirds (63%) of all women reported with AIDS were African American. African American children also represented almost two-thirds (65%) of all reported pediatric AIDS cases. The 1999 rate of reported AIDS cases among African Americans was 66.0 per 100,000 population, more than 2 times greater than the rate for Hispanics and 8 times greater than the rate for whites. Data on HIV and AIDS diagnoses in 25 states with integrated reporting systems show these trends are continuing. In these states, during the period from January 1996 through June 1999, African Americans represented a high proportion (50%) of all AIDS diagnoses, but an even greater proportion (57%) of all HIV diagnoses. And among young people (ages 13 to 24), 65% of the HIV diagnoses were among African Americans.
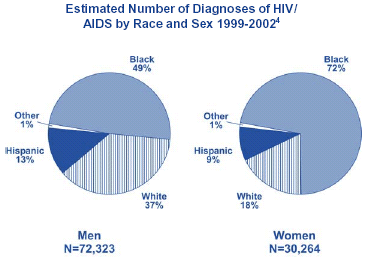 |
Adult/Adolescent Men. Among African American men with AIDS, men who have sex with men (MSM) represent the largest proportion (37%) of reported cases since the epidemic began. The second most common exposure category for African American men is injection drug use (34%), and heterosexual exposure accounts for 8% of cumulative cases.
Adult/Adolescent Women. Among African American women, injection drug use has accounted for 42% of all AIDS case reports since the epidemic began, with 38% due to heterosexual contact.
Looking at select seroprevalence studies among high-risk populations gives an even clearer picture of why the epidemic continues to spread in communities of color. The data suggest that three interrelated issues play a role–the continued health disparities between economic classes, the challenges related to controlling substance abuse, and the intersection of substance abuse with the epidemic of HIV and other sexually transmitted diseases (STDs).
Substance abuse is fueling the sexual spread of HIV in the United States, especially in minority communities with high rates of STDs. Studies of HIV prevalence among patients in drug treatment centers and STD clinics find the rates of HIV infection among African Americans to be significantly higher than those among whites. Sharing needles and trading sex for drugs are two ways that substance abuse can lead to HIV and other STD transmission, putting sex partners and children of drug users at risk as well. Comprehensive programs for drug users must provide the information, skills and support necessary to reduce both injection-related and sexual risks. At the same time, HIV prevention and treatment substance abuse prevention, and sexually transmitted disease treatment and prevention services must be better integrated to take advantage of the multiple opportunities for intervention. Prevention efforts must be improved and sustained for young gay men. In a sample of young men who have sex with men (ages 15-22) in seven urban areas, researchers found that, overall, 7% were infected with HIV (range, 2%-12%). A significantly higher percentage of African American MSM (14%) than white MSM (3%) were infected. It is clear that the public sector alone cannot successfully combat HIV and AIDS in the African American community. Overcoming the current barriers to HIV prevention and treatment requires that local leaders acknowledge the severity of the continuing epidemic among African Americans and play an even greater role in combating HIV/AIDS in their own communities. Additionally, HIV prevention strategies known to be effective (both behavioral and biomedical) must be available and accessible for all populations at risk.
The United States has a large and growing Hispanic population that is heavily affected by the HIV/AIDS epidemics. In 1999, Hispanics represented 13% of the U.S. population (including residents of Puerto Rico), but accounted for 19% of the total number of new U.S. AIDS cases reported that year (9,021 of 46,400 cases). The AIDS incidence rate per 100,000 population (the number of new cases of a disease that occur during a specific time period) among Hispanics in 1999 was 25.6, almost 3 times the rate for whites (7.6) but lower than the rate for African Americans (66.0).
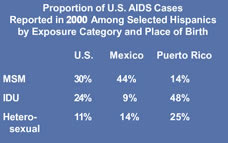 Hispanics in the United States include a diverse mixture of ethnic groups and cultures. As shown in the chart at
left, HIV exposure risks for U.S.-born Hispanics and Hispanics born in other countries vary greatly1, indicating a need for specifically targeted prevention efforts.
Hispanics in the United States include a diverse mixture of ethnic groups and cultures. As shown in the chart at
left, HIV exposure risks for U.S.-born Hispanics and Hispanics born in other countries vary greatly1, indicating a need for specifically targeted prevention efforts.
Between 1992 and 1998, the number of persons living with AIDS increased in all groups, as a result of the 1993 expanded AIDS case definition and, more recently, improved survival among those who have benefited from the new combination drug therapies. During that 6-year period, the characteristics of persons living with AIDS were changing reflecting an expansion of the epidemic, particularly in minority populations. In 1992, 17% of those estimated to be living with AIDS were Hispanic, while in 1998, 19% were Hispanic. In comparison, non-Hispanic whites represented 49% of people estimated to be living with AIDS in 1992, but only 39% in 1998.
Cumulatively, males account for the largest proportion (81%) of AIDS cases reported among Hispanics in the United States, although the proportion of cases among women is rising. Women represent 19% of cumulative AIDS cases among Hispanics, but account for 22% of cases reported in 1999 alone. Fifty-seven percent of Hispanics reported with AIDS in 1999 were born in the U.S.; of those 43% were born in Puerto Rico.U.S. Mexico Puerto Rico MSM 31% 50% 14% IDU 20% 7% 40% Heterosexual 13% 12% 25% From the beginning of the epidemic through December 1999, 107,867 Hispanic men have been reported with AIDS in the United States. Of these cases, men who have sex with men (MSM) represent 43%, injection drug users (IDUs) account for 36%, and 6% of cases were due to heterosexual contact. About 7% of cases were among Hispanic men who both had sex with men and injected drugs. Among men born in Puerto Rico, however, injection drug use accounts for a significantly higher proportion of cases than male-male sex.
For adult and adolescent Hispanic women, heterosexual contact accounts for the largest proportion (47%) of cumulative AIDS cases, most of which are linked to sex with an injection drug user. Injection drug use accounts for an additional 40% of AIDS cases among U.S. Hispanic women.While race and ethnicity alone are not risk factors for HIV infection, underlying social and economic conditions (such as language or cultural diversity, higher rates of poverty and substance abuse, or limited access to healthcare) may increase the risk for infection in some Hispanic American communities.
| Transmission related to substance abuse continues to be a significant problem among Hispanics living in the United States, especially among those of Puerto Rican origin. Studies of HIV prevalence among patients in drug treatment center find the rates of HIV infection among Hispanics to be significantly higher in some regions of the country, particularly the Northeast and Midwest. Comprehensive programs for drug users must provide the information skills, and support necessary to reduce both injection-related and sexual risks. In addition, HIV prevention and treatment, substance abuse prevention, and sexually transmitted disease treatment and prevention services must be better integrated to take advantage of the multiple opportunities for intervention. | |
| Prevention messages must be tailored to the affected communities. Hispanic populations need interventions that (1) are consistent with their values and beliefs and (2) include skills-building activities to facilitate changes in sexual behavior. Further, because the HIV/AIDS epidemic among U.S. Hispanics reflects to a large extent the exposure modes and cultural modes of the individuals 'birthplaces, an understanding of these behaviors and differences is important in targeting prevention efforts. For example, some high-risk behaviors associated with drug abuse (such as use of shooting galleries) may be more predominant among Puerto Rico-born Hispanics than among other Hispanics. Therefore for these populations, prevention strategies should emphasize (1) preventing and treating substance abuse and (2) decreasing needle-sharing and the use of shooting galleries. For Hispanics born in Mexico, Cuba, and Central and South America, CDC data indicate that male-male sex is the primary mode of HIV transmission. Messages targeted to these populations must be based on |
| an understanding of their cultural attitudes toward homosexuality and bisexuality, which may be different from those of other populations at high risk for infection. |
In the United States, HIV-related death has the greatest impact on young and middle-aged adults, particularly racial and ethnic minorities. In 1998, HIV was the fifth leading cause of death for Americans between the ages of 25 and 44. Among African American men in this age group, HIV infection has been the leading cause of death since 1991. In 1998 among black women 25-44 years old, HIV infection was the third leading cause of death. Many of these young adults likely were infected in their teens and twenties. It has been estimated that at least half of all new HIV infections in the United States are among people under 25, and the majority of young people are infected sexually.
In 1999, 1,813 young people (ages 13 to 24) were reported with AIDS, bringing the cumulative total to 29,629 cases of AIDS in this age group. Among young men aged 13- to 24-years, 50% of all AIDS cases reported in 1999 were among men who have sex with men (MSM); 8% were among injection drug users (IDUs); and 8% were among young men infected heterosexually. In 1999, among young women the same age, 47% of all AIDS cases reported were acquired heterosexually and 11% were acquired through injection drug use. Among both males and females in this age group, the proportion of cases with exposure risk not reported or identified (25% for males and 41% for females) will decrease and the proportion of cases attributed to sexual contact and injection drug use will increase as follow-up investigations are completed and cases are reclassified into these categories. Surveillance data analyzed from 25 states with integrated HIV and AIDS reporting systems for the period between January 1996 and June 1999 indicate that young people (aged 13 to 24) accounted for a much greater proportion of HIV (13%) than AIDS cases (3%). These data also show that even though AIDS incidence (the number of new cases diagnosed during a given time period, usually a year) is declining, font there has not been a comparable decline in the number of newly diagnosed HIV cases among youth.Scientists believe that cases of HIV infection diagnosed among 13- to 24-year-olds are indicative of overall trends in HIV incidence (the number of new infections in a given time period, usually a year) because this age group has more recently initiated high-risk behaviors. Females made up nearly half (49%) of HIV cases in this age group reported from the 32 areas with confidential HIV reporting for adults and adolescents in 1999-and in young people between the ages of 13 and 19, a much greater proportion of HIV infections was reported among females (64%) than among males (36%). Cumulatively, young African Americans are
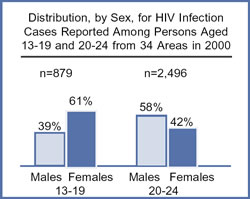 CDC research has shown that early, clear communications between parents and young people about sex is an important
step in helping adolescents adopt and maintain protective sexual behaviors. In addition, a wide range of activities must
be implemented in communities to reduce the toll HIV infection and AIDS takes on young Americans.
CDC research has shown that early, clear communications between parents and young people about sex is an important
step in helping adolescents adopt and maintain protective sexual behaviors. In addition, a wide range of activities must
be implemented in communities to reduce the toll HIV infection and AIDS takes on young Americans.
| School-based programs are critical for reaching youth before behaviors are established. Because risk behaviors do not exist independently, topics such as HIV, STDs, unintended pregnancy, tobacco, nutrition, and physical activity should be integrated and ongoing for all students in kindergarten through high school. The specific scope and content of these school health programs should be locally determined and consistent with parental and community values. Research has clearly shown that the most effective programs are comprehensive ones that include a focus on delaying sexual behavior and provide information on how sexually active young people can protect themselves. Evidence of prevention success can be seen in trends from the Youth Risk Behavior Survey conducted over an 8-year period, which show both a decline in sexual risk behaviors and an increase in condom use among sexually active youth. The percentage of sexually experienced high school students decreased from 54.1% in 1991 to 49.9% in 1999, while condom use among sexually active students increased from 46.2% to 58.0%. These findings represent a reversal in the trend toward increased sexual risk among teens that began in the 1970s and point to the success of comprehensive prevention efforts to both delay first intercourse among teens and increase condom use among young people who are sexually active. | |
| Efforts to reach out-of-school-youth are made by community-based programs. Addressing the needs of adolescents who are most vulnerable to HIV infection, such as homeless or runaway youth, juvenile offenders, or school drop-outs, is critically important. For example, a 1993 serosurveillance survey of females in four juvenile detention centers found that between 1% and 5% were HIV infected (median 2.8%). | |
|
Prevention efforts for young gay and bisexual men must be sustained. Targeted, sustained prevention efforts are urgently needed for young MSM as they come of age and initiate high-risk sexual behavior. Ongoing studies show that both HIV prevalence and risk behaviors remain high among young MSM. In a sample of young MSM ages 15-22 in seven urban areas, researchers found that, overall, 7% were infected with HIV, with higher prevalence among young African American (14%) and Hispanic (7%) men than among young white men (3%). | |
| We must address sexual and drug-related risk. Many students report using alcohol or drugs when they have sex, and 1 in 50 high school students reports having injected an illegal drug. Surveillance data from the 32 states with integrated HIV and AIDS reporting systems suggest that drug injection led to at least 5% of HIV diagnoses reported among those aged 13-24 in 1999, with an additional 49% attributed to sexual transmission (both heterosexual and MSM). |
| STD treatment must play a role in prevention programs for young people. An estimated 12 million cases of STDs other than HIV are diagnosed annually in the United States, and about two-thirds of those are among people under the age of 25. Research has shown that biological factors make people who are infected with an STD more likely to become infected with HIV if exposed sexually; and HIV-infected people with STDs also are more likely to transmit HIV to their sex partners. Expanding STD treatment is critical to reducing the consequences of these diseases and helping to reduce risks of transmitting HIV among youth. | |
| Evaluation of factors influencing risk behavior must be ongoing. Both broad-based surveys of the extent of risk behaviors among young people and focused studies of the factors contributing to the risk and behavioral intent among specific groups of adolescents must be conducted and analyzed. |
Of the adults reported with AIDS in the United States through June 30, 2000, 22,618 had been employed in health care. These cases represented 5.1 percent of the 445,380 AIDS cases reported to CDC for whom occupational information was known (information on employment in the healthcare setting was missing for 299,723 reported AIDS cases).
The type of job is known for 21,261 (94%) of the 22,618 reported healthcare workers with AIDS. The specific occupations are as follows: 1,714 physicians, 114 surgeons, 4,928 nurses, 474 dental workers, 431 paramedics, 2,965 technicians, 1,019 therapists, and 4,985 health aides. The remainder are maintenance workers, administrative staff, etc. Overall, 74% of the healthcare workers with AIDS, including 1,345 physicians, 85 surgeons, 3,660 nurses, 374 dental workers, and 304 paramedics, are reported to have died. CDC is aware of 56 healthcare workers in the United States who have been documented as having seroconverted to HIV following occupational exposures. Twenty-five have developed AIDS. These individuals who seroconverted include 19 laboratory workers (16 of whom were clinical laboratory workers), 23 nurses, 6 physicians, 2 surgical technicians, 1 dialysis technician, 1 respiratory therapist, 1 health aide, 1 embalmer/morgue technician, and 2 housekeepers/maintenance workers. The exposures were as follows: 48 had percutaneous (puncture/cut injury) exposure, 5 had mucocutaneous (mucous membrane and/or skin) exposure, 2 had both percutaneous and mucocutaneous exposure, and 1 had an unknown route of exposure. Forty-nine healthcare workers were exposed to HIV-infected blood, 3 to concentrated virus in a laboratory, 1 to visibly bloody fluid, and 3 to an unspecified fluid. CDC is also aware of 138 other cases of HIV infection or AIDS among healthcare workers who have not reported other risk factors for HIV infection and who report a history of occupational exposure to blood, body fluids, or HIV-infected laboratory material, but for whom seroconversion after exposure was not documented. The number of these workers who acquired their infection through occupational exposures is unknown.Concerning HIV antibody testing remember: Counseling and early diagnosis are important to help prevent the spread of HIV.
Laws about testing are made by each State legislature. HIV antibody tests are extremely accurate. HIV-2 testing of all blood donations became mandatory on June 1, 1992. Targeted counseling and testing programs represent one effective method of controlling the transmission of HIV. Counseling and testing for HIV is being expanded rapidly in hundreds of locations, in cooperation with State and local public health agencies, to reach persons at risk of HIV infection. Counseling and early diagnosis of HIV infection are recommended for the following persons: persons who consider themselves at risk for infection, women of childbearing age who are at risk of infection, persons attending sexually transmitted disease clinics and drug abuse clinics, the spouses and sex or needle-sharing partners of injecting drug users, women seeking family planning services, tuberculosis patients, and selected patients who received transfusions of blood or blood components between early 1978 and mid-1985. The U.S. government has mandated the screening of immigrants entering the United States, foreign service personnel applicants to the armed services, and inmates of Federal prisons. In addition, persons considering marriage should have access to information and educational materials about HIV infection and counseling and testing.Testing for HIV antibody is highly accurate. A repeatedly reactive screening test followed by a positive confirmatory test is necessary before a person is considered seropositive. Antibodies generally appear within 3 months after infection with HIV, but may take up to 6 months in some persons. Testing is recommended at 3 months and again at 6 months after exposure. An HIV-2 enzyme immunoassay or EIA was licensed by the US Food and Drug Administration in 1990. The Food and Drug Administration mandated that by June 1, 1992, U.S. blood centers must begin testing all blood donations for antibodies to HIV-2. Some blood banks and plasma centers began testing potential donors for HIV-2 prior to that date. The HIV-2 EIA should be available from your private physician or through state and local health departments. HIV-2 testing is indicated in persons with epidemiologic risk factors for HIV-2 infection. These include 1. sex and needle-sharing partners of West Africans, 2. persons who have received blood transfusions in West Africa, 3. children born to HIV-2-infected mothers, 4. persons with conditions suggestive of HIV infection (such as an AIDS-associated opportunistic disease) for whom HIV-1 testing is not positive, and 5. persons whose blood specimens are reactive on HIV-1 EIA testing and exhibit certain unusual indeterminate patterns on HIV-1 western blot.>
The standard screening test for antibody to HIV is the enzyme immunoasssay (EIA), which is widely used in the United States and around the world. This test requires serum or plasma, so a blood specimen must be drawn from a vein. Because EIA requires specialized equipment, the specimen must be sent to a laboratory, and test results are usually available several days to several weeks later. A negative screening test means a person is not infected with HIV, and does not require further testing. However, a diagnosis of HIV infection cannot be based on a reactive screening test alone. Thus a reactive EIA is repeated, and repeatedly reactive EIA results are confirmed by a supplemental HIV antibody test–Western blot or immunofluorescence assay (IFA).
Until now, testing required two visits. During the first visit, a client receives pretest counseling, and blood is drawn for HIV testing. During the second visit, test results are communicated to the client, additional counseling is provided, and clients who need them are given referrals for additional services.A rapid test for detecting antibody to HIV is a screening test that produces very quick results, usually in 5 to 30 minutes. Only one rapid HIV test is licensed by the Food and Drug Administration (FDA) for use in the United States.
The rapid HIV test is easier to use and produces results more quickly than the EIA does. The sensitivity and specificity of the rapid HIV test are just as good as those of the EIA.
Currently, only one rapid HIV test is licensed by the FDA.
Several other rapid HIV tests are being used in many other countries. Still others, including one for use with oral
fluids, are being developed. The new generation of rapid HIV tests may be quicker and easier to use.
Rapid HIV testing is suitable for testing any person who would be eligible for HIV testing by EIA. However, the availability of rapid HIV tests may differ from one place to another. (See the list of resources at the end of these questions and answers.)
Yes. The individual kit is more costly then the per-test cost of the EIA. EIA testing was designed for the automated processing of tests in batches (usually using a plate that can process 96 specimens at one time.) However, an analysis done in 1996 by Dr. Paul Farnham and his colleagues at CDC indicated that rapid HIV testing is more cost-effective than the current EIA-based system, because of the number of persons who actually learn their results. In other words although EIA is less expensive, it is a waste of money to perform lab tests if the person tested never learns the test result, if two clinic visits are required to get test results, or if the clinic has to send field staff to locate people for test results. Since an EIA does not yield immediate results, most people must make a second visit to learn their results. Experience at publicly funded testing sites has shown that many persons (26% of those who tested positive for HIV and 33% of those who tested negative in 1996) do not return for their test results.
Probably. It is difficult to know how soon this will take place.
The rapid HIV test is just as accurate as an EIA. As is true of all screening tests (including the EIA), a reactive rapid HIV test result must be confirmed. Studies in countries where more than one type of rapid HIV test is available show that specific combinations of two or more different rapid HIV tests can provide results as reliable as those from an EIA and Western blot or IFA, the combination that is currently used in the United States. A second rapid HIV test for persons whose first rapid HIV test is reactive could significantly improve the predictive value of rapid HIV testing.
Predictive value is the calculated probability that a test result predicts whether a person is truly infected. This calculation produces a number that counselors can use in explaining HIV test results to their clients. For example, a higher predictive value means that a reactive test is more likely to indicate the person is truly infected.
A negative antibody test result, whether it is from a rapid HIV test or an EIA, does not require a confirmatory test. However, a person may have been tested too soon, before antibodies developed. The average time between infection and the development of detectable antibodies is 25 days.
Not necessarily. For most people who are tested, a negative HIV antibody test result does mean that they are not infected. However, in some cases a person may have been tested too soon (before antibodies have developed, which requires an average of 25 days). That is why it is important to assess specific risk behaviors during counseling, and discuss ways to change risky behaviors.
The term reactive is used to describe a test that has detected the presence of antibodies to HIV. It is recommended that all reactive tests be repeated immediately, by using the same test. Repeatedly reactive tests are then further confirmed, by using a different test on the same blood specimen.
The confirmatory tests are usually sent to a laboratory for processing; results are generally available in 1 to 2 weeks.
Prices may be different in different parts of the country. The test kit usually costs $6 to $10, which is more expensive than an EIA. However, the EIA requires expensive equipment and rapid HIV tests do not. Additional costs such as a laboratory or a laboratory technician's time for conducting the tests should also be considered. Rapid HIV tests are simpler to perform and require fewer specialized skills than does an EIA.
The advantage to the clinic is that more people will receive their test results without expensive field visits. Most of the clients at all U.S. publicly funded testing sites, including STD clinics, test negative for HIV. For these persons (approximately 2.1 million in 1996), the need to make a second visit would be eliminated. Of all testing sites, STD clinics have had the lowest proportion of persons who return for HIV test results. Thus, rapid HIV tests have the potential to greatly increase the number of persons who learn their results. In addition, persons who test HIV-positive by the rapid HIV test can be advised immediately of their screening test result, and counseled about the need to take precautions to prevent the possibility of transmitting HIV. These persons of course need to return for their confirmatory test result.
Interviews with persons being tested indicate that most persons prefer rapid HIV testing, and most persons who receive a positive HIV screening test result return on their own to learn the confirmed result (unlike the situation with current testing, in which many persons learn their test results only as a result of outreach). This also means that persons who are truly HIV-positive will learn of their infection sooner. This may help prevent infections that might otherwise have occurred between the time the person was tested and the time the person received results (sometimes as long as several weeks.)
Yes. The progression of HIV disease rarely affects the detection of HIV antibody.
The result of any HIV antibody test performed on an infant less than 15 months of age may reflect the mother's HIV status, because the antibodies are transferred from the mother to the baby. Until these antibodies disappear, only specific virus detection tests can determine the infection status of an infant.
Yes. Batching, or collecting several specimens before testing all of them at the same time, can be done. This process can save money for a busy clinic, because fewer control test kits are required. However, accumulating a sufficient number of tests for a batch can result in excessive waiting time for the client, reducing the main benefit of the rapid HIV test: rapid results.
The rapid HIV test usually takes 15 to 30 minutes. The waiting time depends on how many clients are being tested and whether the clinic is testing individual samples or batching them. Counseling can be performed while the test is being done.
CDC is developing new guidance and training for counselors. (See the list of resources at the end of these questions and answers.)
CDC will assist counselors and others who plan to develop such products. The manufacturers of rapid HIV tests usually have such materials as well.
Current CDC counseling and testing guidelines state that positive HIV results should be communicated by personal contact. Whether this personal contact is established by phone or in person is a decision to be made at the local level.
One of the more challenging counseling issues is how to communicate reactive rapid HIV test results to clients without the benefit of a same-day confirmatory test result. Counselors should be able to discuss with the client the likelihood of whether the rapid HIV test result means the client has HIV infection. This discussion should be based on the prevalence of HIV among persons tested at that clinic coupled with an assessment of the client's risk behaviors. In clinics that usually experience a high prevalence of HIV infection among their clients, a reactive rapid HIV test result is more likely to represent a true infection, especially in persons who report risk behaviors for HIV. Any person whose rapid HIV test is reactive should be counseled about the need to take precautions to prevent any possibility of transmitting HIV infection until their infection status has been determined by a confirmatory HIV test.
Partner notification and referral services should not be initiated until the reactive rapid HIV test result has been confirmed.
A negative rapid HIV test of course means that antiretroviral treatment is not necessary. Deciding what to do about therapy when the rapid HIV test is reactive is more complicated. If the circumstances are not urgent, it would be preferable to wait for the confirmatory test result. In other circumstances (such as a rapid HIV test result for a woman in labor, for whom no other result is available), physicians should base decisions about antiretroviral treatment on the predictive value of the preliminary rapid HIV test results and an assessment of the mother's HIV risk.
As is true of current EIA antibody procedure, an initial reactive rapid HIV test result should be confirmed by Western blot or IFA. For persons who test positive by confirmatory testing, CDC and the Association of State and Territorial Public Health Laboratory Directors recommend that the test sequence be repeated, by using a different sample, to be absolutely certain of the results.
For information on referrals to counseling and testing sites, contact
CDC National AIDS Hotline (English) 800-342-2437 (Spanish) 800-344-7432 (TTY) 800-243-7889 CDC Educator/Trainer Network — HIV Prevention Counseling Trainers and Counselors/p>
Sheila Cort Isoke, MPH CDC, National Center for HIV, STD, and TB Prevention (telephone) 404-639-0962 (fax) 404-639-0944 (e-mail) shc1@cdc.gov CDC-sponsored training for performing HIV testing
National Laboratory Training Network (telephone) 770-488-7811 CDC Division of HIV/AIDS Prevention Internet Homepage http://www.cdc.gov/nchstp/hiv–aids/dhap.htm<
Measurements of HIV- RNA blood levels (viral load) are increasingly being used by healthcare providers to determine when to start antiretroviral therapy and when to change current therapies. This test has become very important in the management of HIV infection because studies have shown that the level of virus in the blood is a predictor of disease progression. In other words, people with high levels of HIV-RNA in their blood, are more likely to rapidly progress to AIDS than people with low levels of the virus.
Because viral load testing is an integral part of the management of HIV disease, it is important to learn what this test is and how it is used.Viral load or viral burden is the quantity of HIV-RNA (HIV virus) that is in the blood. RNA is the genetic material of HIV that contains the information needed to make more virus. Viral load tests measure the amount of HIV-RNA in a small amount of blood (one milliliter, ml).
There are three different viral load tests currently being used:| PCR (polymerase chain reaction) is the most common of these tests and is the only test approved by the FDA. Test results are reported as copies/ml of plasma. | |
| DNA (branched chain DNA assay) is also frequently used. These results are reported as units/ml of plasma. | |
| NASBA (nucleic acid sequence-based amplification) is less frequently used and reports test results as units/ml of plasma. |
Because the tests do not give exactly the same results, it is important to have the same type of viral load test done each time. This will give the physician a baseline measurement against which changes can be evaluated. Since the results of this test can vary greatly, they should be interpreted in the context of clinical management with an experienced physician.When should viral load be measured?
An Expert Panel for the International AIDS Society-USA (1) has issued guidelines indicating when viral load should be measured. The following is the Panel's timetable for testing: Take two different viral load measurements 2-3 weeks apart to determine a baseline measurement.
Repeat every 3-6 months thereafter in conjunction with CD4 counts to monitor viral load and T-cell count. Repeat the test 4-6 weeks after starting or changing antiretroviral therapy to determine the effect on viral load. Avoid measuring viral load in the 3-4 weeks following an immunization (including flu shots) or within one month of an infection. Temporary increases in viral load have been seen in these instances. To minimize misleading results, it's best to avoid testing at these times. Another consideration for measuring viral load is cost. Because the test is expensive (tests can range between $63-$292 with a commonly reported cost of $200 per test), it is important to have them drawn at appropriate times.Changes in viral load are often reported as logarithmic or log changes. This mathematical term denotes a change in the value of what is being measured by a factor of 10. For example, if the baseline viral load by PCR were 20,000 copies/ml plasma, then a 1 log increase equals a 10-fold (10 times) increase or 200,000 copies/ml plasma. A 2 log increase equals 2,000,000 copies/ml plasma, or a 100-fold increase.
Using the same starting point of 20,000 copies/ml plasma, a 1 log decrease means that the viral load has dropped to 2,000 copies/ml. A 2 log decrease equals a viral load of 200 copies/ml plasma. An easy way to figure out log changes is to either drop the last 0or add 0to the original number. Any change of less than one-half log is considered insignificant. More simply, if the viral load measurement has not tripled or dropped to one-third of its previous level, the difference might be unimportant. For example, if the baseline viral load were 20,000 copies, a rise to 60,000 or a fall to 7,000 copies might just be the result of transient changes. Repeat testing of a single specimen may give two quite different results and natural biological day-to-day variability of samples from the same person may cause measurements to vary slightly. Researchers believe that clinical decisions made on the basis of changes in viral load ideally should be based on measurements taken 2-3 weeks apart.Many individuals now have an undetectable level of virus in their blood after taking combination therapy. Undetectable levels do not mean that the person is cured or no longer infectious. Undetectable levels mean that current tests are not sensitive enough to measure very low levels of virus in the blood, for example less than 400 copies/ml. There are experimental assays used in research which are more sensitive and can detect levels of virus as low as 20 copies/ml but they are not generally available in clinics and doctor's offices. Even if the measurement of virus is less than 20 copies/ml in the blood, HIV may be present and infectious in blood genital secretions (such as semen), lymph nodes, other lymphoid tissues, and elsewhere in the body. There are insufficient data to say that individuals with undetectable levels of virus are no longer infectious or no longer at risk of disease progression in the future. We simply do not know yet what an undetectable level of virus means over the long term. Individuals with undetectable levels of virus still need to be monitored by their healthcare providers regularly and they need to practice risk free behaviors.
Even with ongoing monitoring of viral load, it is important to monitor CD4+ levels. CD4+ levels provide information about the status of the immune system. Research has shown that when viral load is lowered, CD4+ levels usually increase. However, research is still ongoing to determine whether the increase in CD4+ levels represent normal, functioning immune cells. CD4+ levels continue to be used as the basis for deciding what type of opportunistic infection prophylaxis a patient should take. Some physicians feel decisions about prophylaxis should be based on the lowest CD4+ level recorded for a person. CD4+ cell levels are also used to measure response to antiretroviral treatment, although most regard viral load as more important indicator. In order to provide the physician with the best possible information about a patient's disease status, it is important to have routine viral load and CD4+ levels measured.
1. Saag MS, Holodniy M, Kuritzkes DR, et al. HIV viral load markers in clinical practice: recommendations of an International AIDS Society-USA Expert Panel. Nature Medicine. 1996;2:625-629.Publicly funded HIV antibody counseling and testing services were initiated in March 1985 to provide an alternative to the donation of blood as a means for high-risk persons to determine their HIV status. At that time, little was known about the prevalence and natural history of HIV infection. Counseling was considered an essential adjunct to HIV testing. The counseling addressed the accuracy and consequences of the test and was designed to help persons interpret the meaning of positive or negative antibody results. HIV counseling was based on the recognition that learning HIV status may be difficult for some clients.
In 1987, with increased understanding about the scope and severity of the HIV epidemic and the predictive value of a positive test, HIV counseling and testing were expanded. Persons seeking care for sexually transmitted infections family planning, childbirth, or substance abuse were counseled and tested in an attempt to reduce their risk for HIV transmission. The primary public health purposes of counseling and testing are to help uninfected individuals initiate and sustain behavioral changes that reduce their risk of becoming infected and to assist infected individuals in avoiding infecting others.(1)Since that time, public awareness about HIV infection has increased, and the reliability and predictive value of the HIV test have been proven. Investigations have demonstrated the benefit of early antiviral and prophylactic treatment for HIV infected persons. These HIV counseling standards and guidelines are the result of increased knowledge about HIV prevention and experience with HIV counseling. Counseling is a direct, personalized, and client-centered intervention designed to help initiate behavior change to avoid infection or, if already infected, to prevent transmission to others and to obtain referral to additional medical care, preventive, psychosocial and other needed services in order to remain healthy. Goals of HIV Counseling, Testing, and Referral Services
The current goals of HIV counseling are as follows: provide a convenient opportunity for persons to learn their current serostatus; allow such persons to receive prevention counseling to help initiate behavior change to prevent the transmission or acquisition of HIV; help persons obtain referrals to receive additional medical-care, preventive, psycho social, and other needed services; provide prevention services and referrals for sex and needle sharing partners of HIV- infected persons.
| Maintenance of Confidentiality | |
| Risk Assessment | |
| Prevention Counseling | |
| Providing Test Results | |
| Provision of Referrals |
HIV Prevention Case Management (PCM) is a one-on-one client service designed to assist both uninfected persons and those living with HIV. PCM provides intensive, individualized support and prevention counseling to assist persons to remain seronegative or to reduce the risk for HIV transmission to others by those who are seropositive. PCM is intended for persons who are having or who are likely to have difficulty initiating and sustaining safer behavior. The client's participation is always voluntary, and services are provided with the client's informed consent.
Prevention Case Management involves the assessment of HIV risk behavior and the assessment of other psychosocial and health service needs in order to provide risk reduction counseling and to assure psychosocial and medical referrals such as housing, drug treatment, and other health and social services. PCM provides an ongoing, sustained relationship with the client in order to assure multiple-session HIV risk reduction counseling and access to service referrals. PCM should not duplicate Ryan White CARE Act case management services for persons living with HIV. Case managers work with clients to assess their HIV risk and psychosocial and medical needs, develop a plan for meeting those needs, facilitate the implementation of the PCM plan through referral and follow-up, provide ongoing HIV risk-reduction counseling, and advocate on behalf of the client to obtain services. HIV Prevention Case Management creates bridges to assist clients in obtaining services with which they are unfamiliar or that pose special barriers to access. Clients are active participants in developing their PCM plan for risk reduction. Prevention Case Management may be carried out in a variety of settings, including the client's home, a community-based organization's office or storefront, clinics, or institutional settings. Referral services may include HIV counseling and testing services (CT), psychosocial assessment and care, other HIV health education and risk reduction programs, medical evaluation and treatment, legal assistance, substance abuse treatment, crisis intervention, and housing and food assistance. Additionally, HIV PCM services should assist the client in obtaining STD prevention and treatment services, women's health services, TB testing and treatment, and other primary health care services. A strong relationship with STD clinics, TB testing sites, HIV counseling and testing clinics, and other health service agencies may be extremely beneficial to successfully recruiting persons at high risk who are appropriate for this type of intervention. PCM services are not intended as substitutes for medical case management extended social services, or long-term psychological care. The case manager needs a thorough knowledge of available community social and medical services as well as HIV prevention, treatment, and related services. This includes specific knowledge of the scope of services available, the protocol for accessing these services, and contact persons working with local agencies. Case managers are usually skilled in providing individual or couples' HIV risk-reduction counseling on an ongoing basis. Case managers usually have an academic background or special training in psychosocial assessment and counseling (e.g., social work, drug and alcohol treatment counseling, nursing, health education). Prevention Case Management supervisors need the academic training and/or experience to adequately develop PCM protocols, case documentation, and policies.Effective case managers are:
| Non-judgmental in addressing the needs of the client. | |
| Empathetic and critical listeners. | |
| Skilled in dispute mediation. | |
| Skilled in individual and relationship counseling. | |
| Skilled in conducting a thorough behavioral risk assessment and psychosocial assessment at client intake and skilled in developing an individualized case plan. | |
| Comfortable working in the home environments of their clients as well as in street settings, if necessary. | |
| Continually concerned about the protection of the client's rights, including confidentiality, and always respectful of guidelines in the agency protocol document. | |
| Sensitive to the client's ability to read literature and comprehend oral presentations. | |
| Responsive to the financial resources of clients in regard to case planning and referrals. |
| Maintain communication with case managers from other agencies working with the client to assure a coordinated treatment plan. | |
| Identify resources and services for the client and assist them in accessing service needs. | |
| Take into account and provide for cognitive impairments that may be related to the health status of the client. |
| Includes specific, measurable, realistic, and time-phased program objectives. | |
| Assures that all services in the plan conform to agency policies and local, State, and Federal laws (for example, confidentiality of information). | |
| Assures the development of a written, formal PCM protocol for service delivery. | |
| Provides for the development of specific, measurable, realistic, and time-phased objectives in each client's case plan. | |
| Provides for regular meetings with each client to assess changing needs, monitor progress, and revise the service plan accordingly. | |
| Assures that each case manager negotiates a risk reduction plan with the client, referring to the plan at each session in order to assess progress. | |
| Assures the development and use of a comprehensive HIV risk assessment instrument to assess the behavioral variables influencing the client's risk taking. | |
| Assures the development and use of a comprehensive psychosocial assessment instrument to assess psychosocial and medical service needs of the client as well as financial resources, language preferences, barriers to accessing these services, etc. | |
| Assures that prevention case managers and their supervisors meet frequently for case presentations and supervision. | |
| Defines collaboration with other local service providers through memoranda of agreement and regular meetings between agencies to facilitate access to other social and health services as well as to discuss and coordinate treatment plans for individual clients. | |
| Assures that the memoranda of understanding among agencies are periodically updated, accurately reflecting collaborative activities. | |
| Assures that the assessment of progress in meeting the case plan is communicated to the client for review and comment. | |
| Assures that case records include documentation that acknowledges voluntary client participation and mutually satisfactory case plans. | |
| Assures that an updated written or computerized database of service referrals and a system for documenting successful referrals are maintained. | |
| Assures that regularly scheduled staff meetings are held to discuss challenges, successes, and barriers encountered by case managers; adequate time must be allocated for staff to share concerns, frustrations, grief, and other emotions experienced through the close work with persons at risk or with persons living with HIV. |
PCM staff training plans usually include the following:
| Staff training in established PCM protocols, agency policies, and referral mechanisms. | |
| Periodic training addressing the local services available for client referral. | |
| Skills training to improve the HIV risk reduction counseling provided to clients. | |
| Training that addresses how to effectively intervene with clients who are in extreme states, such as persons who are combative, in emotional crisis, mentally ill, or under the influence of drugs and/or alcohol. |
Counseling provides a critical opportunity to assist the client in identifying his or her risk of acquiring or transmitting HIV. Counseling also provides an opportunity to negotiate and reinforce a plan to reduce or eliminate the risk. Counseling prior to HIV testing, prevention counseling (pretest counseling), should prepare the client to receive and manage his or her test result.
Prevention counseling should also: 1)facilitate an accurate perception of HIV risk for those who are unaware, uninformed, misinformed, or in denial; 2)translate the client's risk perception into a risk reduction plan that may be enhanced by knowledge of HIV infection status; 3)help clients initiate and sustain behavior changes that reduce their risk of acquiring or transmitting HIV. Unless it is prohibited by state law or regulation, clients should be offered reasonable opportunities to receive HIV-antibody counseling and testing services anonymously. The availability of anonymous services may encourage some persons at risk to seek services who would otherwise be reluctant to do so.
Standards ______________________________________
| Assure the client that test results and other information he or she provides will remain confidential. | |
| Discuss anonymous testing options. | |
| Provide client-centered counseling to: | |
| Discuss clients history of HIV testing and results. | |
| Involve the client in an assessment to determine his or her behaviors which result in a risk of acquiring HIV infection. | |
| Tailor the counseling session to the behaviors, circumstances, and special needs of the client. Assist the client in recognizing those behaviors which put him or her at risk for HIV. | |
| Identify steps already taken by the client to reduce risk and provide positive reinforcement. | |
| Identify barriers/obstacles to the client's previous efforts to reduce risk. | |
| Determine one or two behavioral changes the client may be willing to make to reduce risk. | |
| Discuss the steps necessary to implement these changes. | |
| Address any difficulties the client anticipates in taking these steps. | |
| Respond to the client's concerns. | |
| Provide the client with necessary referrals and a written copy of the risk reduction plan (this plan should not include any personal identifiers). | |
| For clients who cannot read, a verbal summary should be provided. – Assist the client to arrive at an appropriate decision concerning HIV testing. – Obtain informed consent from the client prior to testing. | |
| Establish a plan with the client to receive test results. |
Guidelines __________________________________
| Document the risk assessment in the client's record for use during subsequent care. | |
| Document the risk reduction plan in the client's record. | |
| Ensure that the client understands the risks and benefits of knowing his or her HIV infection status. | |
| Discuss the client's expectations of test results. | |
| Discuss the client's plan to cope while waiting for test results. | |
| Explore with the client support systems that may be available. | |
| Ensure that the client understands what will happen during his or her visit to receive test results. | |
| Discuss the client's responsibility to disclose HIV infection status to sex/needle sharing partners. |
As part of the assessment, the counselor should ascertain the client's understanding of HIV transmission and the meaning of HIV antibody test results. When appropriate and relevant to the client, the counselor may:
| Discuss what the virus is and how it is transmitted. | |
| Assist the client to comprehend transmission of HIVand the delay between infection and development of a positive test | |
| Discuss what the test results mean and how they are used in medical management. |
Negative Result - A negative test means that the person is either (1) not infected, or (2) so recently infected that the test could not detect the infection.
Positive Result - A positive test means that the person is infected with HIV and can transmit it to others.| Discuss need for retest. |
Clients engaging in continued high-risk behavior should be retested 6 months after the last possible exposure to any HIV risk.
| Review risk reduction options with the client, for example: | |
| Advise persons with behavioral risk for HIV not to donate blood and not to use the blood bank as a means of periodic HIV testing. | |
| Discuss related healthy behaviors, for example: | |
| Explain authorized disclosures and antidiscrimination protection. |
Providing HIV antibody test results to a client involves interpretation that is based on the test result and the person's specific risk for HIV infection and dealing with the client's reaction to his/her test result. The client will most often focus on the result itself. Client-centered counseling is required to reassess behavioral risk that may influence the interpretation. When the client receives HIV test results, the primary public health purposes of counseling are (1) to reinforce perception of risk for those who are unaware or uninformed; (2) to help uninfected persons initiate and sustain behavior changes that reduce their risk of becoming infected; (3) to arrange access to necessary medical, prevention, and case management services for persons with a positive test result; (4) to assist those who may be infected to avoid infecting others and remain healthy; and (5) to support and/or assist infected clients to ensure the referral of as many sex or needle sharing partners as possible.
Knowledge of HIV status is an important piece of information a client can use in planning the scope of behavioral changes. Persons who abstain or have sexual relations with others who are known to be free of HIV infection and who do not use injecting drugs can essentially eliminate their risk of acquiring HIV. However, the consistent and correct use of condoms or the adoption of certain non-insertive sexual activities can greatly reduce the risk of acquiring or transmitting HIV. Although methods may be employed to reduce the risk of HIV from injecting drug use (such as the use of new needles), injecting drug use constitutes a health risk even in the absence of HIV and must be avoided. The risk assessment and risk reduction plan developed during counseling prior to HIV testing provide a framework for strengthening efforts the client has already taken toward healthier behaviors and for recommending modifications based upon the HIV test result.Standards________________________________
| Review available documentation including the risk assessment, prior to meeting with the client. | |
| Assure the client that test results and other information he or she provides will remain confidential. | |
| Provide HIV positive test results only by personal contact, assuring a confidential environment. | |
| Provide counseling at the time results are given to: | |
| Discuss with the client the need to appropriately disclose HIV status. | |
| Assess the client's need for subsequent counseling or medical services. | |
| Develop a plan to access necessary resources and appropriate referrals. | |
| For use during subsequent clinical care, document test results, risk reduction plan, and identified need for any resources and referrals in the client's chart. | |
| Ensure that confidentially tested HIV infected clients who do not return for results and counseling are provided appropriate follow-up. Document all follow-up. Exhaustive efforts should be made to ensure that confidentially tested HIV infected clients are offered their HIV test results and counseling. |
Interpretation of HIV antibody test results depends upon the client's risk behaviors. Some recently infected clients may have negative antibody tests. Indeterminate results may represent a recent HIV infection or a biologic false positive. Eliciting specific information about recent risk behavior is essential to accurate interpretation and counseling. The client will likely encounter circumstances where it is appropriate to reveal their HIV infection status (e.g., to healthcare or dental providers; past, present, or potential sex and needle sharing partners). It is important to discuss such situations with the client and assist in developing a plan and skills for appropriate disclosure of HIV infection status.
Guidelines_______________________________
| Ensure that the client understands what the test result means including: | |
| Identify any steps already taken by the client to reduce risk and provide positive reinforcement. | |
| Encourage the client to continue avoiding risk behaviors. | |
| Determine one or two behavioral changes the client may be willing to make to reduce risk and discuss steps to implement these changes. | |
| Assist the client in building skills to negotiate risk reduction activities with current or potential partners through discussion and role plays. | |
| Offer referral for further assistance in avoiding risk behaviors and maintaining low-risk behaviors. | |
| Discuss his/her need and ability to help partners realize they are also at risk for HIV infection. | |
| Reinforce the importance of discussing risk reduction measures with potential partners; identify any difficulties the client perceives. | |
| Advise client about importance of early STD detection and treatment to reduce HIV risk. | |
| Advise client to refrain from donating blood, plasma, and organs. | |
| Advise client on access to other prevention and treatment services (i.e., drug treatment, psychosocial support etc.) |
Some HIV positive clients may be better prepared to receive positive test results than others. Counseling of patients with positive results must be directed to the client's specific circumstances and may require more than one session. Counselors should recognize that the emotional impact of learning about an HIV positive test result often prevents clients from absorbing other information during this encounter. Counselors may need to arrange additional sessions or provide appropriate referrals to meet the client's needs and accomplish the goals of counseling persons who are HIV positive.
| Allow time for the client's emotional response after learning his or her positive HIV result. A subsequent counseling session or follow-up telephone call may be required. | |
| Ensure that the client understands what the test result means. | |
| Assess the client's immediate needs for medical, preventive, and psychosocial support. (e.g., financial personal, and other) | |
| Make the client aware of the need for additional medical evaluation and the availability of treatment. | |
| Establish a plan for continuing medical care and psychological support, including a subsequent prevention counseling session if necessary. As part of the plan, the counselor should: | |
| Reassess the client's risk for transmitting HIV infection. | |
| Help facilitate behavior change and/or reinforce behaviors that minimize or eliminate risk of transmission. | |
| Discuss with the client access and availability to ongoing prevention services including psychosocial and support services. | |
| Discuss with the client the responsibility to assure that sex and/or needle-sharing partners are counseled about their exposure to HIV and the need for them to seek medical evaluation. | |
| Assist the client in developing a plan which ensures that all partners are counseled about their exposure to HIV. | |
| Discuss how the client will notify other persons of his or her HIV status including future sex and needle-sharing partners, healthcare providers, and dental providers. | |
| Discuss with the client his or her specific plans for the next few days and ensure that the client has access to support systems during this time. | |
| Advise client to refrain from donating blood, plasma, and organs. |
The current testing strategy of two EIA determinations followed by a supplemental test for confirmation, such as the Western blot, makes false positive test results extremely unlikely; however, the possibility of a mislabeled sample or laboratory error must be considered, and for a client with no identifiable risk for HIV infection, a repeat test may be appropriate.
| Clients whose results are HIV positive may have specific medical questions. | |
| Considering the complexity of medical questions, responses should be left to clinicians to whom the client is referred, or to counselors or case managers with specific expertise in this area. | |
| Some clients may be at very high risk of transmitting the virus to others. Sites are encouraged to provide either on-site or through referral, additional prevention counseling (individual, couple, group, or peer), as appropriate to the needs of these clients. Counselors should appreciate the complexity of reproductive decision-making for HIV-infected women and must be familiar with the most recent Public Health Service recommendations on antiretroviral therapy to prevent vertical transmission. |
| Explain that the test result is inconclusive and may represent either: | |
| Schedule a repeat test approximately 6 weeks after the date of this inconclusive test. | |
| Emphasize that the client must take the same risk reduction precautions as persons testing HIV positive until the indeterminate finding is resolved. | |
| Assess the client's concerns and anxieties during the waiting period. If necessary, |
Situations where clients need repeat HIV counseling or request repeat HIV testing challenge and pose difficult issues for counselors. These situations include previously counseled persons who continue to place themselves or others at risk for infection, persons with indeterminate test results seronegative persons with no risk who continue to request testing, and persons doubting or disbelieving their seropositive test results. Repeat testing is not advised as a substitute for initiating and maintaining safer behaviors.
Standards _____________________________
| Assess the reasons the client requests repeat testing or continues risk behaviors. | |
| Emphasize that repeated testing for HIV will not prevent infection if the client continues to engage in risk behaviors. | |
| Arrange the specific services to meet the client's needs. | |
| Document all counseling activities, negotiated plans, and referrals in the client's record. |
Guidelines____________________________
The counselor should:
| Review previous risk assessment and risk reduction plan with client. | |
| Proceed with HIV counseling as outlined in the Section, HIV Prevention Counseling. | |
| Provide alternative counseling options (e.g. referral to community based group or individual counseling) to the client to further help him or her understand his or her recidivist risk behavior(s) and modify the behaviors accordingly. | |
| Acknowledge incremental behavior changes, reinforce those which have reduced risk, and document in the client's chart. | |
| Identify obstacles which the client encountered in adopting safe behaviors. | |
| Explain potentially negative impact of HIV reinfection or exposure to other STDs. |
| Explain the continued risk of infecting sex and needle sharing partners | |
| > Negotiate a plan with the client to prevent HIV transmission. | |
| Identify resources and alternative counseling options to ensure that the client implements this plan and to reinforce the importance of practicing safer behaviors to protect himself or herself and others. | |
| Reinforce the importance of informing partners and making risk-reduction decisions with partners. | |
| Ensure that the client understands the adverse impact of STDs and drug use upon immune function. |
The counselor should:
| Arrange a repeat test approximately 6 weeks from the date of this current test; | |
| Assess the client's concerns and level of anxiety during the waiting period. If necessary, arrange psychological referral to assist the client in coping; | |
| Consider persons to be negative for antibodies to HIV if their Western Blot test results continue to be consistently indeterminate for at least 6 months in the absence of any known risk behaviors, clinical symptoms, or other findings (2); | |
| Encourage the client to follow guidelines outlined in the Notification of HIV Results and Prevention Counseling Section. |
The counselor should:
| Counsel the client on modes of HIV transmission and behaviors that place persons at risk for HIV; | |
| Counsel the client on unwarranted fears; | |
| Arrange referral for additional counseling for clients who continue to exhibit unfounded anxiety about HIV. |
The counselor should:
| Assess why the client doubts the accuracy of the test results; | |
| Explain the process of multiple tests to confirm a positive result; | |
| Assist the client in recognizing behaviors that lead to HIV infection |
A thorough client assessment often indicates a need for services that cannot be provided by the counselor. The counselor has two opportunities to make referrals: (1) the HIV prevention counseling session, and (2) the test notification/prevention counseling session.
Standards ____________________________
| Provide appropriate referral resources for: | |
| Provide the client with a written list of referrals including telephone numbers, addresses, hours of operation and services provided. | |
| Document referrals in the client's record. | |
| Referrals made during the initial HIV prevention counseling session should be followed-up during the test notification/prevention counseling session. |
Guidelines __________________________________
| The counselor should: Offer referral to case management provider, if one is available; | |
| Seek feedback from the client about preferences for referrals, the accessibility of the referral, and the client's intention to follow through with the referral; | |
| Provide the client with relevant details about referral sites, e.g., the name of a specific contact person. |
Any HIV positive or negative client who continues to engage in risk behaviors should know where and how to access STD 11 examination and treatment services.
Partner notification, a component of sexually transmitted disease (STD) control programs for many years , is a means to identify and target risk-reduction education to individuals at high risk for contracting or transmitting HIV infection. When applied to HIV infection, the term partner includes not only sex partners but also intravenous drug users who share needles. Partner notification for HIV infection or acquired immunodeficiency syndrome (AIDS), as for all STDs, is highly confidential and depends upon the voluntary cooperation of the patient. CDC currently recommends the following: Persons who are HIV-antibody positive should be instructed in how to notify their partners and to refer them for counseling and testing. If they are unwilling to notify their partners or if it cannot be assured that their partners will seek counseling, physicians or health department personnel should use confidential procedures to assure that the partners are notified. Two complementary notification processes can be used to identify partners, patient referral and provider referral.
With patient referral, HIV-infected patients choose to inform their own partners directly of their risk of infection. Trained health department personnel can help instruct patients how to inform sex and needle- sharing partners sensitively about their potential risk for infection. With provider referral, infected patients request assistance in notifying some or all of their partners; they voluntarily provide names, descriptions, and addresses so that the notification process can be carried out by trained health department staff. This process is designed to protect the anonymity of patients; their names are never revealed to sex or needle-sharing partners. When the partner-notification model is applied to the control of HIV infection, certain differences must be considered. The incubation period for HIV is long; therefore, sex partners or needle-sharing partners from months or years earlier may potentially have been the sources of infection. Partner notification for patients with hepatitis B, which has an epidemiologic pattern similar to that of HIV infection, has proven difficult because of the prolonged period of infectivity, the large number of anonymous sex partners among many homosexual men, and the inaccessibility of the intravenous drug-using population.The assurance of confidentiality and protection against discrimination, which are critical in dealing with any STD have become legal issues in the case of HIV infection. These issues may influence the success of programs based on patient referral alone. Confidentiality is essential to ensure that individuals at risk continue to seek counseling, testing, or partner-notification services.
Partner-notification data from several states reveal a high seroprevalence rate, ranging from 11% to 39%, among persons identified as sex or needle-sharing partners, many of whom are themselves engaging in high-risk behavior. By identifying such individuals, the partner-notification process can target risk-reduction messages to those at greatest risk of acquiring or transmitting infection. Thus, partner notification provides both primary and secondary prevention of HIV infection. Notification of unsuspecting partners is especially important because it enables persons who may not have been reached through other AIDS education programs to receive risk-reduction education. For example, the partner-notification process can identify female and male partners of intravenous drug users or female partners of bisexual males who may have been exposed to HIV infection but who may be unaware of their risk. Partner-notification activities targeted toward women of childbearing age contribute additionally by potentially preventing the perinatal transmission of HIV. Homosexual men who voluntarily request counseling and HIV testing may be at lower risk for infection than those who have refused testing. Through the partner-notification process, these high-risk partners who otherwise might not request risk-reduction education can receive counseling. Also, counseling of partners provides an opportunity to offer other beneficial services to those at risk, including drug treatment, STD treatment, tuberculosis testing and treatment, adult immunizations, psychosocial support services, and contraceptive counseling. The type of partner-notification services provided by different health departments will depend on local resources and the number of seropositive persons identified. In San Francisco, which has high rates of infection among homosexual men, provider referral for all partners of homosexual men was not thought to be feasible because of the excessive cost and personnel required. However, the San Francisco Health Department did notify heterosexual sex partners of AIDS patients and received excellent cooperation from both patients and named partners.ABSTINENCE: Refraining from participating in something. When talking about HIV, abstinence refers to not engaging in sexual intercourse or injecting drugs.
AIDS: The acronym for acquired immunodeficiency syndrome. AIDS can affect the immune and central nervous systems and can result in neurological problems, infections, or cancers. It is caused by HIV.
Anal Sex: A type of sexual intercourse in which a man's penis enters his partner's anus.
Anonymous: Without any identification. The term is used in regard to HIV testing when the persons ordering and performing the test do not maintain a record of the name or identity of the person whose blood they are testing.
Antibodies: Proteins that are manufactured by the immune system in response to foreign substances. Antibody Test: A laboratory procedure which detects antibodies to specific microorganisms. An HIV antibody test determines if a person's body has produced antibodies to HIV but does not detect the virus itself.
Antidiscrimination Protection: Provisions of laws that impose penalties for discrimination because of a person's HIV infection or perceived risk of infection.
ANTIVIRAL: Pertaining to something that inhibits the actions of a virus. Antiviral therapy refers to a treatment that works against the virus itself.
ANUS: The opening of the body through which feces or bowel movements pass. The anus is the part of the body which is penetrated during anal sex.
APPROPRIATE DISCLOSURE: Notifying specific people of a client's HIV risks or infection to other people because of their risk of exposure or their ability to provide medical assistance or support.
ASYMPTOMATIC: Being infected but having no symptoms of infection.
BISEXUAL: A person whose sex partners are both men and women. A bisexual can be a man or a woman.
CD4 TESTING: A laboratory blood test that counts a subset of white blood cells as an aid to determining immune function. Certain counts are indications for starting medications for persons with HIV infection.
CLIENT: A person to whom professional services are rendered.
CLIENT-CENTERED APPROACH: Refers to counseling conducted in an interactive manner responsive to individual client needs. Avoids a preconceived set of points to be made by the counselor and encourages the client to do most of the talking. Focuses on developing goals with the client rather than simply providing information or imposing counselor goals.
CONDOM: Commonly called rubbers, condoms are sheaths that fit over a man's penis or into a woman's vagina to prevent semen from entering the partner's body after ejaculation. Condoms also prevent a man's penis from coming in contact with his partner's body fluids.
CONFIDENTIAL: Kept private. In regard to HIV testing, it means that the results of a test are known only to the person who is being tested and the immediate group of people who provide care and prevention services for that person.
COUNSELING: Helping people plan actions that will benefit themselves or others. Unless designated as group counseling or couple counseling, the word is used here to describe one-on-one discussions.
DISCORDANT: Conflicting. Used to describe the circumstances in which one partner is infected with HIV and the other is not.
EIA: See ELISA.
EARLY INTERVENTION: The set of medical, preventive and psychosocial services provided to persons upon diagnosis of HIV infection. Involves monitoring indicators of immune function as signals to provide interventions to delay the onset of illness, psychosocial support, and measures to prevent transmission.
ELISA: Acronym for enzyme-linked immunosorbent assay. The laboratory test most commonly used to screen for antibodies to HIV. See Positive Test.
FALSE-NEGATIVE: A negative test result for a person who is actually infected.
FALSE-POSITIVE: A positive test result for a person who is actually not infected.
HETEROSEXUAL: A person whose sex partners are exclusively persons of the opposite sex.
HIV: Human immunodeficiency virus; the virus that causes AIDS.
HOMOSEXUAL: A person whose sex partners are exclusively members of the same sex. A homosexual man is called a gay man. A homosexual woman is called a lesbian.
IMMUNE STATUS: The state of the body's natural ability to fight diseases.
IMMUNE SYSTEM: The body's mechanism to identify and fight off infections and other foreign substances.
INJECTED DRUGS: Drugs that are introduced directly into a person's body or bloodstream through a needle. These include cocaine, crack, heroin, and steroids.
INDETERMINATE: Not determined one way or another. Inconclusive test results; the laboratory is unable to state whether antibody is present or not.
INTERVENTION: An action taken to change an outcome.
MASTURBATION: Stimulating a man's penis or a woman's clitoris. MONOGAMOUS: Having an exclusive sexual relationship with only one partner. Mutual monogamy means neither partner has sex with other people.
MORBIDITY: Illness or disease.
MORTALITY: Death.
NEGOTIATED RISK
REDUCTION PLAN: Discussions that result in identifying the steps that a client thinks he or she will take to reduce the chances of acquiring HIV. The counselor's role is to assist the client in developing a realistic plan.
OUTREACH SERVICES: Usually refers to services provided outside the walls of an agency. An outreach worker might go to a client's home or neighborhood.
PARENTERAL: Taken into the body through intravenous or intramuscular injection.
PHLEBOTOMY: Collecting a blood sample for laboratory testing by inserting a needle into a person's vein.
POSITIVE REINFORCEMENT: Acknowledging healthy behaviors or intentions through some mechanism that indicates approval intended to be perceived as rewarding.
POSITIVE TEST: For HIV, a sample of blood that is reactive on an initial ELISA test, repeatedly reactive on a second
ELISA run on the same specimen, and confirmed positive on Western blot or other supplemental test. PREVALENCE: The total number of persons in a given population with a disease or condition at a given point in time.
PREVENTION COUNSELING: Counseling which is designed to facilitate the client's perception of risk, identify behavior changes that the client has already implemented and barriers to the client's previous efforts to reduce risk, and to assist the client in developing a plan to reduce risk regardless of whether or not he or she takes the test. Prevention counseling that takes place prior to HIV testing should prepare the client for receiving and managing his or her test results.
PROBLEM-SOLVING TECHNIQUES: A process by which a counselor tries to discover the basis of barriers indicated by some verbal or nonverbal communication from the client. After the barriers have been identified, possible solutions are discussed.
PROPHYLACTIC TREATMENT: Medications given to help prevent infection or its consequences.
PROBLEM-SOLVING TECHNIQUES: A process by which a counselor tries to discover the basis of barriers indicated by some verbal or nonverbal communication from the client. After the barriers have been identified, possible solutions are discussed.PROPHYLACTIC TREATMENT: Medications given to help prevent infection or its consequences. RETROVIRUS: One of a group of RNA viruses. HIV is a retrovirus.
RISK ASSESSMENT: Used in this document, risk assessment is that portion of a client-centered discussion that encourages the client to identify and acknowledge his or her personal risk for acquiring HIV.
SENSITIVITY: The probability that a test will be positive when infection is present.
SPECIFICITY: The probability that a test will be negative when the infection is not present.
SPERMICIDE: A substance that kills sperm.
TRIAGE ASSESSMENT: The process that determines whether someone should be referred to counseling. Triage assessment facilitates prevention counseling services for those persons at increased risk for HIV.
WESTERN BLOT: A laboratory test that detects specific antibodies to components of a virus. Often used to confirm HIV antibodies in specimens found repeatedly reactive using the ELISA test for HIV antibodies.
The interconnectedness of HIV and other sexually transmitted diseases (STDs) grows increasingly apparent as biomedical and behavioral scientists learn more about people's susceptibility and risks. CDC is applying new research to the prevention of all major STDs, including HIV infection, and is working to ensure communities have the information they need to design, implement, and evaluate comprehensive approaches to HIV and STD prevention.
Globally, an estimated 333 million new cases of curable STDs occur each year among adults, according to 1995 estimates by the World Health Organization. STDs in the United States have reached epidemic proportions with an estimated 12 million new cases each year. Of these, 3 million occur among teenagers, 13 to 19 years old. STDs are the most common reportable diseases in the United States.
The sexual spread of HIV in the United States has paralleled that of other STDs. For example, the geographic distribution of heterosexual HIV transmission closely parallels that of other STDs. Most of the health districts with the highest syphilis and gonorrhea rates in the United States are concentrated in the South, the same part of the nation with the highest HIV prevalence among childbearing women. Researchers have long recognized that the risk behaviors which place individuals at risk for other STDs also increase a person's risk of becoming infected with HIV. STD surveillance can provide important indications of where HIV infection may spread, and where efforts to promote safer sexual behaviors should be targeted.There is now strong evidence that other STDs increase the risk of HIV transmission and, conversely, that STD treatment reduces the spread of HIV.
Epidemiological studies: Studies have repeatedly demonstrated that people are 2-5 times more likely to become infected with HIV when other STDs are present. Biological studies: Biological studies suggest both increased susceptibility to HIV infection and increased likelihood of infecting other people when other STDs are present. a) Increased susceptibility–STDs that cause genital lesions can create a portal of entry for HIV. And even without lesions, STDs increase the number of HIV target cells (CD4 cells) in cervical secretions, thereby likely increasing HIV susceptibility in women. b) Increased infectiousness–Studies have demonstrated that co-infection with HIV and other STDs results both in more shedding of HIV and in greater concentrations of HIV being shed. For example, in African studies, co-infection with gonorrhea and HIV more than doubles the proportion of HIV-infected individuals with HIV RNA detectable in genital secretions. Furthermore, the median concentration of HIV RNA in semen is dramatically increased in co-infected men compared with men infected with HIV alone.Intervention studies: New evidence indicates that STD detection and treatment can substantially reduce HIV transmission. For example: a) STD treatment reduces the prevalence and magnitude of HIV shedding–Treatment of gonorrhea in HIV-infected men resulted in a reduction in the number of men who shed HIV, as well as a lower concentration of HIV shed. With STD treatment, the level of shedding among co-infected men returns to the level seen in men who are not co-infected.
b) STD treatment reduces the spread of HIV infection in communities– A community-level, randomized trial in a rural African community in Tanzania demonstrated a 42% decrease in new, heterosexually transmitted HIV infections in communities with improved STD treatment. An ongoing study in Uganda is further exploring the impact of mass STD treatment in slowing the spread of HIV. These studies will be critical in more clearly defining the role STD treatment can play in HIV prevention efforts in the developing world and in industrialized nations.With nearly 1 million Americans infected with HIV, most of them through sexual transmission, and an estimated 15 million cases of other sexually transmitted diseases (STDs) occurring each year in the United States, effective strategies for preventing these diseases are critical. Refraining from having sexual intercourse with an infected partner is the best way to prevent transmission of HIV and other STDs. But for those who have sexual intercourse, latex condoms are highly effective when used consistently and correctly.
The correct and consistent use of latex condoms during sexual intercourse- vaginal, anal, or oral-can greatly reduce a person' s risk of acquiring or transmitting most STDs, including HIV infection, gonorrhea, chlamydia, trichomonas human papilloma virus infection (HPV), and hepatitis B. Protecting yourself and others against STDs is important because many of these diseases have serious complications. Protecting yourself and others against HIV is important because it is life threatening and has no cure.
 Laboratory studies show that latex condoms are effective barriers to HIV and other STDs. In addition, several studies
provide compelling evidence that latex condoms are highly effective in protecting against HIV infection when used for
every act of intercourse. This protection is most evident from studies of couples in which one member is infected with
HIV and the other is not, i.e., discordant couples.
Laboratory studies show that latex condoms are effective barriers to HIV and other STDs. In addition, several studies
provide compelling evidence that latex condoms are highly effective in protecting against HIV infection when used for
every act of intercourse. This protection is most evident from studies of couples in which one member is infected with
HIV and the other is not, i.e., discordant couples.
In a 2-year study of discordant couples in Europe, among 124 couples who reported consistent use of latex condoms none of the uninfected partners became infected. In contrast, among the 121 couples who used condoms inconsistently, 12 (10%) of the uninfected partners became infected. In another study, among a group of 134 discordant couples who did not use condoms at all or did not use them consistently, 16 partners (12%) became infected. This contrasts markedly with infections occurring in only 3 partners (2%) of the 171 couples in this study who reported consistently using condoms over the 2-year period. Similarly, in a recent study among discordant couples in Haiti, 1 of 42 uninfected partners (2%) became infected with consistent condom use and 19 of 135 who used condoms inconsistently (14%) became infected.
Condoms are classified as medical devices and are regulated by the Food and Drug Administration (FDA). Every latex condom manufactured in the United States is tested for defects before it is packaged. During the manufacturing process, condoms are double-dipped in latex and tested electronically for holes. Several studies clearly show that condom breakage rates in this country are less than 2%. Most of the breakage and slippage likely is due to incorrect use rather than poor condom quality. Using oil-based lubricants can weaken latex, causing the condom to break. In addition, condoms can be weakened by exposure to heat or sunlight or by age, or they can be torn by teeth or fingernails. Studies also indicate that condoms slip off the penis in about 1-5% of acts of vaginal intercourse and slip down (but not off) about 3-13% of the time.
Some persons have expressed concern about studies that report higher failure rates among couples using condoms for pregnancy prevention. Analysis of these studies indicates that the large range of efficacy rates is related to incorrect or inconsistent use. In fact, latex condoms are highly effective for pregnancy prevention, but only when they are used properly. Research indicates that only 30-60% of men who claim to use condoms for contraception actually use them for every act of intercourse. Further, even people who use condoms every time may not use them correctly from start to finish. Incorrect use contributes to the possibility that the condom could leak at the base or break.
As mentioned previously, the primary reason that condoms sometimes fail to prevent HIV/STD infection or pregnancy is incorrect or inconsistent use, not failure of the condom itself. Consistent use means using a condom with each act of intercourse. Correct condom use includes all of the following steps:
Use a new condom for each act of vaginal, anal, or oral intercourse.
| |||
| Use the condom throughout sex–from start to finish. | |||
| Put on the condom as soon as erection occurs and before any vaginal, anal, or oral contact with the penis. Hold the tip of the condom and unroll it onto the erect penis, leaving space at the tip of the condom, yet ensuring that no air is trapped in the condom' s tip. | |||
| Adequate lubrication is important to prevent condom breakage, but use only water-based lubricants, such as glycerine or lubricating jellies (which can be purchased at any pharmacy). Do not use oil-based lubricants such as petroleum jelly, cold cream, hand lotion, or baby oil, which can weaken the condom. |
Condom users have product options

|
|
Condoms and latex sheets |
Latex condoms for men. Latex condoms are made of a particular kind of rubber. Laboratory studies show that intact latex condoms provide a highly effective barrier to sperm and micro-organisms, including HIV and the much smaller hepatitis B virus. Their effectiveness has been proven over many years.
Synthetic condoms. For people who are allergic to latex, several new types of materials are being used to make condoms. One new type is polyurethane, a soft plastic. Another new type is Tactylon TM, a synthetic latex. Lab tests have shown that both these materials provide an effective barrier against sperm, bacteria, and viruses such as HIV.Polyurethane condoms for women. The female condom (Reality TM* ) fits inside the vagina and covers some of the area outside of the vagina. It also is made of polyurethane. When a male condom cannot be used, couples should consider using a female condom. Unlike latex condoms, synthetic condoms such as male and female polyurethane condoms can be used with either water-based or oil-based lubricants. Although not as thoroughly tested as latex condoms, synthetic condoms likely provide similar protection.
Lambskin condoms. These condoms are made from animal membranes that contain tiny holes. While they can prevent pregnancy, they should not be used for STD or HIV prevention because viruses may be able to pass through these holes.
Novelty condoms. Novelty (play) condoms are for sexual amusement only. The FDA does not allow them to be labeled as condoms, and they should never be used for STD/HIV or pregnancy prevention.
Spermicides. Although studies indicate that nonoxynol-9, a spermicide, kills HIV in laboratory testing, it is not clear whether spermicides used alone or with condoms during intercourse provide protection against HIV. Therefore, latex condoms with or without spermicides should be used to prevent sexual transmission of HIV.
Oral protection. Even though their risk is less than with unprotected anal and vaginal sex, people who engage in oral sex can reduce their risk of getting HIV or another STD by placing a barrier over the vagina or anus. In addition to the male condom, a product designed to reduce the risk of acquiring an STD during oral sex is now being sold in the United States. The Sheer Glyde Dam TM* is a 10 x 6 latex sheet that the FDA has authorized for marketing in the United States. Plastic food wrap, dental dams (pieces of latex used by dentists), and condoms that have been cut open all have been used to cover the vagina or anus during oral sex, although there is no information about how well these materials work.
Education about condom efficacy does not promote sexual activity
Five U.S. studies of specific sex education programs have demonstrated that HIV education and sex education that included condom information either had no effect upon the initiation of intercourse or resulted in delayed onset of intercourse; five studies of specific programs found that HIV/sex education did not increase frequency of intercourse, and a program that included development of skills to negotiate safer sexual behaviors actually resulted in a decrease in the number of youth who initiated sex. In addition, a World Health Organization (WHO) review cited 19 studies of sex education programs that found no evidence that sex education leads to earlier or increased sexual activity in young people. In fact, five of the studies cited by WHO showed that such programs can lead to a delay or decrease in sexual activity. In a recent study of youth in Los Angeles, an HIV prevention program focusing on condom use did not increase sexual activity or the number of sex partners. But condom use did increase among those who were already sexually active. A 1987 study of young U.S. men who were sent a pamphlet discussing STDs with an offer of free condoms also did not find any increase in the youths' reported sexual activity.
Prevention is cost-effective
In summary, STDs, including HIV infection, are preventable, and condoms represent an effective prevention tool. A recent analysis estimated that, for high-risk heterosexual men, the societal savings (in health care costs and productivity) per condom was $27, and for men who have sex with men, the savings per condom was more than $530 when condoms were used consistently and correctly with multiple partners.
Latex condoms for men. Latex condoms are made of a particular kind of rubber. Laboratory studies show that intact latex condoms provide a highly effective barrier to sperm and micro-organisms, including HIV and the much smaller hepatitis B virus. Their effectiveness has been proven over many years.
Synthetic condoms. For people who are allergic to latex, several new types of materials are being used to make condoms. One new type is polyurethane, a soft plastic. Another new type is Tactylon TM, a synthetic latex. Lab tests have shown that both these materials provide an effective barrier against sperm, bacteria, and viruses such as HIV.Polyurethane condoms for women. The female condom (Reality TM* ) fits inside the vagina and covers some of the area outside of the vagina. It also is made of polyurethane. When a male condom cannot be used, couples should consider using a female condom. Unlike latex condoms, synthetic condoms such as male and female polyurethane condoms can be used with either water-based or oil-based lubricants. Although not as thoroughly tested as latex condoms, synthetic condoms likely provide similar protection.
Lambskin condoms. These condoms are made from animal membranes that contain tiny holes. While they can prevent pregnancy, they should not be used for STD or HIV prevention because viruses may be able to pass through these holes.
Novelty condoms. Novelty (play) condoms are for sexual amusement only. The FDA does not allow them to be labeled as condoms, and they should never be used for STD/HIV or pregnancy prevention.
Spermicides.Although studies indicate that nonoxynol-9, a spermicide, kills HIV in laboratory testing, it is not clear whether spermicides used alone or with condoms during intercourse provide protection against HIV. Therefore, latex condoms with or without spermicides should be used to prevent sexual transmission of HIV.
Oral protection.Even though their risk is less than with unprotected anal and vaginal sex, people who engage in oral sex can reduce their risk of getting HIV or another STD by placing a barrier over the vagina or anus. In addition to the male condom, a product designed to reduce the risk of acquiring an STD during oral sex is now being sold in the United States. The Sheer Glyde Dam TM* is a 10 x 6 latex sheet that the FDA has authorized for marketing in the United States. Plastic food wrap, dental dams (pieces of latex used by dentists), and condoms that have been cut open all have been used to cover the vagina or anus during oral sex, although there is no information about how well these materials work.
Education about condom efficacy does not promote sexual activity
Five U.S. studies of specific sex education programs have demonstrated that HIV education and sex education that included condom information either had no effect upon the initiation of intercourse or resulted in delayed onset of intercourse; five studies of specific programs found that HIV/sex education did not increase frequency of intercourse, and a program that included development of skills to negotiate safer sexual behaviors actually resulted in a decrease in the number of youth who initiated sex. In addition, a World Health Organization (WHO) review cited 19 studies of sex education programs that found no evidence that sex education leads to earlier or increased sexual activity in young people. In fact, five of the studies cited by WHO showed that such programs can lead to a delay or decrease in sexual activity. In a recent study of youth in Los Angeles, an HIV prevention program focusing on condom use did not increase sexual activity or the number of sex partners. But condom use did increase among those who were already sexually active. A 1987 study of young U.S. men who were sent a pamphlet discussing STDs with an offer of free condoms also did not find any increase in the youths' reported sexual activity.
Prevention is cost-effective
In summary, STDs, including HIV infection, are preventable, and condoms represent an effective prevention tool. A recent analysis estimated that, for high-risk heterosexual men, the societal savings (in health care costs and productivity) per condom was $27, and for men who have sex with men, the savings per condom was more than $530 when condoms were used consistently and correctly with multiple partners.
The likelihood of transmission of HIV from an infected person to an uninfected person varies significantly depending on the type of exposure or contact involved. The risk of becoming infected with HIV through unprotected (without a condom) oral sex is lower than that of unprotected anal or vaginal sex. However, even a lower risk activity can become an important way people get infected if it is done often enough. One study sponsored by the Centers for Disease Control found that 7.8% (8 of 102) of recently infected men who have sex with men in San Francisco were probably infected through oral sex. Most of these men believed that the risk was minimal or non-existent.
Nearly half (3 of 8) of these cases reported oral problems, including occasional bleeding gums. Almost all (7 of 8) of these men reported to have had oral contact with pre-semen or semen.
Oral transmission of HIV is very difficult to single out as the only way that HIV is transmitted because few people engage exclusively in oral sex. A number of specific questions were asked by a trained evaluator. The participants' risk behaviors were assessed by using clinical interviews, counselor intervention, epidemiologic interview, partner interview when possible, and final disposition of transmission risk. Of the 8 cases, 4 reported protected anal intercourse without the condom breaking, with persons who were either HIV infected or had an unknown serostatus. Men in this study who reported that they were uncertain if the condom was used properly were eliminated from this study.
Yes and No. The percentage of recently infected men enrolled in this study who were probably infected through oral sex (8%) was higher than many researchers had thought likely or found in other studies. More media attention appeared to be placed on this particular study, probably because of the higher number of study participants. There appears to be evidence that higher risk activities (anal sex) among men who have sex with men is decreasing while lower risk activities (oral sex) among these men is increasing. Oral sex has always been considered a lower risk activity but is certainly not risk free.
The study results emphasize that any type of sexual activity with an infected person is a risk of HIV transmission. Oral sex with someone who is infected with HIV is certainly not risk free. Prevention of HIV is more important than ever. Some persons have indicated that they are less concerned about HIV because of new treatments and are being less careful. This study presents a wake-up call to everyone- that HIV is far from over and remains a serious, lifelong disease that is best to prevent. CDC's recommendations on how to prevent sexual transmission of HIV remain the same. Protection requires abstaining from sexual activity or taking precautions with all types of intercourse- either having sex with only one uninfected partner, using condoms for sexual intercourse and oral sex, and using lower risk activities such as mutual masturbation.
In the United States, HIV-related illness and death historically have had a tremendous impact on men who have sex with men (MSM). Even though the toll of the epidemic among injection drug users (IDUs) and heterosexuals has increased during the last decade, MSM continue to account for the largest number of people reported with AIDS each year. In 1999 alone, 15,464 AIDS cases were reported among MSM, compared with 10,138 among IDUs and 7,139 among men and women who acquired HIV heterosexually.
Overall, the number of MSM of all races and ethnicities who are living with AIDS has increased steadily,partly as a result of the 1993 expanded AIDS case definition and, more recently, improved survival. (See chart at right.)
Abundant evidence shows a need to sustain prevention efforts for each generation of young gay and bisexual men. We cannot assume that the positive attitudinal and behavioral change seen among older men also applies to younger men. Recent data on HIV prevalence and risk behaviors suggest that young gay and bisexual men continue to place themselves at considerable risk for infection with HIV and other sexually transmitted diseases (STDs).
| Ongoing studies show that both HIV prevalence (the proportion of people living with HIV in a population) and risk behaviors remain high among young MSM. In a sample of MSM 15-22 years old in seven urban counties, CDC researchers found that, overall, 7% already were infected with HIV. Higher percentages of African Americans (14%) and Hispanics (7%) were infected than were whites (3%). | |
| In the 32 states with confidential HIV reporting, data show that substantial numbers of MSM still are being infected, especially young men. In 1999, 46% of reported HIV infections among adolescent males aged 13-19 and 51% of cases among men aged 20-24 were attributed to male-to-male sexual contact. | |
| Research among gay and bisexual men suggests that some individuals are now less concerned about becoming infected than in the past and may be inclined to take more risks. This is backed up by reported increases in gonorrhea among gay men in several large U.S. cities between 1993 and 1996. Despite medical advances, HIV remains a serious usually fatal disease that requires complex, costly, and difficult treatment regimens that do not work for everyone. As better treatment options are developed, we must not lose sight of the fact that preventing HIV infection in the first place precludes the need for people to undergo these difficult and expensive therapies. |
Studies among MSM who are treated in STD clinics have shown consistently high rates of HIV infection, ranging from nearly 4% in Seattle to a high of almost 36% in Atlanta. Scientists know that the likelihood of both acquiring and spreading HIV is 2-5 times greater in people with STDs, and that aggressively treating STDs in a community can help to reduce the rate of new HIV infections. Along with prompt attention to and treatment of STDs, efforts to reduce the behaviors that spread STDs are critical.
Research has shown that high-risk behavior is continuing in some populations of MSM, including those who are infected with HIV. As the number of gay and bisexual men living with HIV increases, greater efforts must be made to reach them with behavioral interventions that can help them protect their own health and prevent transmission to others.
Female-to-female transmission of HIV appears to be a rare occurrence. However, case reports of female-to-female transmission of HIV and the well documented risk of female-to-male transmission of HIV indicate that vaginal secretions and menstrual blood are potentially infectious and that mucous membrane (e.g., oral, vaginal) exposure to these secretions have the potential to lead to HIV infection.
Through December 1998, 109,311 women were reported with AIDS. Of these, 2,220 were reported to have had sex with women; however, the vast majority had other risks (such as injection drug use, sex with high-risk men, or receipt of blood or blood products). Of the 347 (out of 2,220) women who were reported to have had sex only with women, 98% also had another risk–injection drug use in most cases.
Note: Information on whether a woman had sex with women is missing in half of the 109,311 case reports, possibly because the physician did not elicit the information or the woman did not volunteer it.Women with AIDS whose only reported risk initially is sex with women are given high priority for follow-up investigation. As of December 1998, none of these investigations had confirmed female-to-female HIV transmission, either because other risks were subsequently identified or because, in a few cases, women declined to be interviewed. A separate study of more than 1 million female blood donors found no HIV-infected women whose only risk was sex with women. These findings suggest that female-to-female transmission of HIV is uncommon. However, they do not negate the possibility because it could be masked by other behaviors.
Surveys of risk behaviors have been conducted in groups of WSW. These surveys have generally been surveys of convenient samples of WSW that differ in sampling, location, and definition of WSW. As a result, their findings are not generalizable to all populations of WSW. These surveys suggest that some groups of WSW have relatively high rates of high-risk behaviors, such as injection drug use and unprotected vaginal sex with gay/bisexual men and injection drug users.
Although female-to-female transmission of HIV apparently is rare, female sexual contact should be considered a possible means of transmission among WSW. These women need to know:
| that exposure of a mucous membrane, such as the mouth, (especially nonintact tissue) to vaginal secretions and menstrual blood is potentially infectious, particularly during early and late-stage HIV infection when the amount of virus in the blood is expected to be highest. | |
| that condoms should be used consistently and correctly each and every time for sexual contact with men or when using sex toys. Sex toys should not be shared. No barrier methods for use during oral sex have been evaluated as effective or approved by the FDA. However, women can use dental dams, cut-open condoms, or plastic wrap to help protect themselves from contact with body fluids during oral sex. | |
| their own and their partner's HIV status. This knowledge can help uninfected women begin and maintain behavioral changes that reduce their risk of becoming infected. For women who are found to be infected, it can assist in getting early treatment and avoiding infecting others. |
| that sexual identity does not necessarily predict behavior, and that women who identify as lesbian may be at risk for HIV through unprotected sex with men. | |
| that prevention interventions targeting WSW must address behaviors that put WSW at risk for HIV infection including injection drug use and unprotected vaginal-penile intercourse. |
; Preventing drug use and providing substance abuse treatment for persons who inject illicit drugs are crucial to preventing many blood-borne infections, including human immunodeficiency virus (HIV). However, many drug users are not currently in substance abuse treatment programs because of multiple factors including the limited availability of these programs and the lack of readiness or willingness of some drug users to enter substance abuse treatment. Consequently substantial numbers of drug users continue to inject drugs.
This chapter summarizes new information on preventing transmission of HIV and other blood-borne infections among persons who inject drugs. Various studies indicate that persons who inject drugs should use sterile syringes to prevent the transmission of HIV and other blood-borne infectious diseases. This conclusion should be considered by clinicians providing healthcare to persons who use or inject drugs and by public health professionals planning and carrying out HIV prevention programs for injection drug users (IDUs). Health professionals should inform IDUs that using sterile syringes is safer than reusing syringes, including syringes that have been disinfected with bleach. The information in this chapter has been prepared for health professionals involved in programs serving persons who inject drugs.The reuse and sharing of blood-contaminated injection equipment and blood- contaminated dissolved drugs play substantial roles in the transmission of HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and other blood-borne infections. These infections cause illness and death among drug users, their sex partners, and, through mother-to-infant transmission, their children. More than one third (35%) of all AIDS cases reported in the United States in 1995 were directly or indirectly associated with injection drug use.
Blood is introduced into needles and syringes at the start of every intravenous injection. The reuse of a blood-contaminated syringe by another drug injector (sometimes called direct syringe sharing) carries a substantial risk of transmission of blood-borne infections, including HIV, HBV, and HCV. In addition, blood and blood-borne infections can be introduced into drug solutions by the use of blood-contaminated syringes to prepare drugs; the reuse of water; the reuse of bottle caps, spoons, or other containers (spoons and cookers) used to dissolve drugs in water and to heat drug solutions; and the reuse of small pieces of cotton or cigarette filters (cottons) used to filter out particles that could block the needle. Multiperson use of contaminated water, dissolved drugs, and drug preparation equipment is sometimes called indirect sharing. Because some street sellers of syringes repackage used syringes and sell them as sterile syringes persons who continue to inject drugs should obtain syringes from reliable sources of sterile syringes, such as pharmacies. In addition to HIV, HBV, HCV and other blood-borne infections, persons who inject drugs are at risk of other serious infections. Use of alcohol swabs to clean the injection site prior to injection has been shown to reduce the occurrence of cellulitis, injection site abscesses, and, possibly, endocarditis among persons who inject drugs.The risks of transmission of blood-borne illnesses are compelling reasons for strengthening public health and community efforts to help persons avoid starting drug injection and to help IDUs stop using drugs. Addiction is a major factor in the use of drugs such as heroin, cocaine, and amphetamines. While the recommendations here will help reduce the individual and public health risks associated with injection drug use, the ultimate goals are to prevent at-risk individuals from initiating injection drug use and to help drug injectors stop drug injection through substance abuse treatment and recovery from addiction. For most persons who are addicted to drugs, admission to drug and alcohol treatment programs is a key step in reducing and/or stopping their drug use.
Based on the findings from various studies, the new recommendations for drug users who continue to inject drugs include:| substance abuse treatment to reduce or eliminate drug injection; | |
| the use of sterile syringes to reduce the spread of blood-borne infections; | |
| the use of new, ideally, sterile water and equipment to prepare drugs; and | |
| adequate disinfection of the injection site by drug users to prevent local infection and endocarditis. |
To minimize the risk of disease transmission, persons who continue to inject drugs should be advised to always use sterile injection equipment; warned never to reuse needles, syringes, and other injection equipment; and told that using syringes that have been cleaned with bleach or other disinfectant is not as safe as using new, sterile syringes. The National Academy of Sciences report stated: For injection drug users who cannot or will not stop injecting drugs, the once-only use of sterile needles and syringes remains the safest, most effective approach for limiting HIV transmission. CDC recommends that all syringes used for parenteral injections be sterile. Drug preparation equipment, such as cottons, cookers, water, and syringes should not be reused because they are usually contaminated with blood.
Most syringes and needles used by drug injectors were not designed for reuse. Boiling needles and syringes for 15 minutes between uses can disinfect the equipment. However, boiling may alter the shape and functioning of the plastic syringes widely used by drug injectors in the United States. Disinfecting previously used needles and syringes with bleach (or other chemicals) can reduce the risk of HIV transmission, but using disinfected syringes is not as safe as using a new, sterile needle and syringe. The National Academy of Sciences report found that bleach disinfection is likely to be effective but . . . is clearly an intervention to be used when injection drug users have no safer alternatives.Healthcare workers involved in programs that serve drug users should communicate the following recommendations to drug users who continue to inject. Adhering to these drug preparation and injection procedures will reduce the public health and individual health risks associated with drug injection for both drug users and other persons in their communities.
Persons who inject drugs should be regularly counseled to: I. Stop using and injecting drugs. II. Enter and complete substance abuse treatment, including relapse prevention. III. Take the following steps to reduce personal and public health risks, if they continue to inject drugs: ü Never reuse or share syringes, water, or drug preparation equipment. ü Use only syringes obtained from a reliable source (e.g., pharmacies). ü Use a new, sterile syringe to prepare and inject drugs. ü If possible, use sterile water to prepare drugs; otherwise use clean water from a reliable source (such as fresh tap water). ü Use a new or disinfected container (cooker) and a new filter (cotton) to prepare drugs. ü Clean the injection site prior to injection with a new alcohol swab. ü Safely dispose of syringes after one use. The availability of new, sterile syringes varies, depending on state and local regulations regarding the sale and possession of syringes and on other factors, such as the existence of syringe exchange programs sponsored by local HIV prevention organizations. If new, sterile syringes and other drug preparation and injection equipment are not available then previously used equipment should be boiled or disinfected with bleach. In addition, drug users should be provided information on how to prevent HIV transmission through sexual contact and for women, information on reducing the risk of mother-to-infant HIV transmission.In 1983, CDC published a document entitled Guideline for Isolation Precautions in Hospitals (2) that contained a section entitled Blood and Body Fluid Precautions. The recommendations in this section called for blood and body fluid precautions when a patient was known or suspected to be infected with bloodborne pathogens. In August 1987, CDC published a document entitled Recommendations for Prevention of HIV Transmission in Healthcare Settings (1). In contrast to the 1983 document, the 1987 document recommended that blood and body fluid precautions be consistently used for all patients regardless of their bloodborne infection status. This extension of blood and body fluid precautions to all patients is referred to as Universal Blood and Body Fluid Precautions or Universal Precautions. Under universal precautions, blood and certain body fluids of all patients are considered potentially infectious for human immunodeficiency virus (HIV), hepatitis B virus (HBV), and other bloodborne pathogens.
Universal precautions are intended to prevent parenteral, mucous membrane, and nonintact skin exposures of healthcare workers to bloodborne pathogens. In addition, immunization with HBV vaccine is recommended as an important adjunct to universal precautions for healthcare workers who have exposures to blood (3,4). Body Fluids to Which Universal Precautions ApplyUniversal precautions apply to blood and to other body fluids containing visible blood. Occupational transmission of HIV and HBV to healthcare workers by blood is documented (4,5). Blood is the single most important source of HIV, HBV, and other bloodborne pathogens in the occupational setting. Infection control efforts for HIV, HBV, and other bloodborne pathogens must focus on preventing exposures to blood as well as on delivery of HBV immunization.
Universal precautions also apply to semen and vaginal secretions. Although both of these fluids have been implicated in the sexual transmission of HIV and HBV, they have not been implicated in occupational transmission from patient to healthcare worker. This observation is not unexpected, since exposure to semen in the usual healthcare setting is limited, and the routine practice of wearing gloves for performing vaginal examinations protects healthcare workers from exposure to potentially infectious vaginal secretions. Universal precautions also apply to tissues and to the following fluids: cerebrospinal fluid (CSF), synovial fluid pleural fluid, peritoneal fluid, pericardial fluid, and amniotic fluid. The risk of transmission of HIV and HBV from these fluids is unknown; epidemiologic studies in the healthcare and community setting are currently inadequate to assess the potential risk to healthcare workers from occupational exposures to them. However, HIV has been isolated from CSF, synovial, and amniotic fluid (6-8), and HBsAg has been detected in synovial fluid, amniotic fluid, and peritoneal fluid (9-11). One case of HIV transmission was reported after a percutaneous exposure to bloody pleural fluid obtained by needle aspiration (12). Whereas aseptic procedures used to obtain these fluids for diagnostic or therapeutic purposes protect healthcare workers from skin exposures, they cannot prevent penetrating injuries due to contaminated needles or other sharp instruments.Universal precautions do not apply to feces, nasal secretions, sputum, sweat, tears, urine, and vomitus unless they contain visible blood. The risk of transmission of HIV and HBV from these fluids and materials is extremely low or nonexistent. HIV has been isolated and HBsAg has been demonstrated in some of these fluids; however, epidemiologic studies in the healthcare and community setting have not implicated these fluids or materials in the transmission of HIV and HBV infections (13,14). Some of the above fluids and excretions represent a potential source for nosocomial and community-acquired infections with other pathogens, and recommendations for preventing the transmission of nonbloodborne pathogens have been published(2).
Human breast milk has been implicated in perinatal transmission of HIV, and HBsAg has been found in the milk of mothers infected with HBV (10,13). However, occupational exposure to human breast milk has not been implicated in the transmission of HIV nor HBV infection to healthcare workers. Moreover, the healthcare worker will not have the same type of intensive exposure to breast milk as the nursing neonate. Whereas universal precautions do not apply to human breast milk, gloves may be worn by healthcare workers in situations where exposures to breast milk might be frequent, for example, in breast milk banking.
Saliva of some persons infected with HBV has been shown to contain HBV-DNA at concentrations 1/1,000 to 1/10,000 of that found in the infected person's serum (15). HBsAg-positive saliva has been shown to be infectious when injected into experimental animals and in human bite exposures (16-18). However, HBsAg-positive saliva has not been shown to be infectious when applied to oral mucous membranes in experimental primate studies (18) or through contamination of musical instruments or cardiopulmonary resuscitation dummies used by HBV carriers (19,20). Epidemiologic studies of nonsexual household contacts of HIV-infected patients, including several small series in which HIV transmission failed to occur after bites or after percutaneous inoculation or contamination of cuts and open wounds with saliva from HIV-infected patients, suggest that the potential for salivary transmission of HIV is remote (5,13,14,21,22). One case report from Germany has suggested the possibility of transmission of HIV in a household setting from an infected child to a sibling through a human bite (23). The bite did not break the skin or result in bleeding. Since the date of seroconversion to HIV was not known for either child in this case, evidence for the role of saliva in the transmission of virus is unclear (23). Another case report suggested the possibility of transmission of HIV from husband to wife by contact with saliva during kissing (24). However, follow-up studies did not confirm HIV infection in the wife (21). Universal precautions do not apply to saliva. General infection control practices already in existence–including the use of gloves for digital examination of mucous membranes and endotracheal suctioning, and handwashing after exposure to saliva–should further minimize the minute risk, if any, for salivary transmission of HIV and HBV (1,25). Gloves need not be worn when feeding patients and when wiping saliva from skin. Special precautions, however, are recommended for dentistry (1). Occupationally acquired infection with HBV in dental workers has been documented (4), and two possible cases of occupationally acquired HIV infection involving dentists have been reported (5,26). During dental procedures, contamination of saliva with blood is predictable, trauma to healthcare workers' hands is common, and blood spattering may occur. Infection control precautions for dentistry minimize the potential for nonintact skin and mucous membrane contact of dental healthcare workers to blood-contaminated saliva of patients. In addition, the use of gloves for oral examinations and treatment in the dental setting may also protect the patient's oral mucous membranes from exposures to blood, which may occur from breaks in the skin of dental workers' hands.Protective barriers reduce the risk of exposure of the healthcare worker's skin or mucous membranes to potentially infective materials. For universal precautions, protective barriers reduce the risk of exposure to blood, body fluids containing visible blood, and other fluids to which universal precautions apply. Examples of protective barriers include gloves, gowns, masks, and protective eyewear. Gloves should reduce the incidence of contamination of hands, but they cannot prevent penetrating injuries due to needles or other sharp instruments. Masks and protective eyewear or face shields should reduce the incidence of contamination of mucous membranes of the mouth, nose, and eyes.
Universal precautions are intended to supplement rather than replace recommendations for routine infection control such as handwashing and using gloves to prevent gross microbial contamination of hands (27). Because specifying the types of barriers needed for every possible clinical situation is impractical, some judgment must be exercised. The risk of nosocomial transmission of HIV, HBV, and other bloodborne pathogens can be minimized if healthcare workers use the following general guidelines:Gloves should reduce the incidence of blood contamination of hands during phlebotomy (drawing blood samples), but they cannot prevent penetrating injuries caused by needles or other sharp instruments. The likelihood of hand contamination with blood containing HIV, HBV, or other bloodborne pathogens during phlebotomy depends on several factors: 1) the skill and technique of the healthcare worker, 2) the frequency with which the healthcare worker performs the procedure (other factors being equal, the cumulative risk of blood exposure is higher for a healthcare worker who performs more procedures), 3) whether the procedure occurs in a routine or emergency situation (where blood contact may be more likely), and 4) the prevalence of infection with bloodborne pathogens in the patient population.
The likelihood of infection after skin exposure to blood containing HIV or HBV will depend on the concentration of virus (viral concentration is much higher for hepatitis B than for HIV), the duration of contact, the presence of skin lesions on the hands of the healthcare worker, and–for HBV–the immune status of the healthcare worker. Although not accurately quantified, the risk of HIV infection following intact skin contact with infective blood is certainly much less than the 0.5% risk following percutaneous needlestick exposures (5). In universal precautions, all blood is assumed to be potentially infective for bloodborne pathogens, but in certain settings (e.g., volunteer blood-donation centers) the prevalence of infection with some bloodborne pathogens (e.g., HIV HBV) is known to be very low. Some institutions have relaxed recommendations for using gloves for phlebotomy procedures by skilled phlebotomists in settings where the prevalence of bloodborne pathogens is known to be very low. Institutions that judge that routine gloving for all phlebotomies is not necessary should periodically reevaluate their policy. Gloves should always be available to healthcare workers who wish to use them for phlebotomy. In addition the following general guidelines apply:The Center for Devices and Radiological Health, FDA, has responsibility for regulating the medical glove industry. Medical gloves include those marketed as sterile surgical or nonsterile examination gloves made of vinyl or latex. General purpose utility (rubber) gloves are also used in the healthcare setting, but they are not regulated by FDA since they are not promoted for medical use. There are no reported differences in barrier effectiveness between intact latex and intact vinyl used to manufacture gloves. Thus, the type of gloves selected should be appropriate for the task being performed.
The following general guidelines are recommended:Universal precautions are not intended to change waste management programs previously recommended by CDC for healthcare settings (1). Policies for defining, collecting, storing, decontaminating, and disposing of infective waste are generally determined by institutions in accordance with state and local regulations. Information regarding waste management regulations in healthcare settings may be obtained from state or local health departments or agencies responsible for waste management.
Healthcare workers are at risk for occupational exposure to bloodborne pathogens, including hepatitis B virus (HBV) hepatitis C virus (HCV), and human immunodeficiency virus (HIV). Exposures occur through needlesticks or cuts from other sharp instruments contaminated with an infected patient's blood or through contact of the eye, nose, mouth, or skin with a patient's blood. Important factors that may determine the overall risk for occupational transmission of a bloodborne pathogen include the number of infected individuals in the patient population, the chance of becoming infected after a single blood contact from an infected patient, and the type and number of blood contacts.
The risk of healthcare workers getting HIV on the job is very low, especially if they carefully follow universal precautions (i.e., using protective practices and personal protective equipment to prevent HIV and other blood-borne infections). It is important to remember that casual, everyday contact with an HIV-infected person does not expose healthcare workers or anyone else to HIV. For healthcare workers on the job, the main risk of HIV transmission is through accidental injuries from needles and other sharp instruments that may be contaminated with the virus. Even this risk is small, however. Scientists estimate that the risk of infection from a needle jab is less than 1 percent, a figure based on the findings of several studies of healthcare workers who received punctures from HIV-contaminated needles or were otherwise exposed to HIV-contaminated blood.
Most exposures do not result in infection. Following a specific exposure, the risk of infection may vary with factors such as these:| The pathogen involved | |
| The type of exposure | |
| The amount of blood involved in the exposure | |
| The amount of virus in the patient's blood at the time of exposure |
Many needlesticks and other cuts can be prevented by using safer techniques (e.g., not recapping needles by hand) disposing of used needles in appropriate sharps disposal containers, and using medical devices with safety features designed to prevent injuries. Many exposures to the eyes, nose, mouth, or skin can be prevented by using appropriate barriers (e.g., gloves, eye and face protection, gowns) when contact with blood is expected.
| Wash needlesticks and cuts with soap and water u Flush splashes to the nose, mouth, or skin with water | |
| Irrigate eyes with clean water, saline, or sterile irrigants No scientific evidence shows that using antiseptics or squeezing the wound will reduce the risk of transmission of a bloodborne pathogen. Using a caustic agent such as bleach is not recommended. |
| Report the exposure to the department (e.g., occupational health, infection control) responsible for managing exposures. Prompt reporting is essential because, in some cases, postexposure treatment may be recommended and it should be started as soon as possible. Discuss the possible risks of acquiring HBV, HCV, and HIV and the need for postexposure treatment with the provider managing your exposure. You should have already received hepatitis B vaccine, which is extremely safe and effective in preventing HBV infection. |
HBV Healthcare workers who have received hepatitis B vaccine and have developed immunity to the virus are at virtually no risk for infection. For an unvaccinated person, the risk from a single needlestick or a cut exposure to HBV-infected blood ranges from 6-30% and depends on the hepatitis B e antigen (HBeAg) status of the source individual. Individuals who are both hepatitis B surface antigen (HBsAg) positive and HBeAg positive have more virus in their blood and are more likely to transmit HBV.
HCV Based on limited studies, the risk for infection after a needlestick or cut exposure to HCV-infected blood is approximately 1.8%. The risk following a blood splash is unknown, but is believed to be very small; however, HCV infection from such an exposure has been reported. HIV u The average risk of HIV infection after a needlestick or cut exposure to HlV-infected blood is 0.3% (i.e. three-tenths of one percent, or about 1 in 300). Stated another way, 99.7% of needlestick/cut exposures do not lead to infection.The risk after exposure of the eye, nose, or mouth to HIV-infected blood is estimated to be, on average, 0.1% (1 in 1,000).
| The risk after exposure of the skin to HlV-infected blood is estimated to be less than 0.1%. A small amount of blood on intact skin probably poses no risk at all. There have been no documented cases of HIV transmission due to an exposure involving a small amount of blood on intact skin (a few drops of blood on skin for a short period of time). The risk may be higher if the skin is dam-aged (for example, by a recent cut) or if the contact involves a large area of skin or is prolonged (for example, being covered in blood for hours). |
HBV The annual number of occupational infections has decreased sharply since hepatitis B vaccine became available in 1982 (i.e., there has been a 90% decrease in the number of estimated cases from 1985 to1996). Nonetheless, approximately 800 healthcare workers become infected with HBV each year following an occupational exposure.
HCV There are no exact estimates on the number of healthcare workers occupationally infected with HCV. However, studies have shown that 1% of hospital healthcare workers have evidence of HCV infection (about 1.8% of the U.S. population has evidence of infection). The number of these workers who may have been infected through an occupational exposure is unknown. HIV As of December 1998, CDC had received reports of 54 documented cases and 134 possible cases of occupationally acquired HIV infection among healthcare workers in the United States since reporting began in 1985.
HBV As mentioned above, hepatitis B vaccine has been available since 1982 to prevent HBV infection. All healthcare workers who have a reasonable chance of exposure to blood or body fluids should receive hepatitis B vaccine. Vaccination ideally should occur during the healthcare worker's training period. Workers should be tested 1-2 months after the vaccine series to make sure that vaccination has provided immunity to HBV infection.
Hepatitis B immune globulin (HBIG) is effective in preventing HBV infection after an exposure. The decision to begin treatment is based on several factors, such as:
| Whether the source individual is positive for hepatitis B surface antigen. | |
| Whether you have been vaccinated. | |
| Whether the vaccine provided you immunity. HCV There is no vaccine against hepatitis C, and no treatment after an exposure that will prevent infection. Immune globulin is not recommended. For these reasons, following recommended infection control practices is imperative. |
HIV There is no vaccine against HIV. However, results from a small number of studies suggest that the use of zidovudine after certain occupational exposures may reduce the chance of HIV transmission. Postexposure treatment is not recommended for all occupational exposures to HIV because most exposures do not lead to HIV infection and because the drugs used to prevent infection may have serious side effects. Taking these drugs for exposures that pose a lower risk for infection may not be worth the risk of the side effects. You should discuss the risks and side effects with a healthcare provider before starting postexposure treatment for HIV.
HBV–HCV–HIV If the source individual cannot be identified or tested, decisions regarding follow-up should be based on the exposure risk and whether the source is likely to be a person who is infected with a bloodborne pathogen. Follow-up testing should be available to all workers who are concerned about possible infection through occupational exposure.
HBV If you have not been vaccinated, then hepatitis B vaccination is recommended for any exposure regardless of the source person's hepatitis B status. HBIG and/or hepatitis B vaccine may be recommended depending on your immunity to hepatitis B and the source person's infection status.
HCV Currently there is no recommended postexposure treatment that will prevent HCV infection. HIV The Public Health Service recommends a 4-week course of two drugs (zidovudine and lamivudine) for most HIV exposures or zidovudine and lamivudine plus a protease inhibitor (indinavir or nelfinavir) for exposures that may pose a greater risk for transmitting HIV (such as those involving a larger volume of blood with a larger amount of HIV or a concern about drug-resistant HIV). Differences in side effects associated with the use of these two drugs may influence which drug is selected in a specific situation.These recommendations are intended to provide guidance to clinicians and may be modified on a case-by-case basis. Determining which drugs and how many drugs to use or when to change a treatment regimen is largely a matter of judgement. Whenever possible, consulting an expert with experience in the use of antiviral drugs is advised, especially if a recommended drug is not available, if the source patient's virus is likely to be resistant to one or more recommended drugs, or if the drugs are poorly tolerated.
HBV Postexposure treatment should begin as soon as possible after exposure, preferably within 24 hours, and no later than 7 days.
HIV Treatment should be started promptly, preferably within hours as opposed to days, after the exposure. Although animal studies suggest that treatment is not effective when started more than 24-36 hours after exposure, it is not known if this time frame is the same for humans. Starting treatment after a longer period (e.g., 1-2 weeks) may be considered for the highest risk exposures; even if HIV infection is not prevented, early treatment of initial HIV infection may lessen the severity of symptoms and delay the onset of AIDS.HBV Yes. Both hepatitis B vaccine and HBIG are approved for this use.
HIV No. The FDA has approved these drugs for the treatment of existing HIV infection, but not as a treatment to prevent infection. However, physicians may prescribe any approved drug when, in their professional judgment, the use of the drug is warranted.HBV Hepatitis B vaccine is very safe. There is no information that the vaccine causes any chronic illnesses. Most illnesses reported after an HBV vaccination are often related to other causes and not the vaccine. However, you should report any unusual reaction after a hepatitis B vaccination to your healthcare provider. HIV All of the antiviral drugs for HIV have been associated with side effects. The most common side effects include upset stomach (nausea, vomiting, diarrhea), tiredness, or headache. The few serious side effects that have been reported in healthcare workers using combination postexposure treatment have included kidney stones, hepatitis, and suppressed blood cell production. Protease inhibitors (indinaivir and nefinavir) may interact with other medicines and cause serious side effects and should not be used in combination with certain other drugs, such as prescription antihistamines. It is important to tell the healthcare provider managing your exposure about any medications you are currently taking, if you need to take antiviral drugs for an HIV exposure.
HBV Yes. Women who are pregnant or breast feeding can be vaccinated against HBV infection and/or get HBIG. Pregnant women who are exposed to blood should be vaccinated against HBV infection, because infection during pregnancy can cause severe illness in the mother and a chronic infection in the newborn. The vaccine does not harm the fetus.
HIV Pregnancy should not rule out the use of postexposure treatment when it is warranted. If you are pregnant you should understand what is known and not known regarding the potential benefits and risks associated with the use of antiviral drugs in order to make an informed decision about treatment.
HBV Because postexposure treatment is highly effective in preventing HBV infection, CDC does not recommend routine follow-up after treatment. However, any symptoms suggesting hepatitis (e.g., yellow eyes or skin, loss of appetite nausea, vomiting, fever, stomach or joint pain, extreme tiredness) should be reported to your healthcare provider.
HCV You should have an antibody test for hepatitis C virus and a liver enzyme test (alanine aminotransferase activity) as soon as possible after the exposure (baseline) and at 4-6 months after the exposure. Some clinicians may also recommend another test (HCV RNA) to detect HCV infection 4-6 weeks after the exposure. Report any symptoms suggesting hepatitis (mentioned above) to your healthcare provider. HIV You should be tested for HIV antibody as soon as possible after exposure (baseline) and periodically for at least 6 months after the exposure (e.g., at 6 weeks, 12 weeks, and 6 months). If you take antiviral drugs for postexposure treatment, you should be checked for drug toxicity by having a complete blood count and kidney and liver function tests just before starting treatment and 2 weeks after starting treatment. You should report any sudden or severe flu-like illness that occurs during the follow-up period, especially if it involves fever, rash, muscle aches, tiredness, malaise, or swollen glands. Any of these may suggest HIV infection, drug reaction, or other medical conditions. You should contact the healthcare provider managing your exposure if you have any questions or problems during the follow-up period.
HBV If you are exposed to HBV and receive postexposure treatment, it is unlikely that you will become infected and pass the infection on to others. No precautions are recommended.
HCV Because the risk of becoming infected and passing the infection on to others after an exposure to HCV is low, no precautions are recommended. HIV During the follow-up period, especially the first 6-12 weeks when most infected persons are expected to show signs of infection, you should follow recommendations for preventing transmission of HIV. These include not donating blood semen, or organs and not having sexual intercourse. If you choose to have sexual intercourse, using a condom consistently and correctly may reduce the risk of HIV transmission. In addition, women should consider not breast-feeding infants during the follow-up period to prevent exposing their infants to HIV in breast milk.Although HIV transmission is possible in health care settings, it is extremely rare. Medical experts emphasize that the careful practice of infection control procedures, including universal precautions, protects patients as well as health care providers from possible HIV infection in medical and dental offices.
In 1990, the CDC reported on an HIV-infected dentist in Florida who apparently infected some of his patients while doing dental work. Studies of viral DNA sequences linked the dentist to six of his patients who were also HIV-infected. The CDC has as yet been unable to establish how the transmission took place. Further studies of more than 22,000 patients of 63 health care providers who were HIV-infected have found no further evidence of transmission from provider to patient in health care settings.
Past experiences in planning, implementing, and evaluating efforts to stem the U.S. epidemic have clearly shown that preventing HIV infection depends on two equally important factors– studying and implementing biomedical interventions to thwart the virus, and influencing millions of individuals in diverse populations to adopt or maintain safe behaviors. Comprehensive, sustained prevention activities offer the best hope for slowing the epidemic's spread.
In the United States, as elsewhere, the AIDS epidemic is composed of diverse multiple sub-epidemics that vary by region and community. The foundation of all HIV prevention programs is that those closest to the problem, equipped with needed information and tools, are best able to solve it.
Essential elements of comprehensive programs
Comprehensive programs should be based on several key principles and include a number of essential activities. These are highlighted below.
| A community planning process to ensure efforts are directed to communities at greatest risk | |
| Epidemiologic and behavioral surveillance to effectively guide prevention efforts | |
| Voluntary HIV counseling, testing, referral, and partner counseling to provide a pathway to needed prevention and treatment services | |
| Health education and risk-reduction activities, including individual-, group-, and community-level programs to provide the skills and support necessary for reducing risks | |
| Accessible diagnosis and treatment of other sexually transmitted diseases to decrease risk of HIV transmission | |
| School-based prevention efforts for youth to provide young people the skills and support they need to keep from initiating risky behaviors and to adopt healthy ones | |
| Public information programs to ensure that knowledge and awareness of how to prevent HIV remain high | |
| Training and quality assurance to provide those implementing programs needed skills | |
| Laboratory support to keep pace with diagnostic and testing services and related research efforts | |
| HIV prevention capacity-building activities to support organizations in expanding their abilities to implement effective programs | |
| An HIV prevention technical assistance assessment and plan to ensure that programs keep pace with prevention technologies | |
| Evaluation of major program activities, interventions, and servicesto ensure efforts are effective |
Voluntary HIV testing is an important part of comprehensive HIV prevention programs. However, testing alone will not result in behavior change, nor will it prevent transmission - sexual, drug-related, or perinatal. To have a chance of preventing HIV transmission and ensuring that necessary services and care are provided to infected individuals, the focus of HIV testing must be on counseling. Voluntary, rather than mandatory, HIV testing is recommended because it fosters the development of trust between patients and healthcare providers. A trusting atmosphere facilitates better understanding of what the test means for patients, their partners, and their families and helps ensure that they are linked to needed services and care.
Anonymous HIV testing should be available to increase options for individuals seeking to learn their HIV status. In this age of effective treatment, it is increasingly important for people to know their HIV status. Recent studies show that eliminating the availability of anonymous HIV testing services has a deterrent effect on some people's willingness to come forward for testing. People with legitimate concerns about discrimination or people who are unfamiliar with or distrust the public health system are able to gain access to the system through anonymous testing and subsequently receive referrals for needed treatment, care, or prevention services. Partner counseling also can be provided following anonymous testing, if requested.
Partner counseling. CDC considers voluntary, confidential notification of potentially exposed partners to be an essential component of a comprehensive HIV prevention program. Partner counseling is a primary prevention service with the following objectives:
| To provide prevention information to people who are at very high risk of becoming HIV infected, but who may be unaware of or misunderstand their risks | |
| To assist these individuals in obtaining HIV prevention counseling and voluntary testing, and referral | |
| To provide access to partners who are already infected to prevention and treatment services that can improve their health and quality of life |
Partner counseling services can be provided in both anonymous and confidential testing sites. For areas lacking the resources to perform partner counseling services themselves, CDC recommends they provide the necessary training to conduct these services for physicians or hospitals that provide HIV counseling and testing.
Reaching HIV-infected individuals and linking them with care and treatment services is a priority
The availability of effective drug therapies makes it more important than ever for HIV-infected persons to know their serostatus. Early recognition of their infection allows patients to consider treatment options that will keep them healthy longer and protect the quality of their lives. It also allows them to take steps to prevent transmitting the virus to others.
Comprehensive efforts are needed for reducing sexual risk behaviors
In all prevention activities, CDC supports the incorporation of prevention messages and programs that strongly emphasize that young people should postpone sexual activity, and that sexually active adults should maintain a monogamous relationship with an uninfected partner. However, some young people and adults will still engage in sexual intercourse that puts them at risk for HIV and other sexually transmitted diseases (STDs). To ensure these individuals acquire the knowledge and skills necessary to protect themselves from HIV, CDC also supports prevention messages and programs that encourage consistent and correct use of latex condoms among those who are sexually active. Research has conclusively shown that latex condoms are highly effective barriers to HIV and many other sexually transmitted diseases when used consistently and correctly.
Condom availability as a prevention strategy.
Individuals in some populations, especially sexually active young people, may experience problems accessing condoms because of several factors, including cost, convenience, and embarrassment. For these individuals, the fact that condoms are not readily accessible may be a significant barrier to consistent use. To eliminate this barrier, many local communities actively support programs that make condoms available to populations most vulnerable to HIV infection, including sexually active young people. Research shows that providing access to condoms can increase their use among some sexually active young people. And, despite some fears to the contrary, research clearly demonstrates that young people who participate in comprehensive HIV prevention programs that include access to condoms are no more likely to initiate or increase sexual activity than other young people. No single approach by itself–prevention counseling and health education, abstinence promotion, condom education condom availability, STD treatment–can eliminate HIV. Each affected community can best determine which combination of approaches will be most appropriate and effective for preventing HIV infection under local circumstances.Comprehensive efforts are needed for reducing drug-related behaviors
Numerous studies have documented that drug users are at risk for HIV through both drug-related and sexual behaviors which places their partners at risk as well. Comprehensive programs must provide the information, skills, and support necessary to reduce both risks. Researchers have found that many interventions aimed at reducing sexual risk behaviors among drug users have significantly increased the practice of safer sex (e.g., using condoms, avoiding unprotected sex) among participants. p>In the United States, drug use and dependence are widespread in the general population. Experts generally agree that there are about 1 million active IDUs in this country, as well as many others who use noninjection drugs or abuse alcohol. Clearly, the need for substance abuse treatment vastly exceeds our capacity to provide it. Effective substance abuse treatment that helps people stop using drugs not only eliminates the risk of HIV transmission from sharing contaminated syringes, but, for many, reduces the risk of engaging in risky behaviors that might result in sexual transmission.Substance abuse prevention and treatment programs are the key to slowing the spread of HIV in this population and efforts in this area must be increased and strengthened nationwide. To further minimize the risk of HIV transmission, IDUs who continue to inject must have access to interventions that can help them protect their health. They must be advised to always use sterile injection equipment; warned never to reuse needles, syringes, and other injection equipment; and told that using syringes that have been cleaned with bleach or other disinfectant is not as safe as using new, sterile syringes. Drug users not only need access to this information, but also the skills and support necessary for them to adopt and maintain safer behaviors.
Increasing syringe availability as a prevention strategy. To reduce the risk of HIV transmission through needle sharing, prevention strategies for IDUs who continue to inject drugs have included various approaches to increasing the availability of sterile syringes. In some communities, drug paraphernalia laws have been modified to exclude syringes syringe prescription laws have been repealed, and pharmacy regulations and practice guidelines restricting the sale of sterile syringes have been changed. In other communities, needle exchange programs have been established and are contributing to reductions in HIV transmission among drug users without encouraging the use of illegal drugs. Needle exchange programs should also provide drug users with risk-reduction education and referrals to drug counseling and treatment and other medical services.
For injection drug users who cannot or will not stop injecting drugs, using sterile needles and syringes only once remains the safest, most effective approach for limiting HIV transmission.
Having access to sterile injection equipment is important, but it is not enough. Better integration of all prevention and treatment services, including for STDs and substance abuse, is critically needed.
Preventing the spread of HIV through injection drug use requires a comprehensive approach that incorporates several basic principles:
| ensure coordination and collaboration among all providers of services to IDUs, their sex partners, and their children, | |
| ensure coverage, access to, and quality of interventions, | |
| recognize and overcome stigma associated with injection drug use, and | |
| tailor services and programs to the diverse populations and characteristics of IDUs. |
Strategies for prevention should include:
| preventing initiation of drug injection, | |
| using community outreach programs to reach drug users on the streets | |
| improving access to high quality substance abuse treatment programs, | |
| instituting HIV prevention programs in jails and prisons, | |
| providing healthcare for HIV-infected IDUs, and | |
| making HIV risk-reduction counseling and testing available for IDUs and their sex partners. |
During the early 1990s, before perinatal preventive treatments were available, an estimated 1,000-2,000 infants were born with HIV infection each year in the United States. Today, the United States has seen dramatic reductions in mother-to-child, or perinatal, HIV transmission rates. These declines reflect the widespread success of Public Health Service (PHS) recommendations made in 1994 and 1995 for routinely counseling and voluntarily testing pregnant women for HIV, and for offering zidovudine (AZT) to infected women during pregnancy and delivery, and for the infant after birth.
Perinatal prevention saves lives and dollars
On a national level, HIV/AIDS surveillance and other studies continue to demonstrate that perinatal HIV prevention is making a difference, both in terms of lives and resources saved:
| Between 1992 and 1997, perinatally acquired AIDS cases declined 66% in the United States. | |
| A study conducted in four states (Michigan, New Jersey, Louisiana, and South Carolina) found that the proportion of pregnant women voluntarily tested for HIV increased from 68% in 1993 to 79% in 1996. The percentage of women offered AZT increased from 27% in 1993 to 85% in 1996. However, the study also found that 15% of HIV-infected pregnant women in these states did not receive prenatal care and therefore could not be offered this intervention. | |
| Among women in CDC's Perinatal AIDS Collaborative Transmission Study (PACTS), AZT use increased following the publication of PHS guidelines, and the rate of perinatal transmission dropped from 21% to 11%. The PACTS study includes women from four cities -New York City, Newark, Atlanta, and Baltimore. | |
| Prenatal care that includes HIV counseling and testing and AZT treatment for infected mothers and their children saves lives and resources. Without intervention, a 25% mother-to-infant transmission rate would result in the birth of an estimated 1,750 HIV-infected infants annually in the United States, with lifetime medical costs of $282 million. | |
| The estimated annual cost of perinatal prevention in the United States is $67.6 million. This investment prevents 656 HIV infections and saves $105.6 million in medical care costs alone a net cost-savings of $38.1 million annually. |
HIV transmission from mother to child during pregnancy, labor, and delivery or by breast-feeding has accounted for 91% of all AIDS cases reported among U.S. children. The best ways to prevent infection in children are to prevent infection in women and to encourage early prenatal care that includes HIV counseling and testing.
Women of color and their children have always been disproportionately affected by the HIV epidemic. In 1998, of the 10,998 total AIDS cases reported among U.S. women, 8,830 (80%) were among African American and Hispanic women. Of the 382 children reported with AIDS in 1998, 321 (84%) were African American and Hispanic (see chart below). We must continue to improve HIV prevention efforts for women of color and ensure that interventions provide the information, skills, and support needed to reduce their HIV-related risks.
| Perinatal HIV prevention activities must help ensure that all HIV-infected women are reached early in pregnancy with prenatal care and with the opportunity to learn their HIV status. If infected, they should be offered preventive therapy to improve the chances that their children will be born free of infection and to ensure quality HIV care and treatment for mothers and their babies. Achieving this goal will required increased access to and use of prenatal care. | |
| Women who use drugs during pregnancy are the least likely to get prenatal care. Increased efforts are needed at all levels (community, state, national) to integrate substance abuse and HIV prevention activities and assist pregnant women in accessing needed services to improve their own health and the health of their babies. |
Comprehensive programs for youth are essential
School-based programs.It is estimated that half of new HIV infections in the United States–about 40,000 annually–are among people younger than 25. Prevention activities and interventions that begin well before young adulthood are desperately needed to stem this tide. To that end, CDC supports comprehensive school-based HIV prevention programs. CDC does not endorse any specific curriculum, but recommends that the scope and content of school health programs be locally determined and consistent with parental and community values. At the same time, CDC has identified curricula that have credible evidence of reducing health risk behaviors among youth and provides resources to ensure that the interventions, including training, are available for those who want to use them.
Programs for out-of-school youth. Many youth at very high HIV risk, such as homeless or runaway youth juvenile offenders, or school drop-outs, can only be reached through intensive community-based programs. Integrating HIV prevention programs with ongoing community efforts to provide shelter, medical care, or other services to out-of-school youth is essential.
Improved prevention programs in correctional facilities are needed
Correctional facilities are critical settings for monitoring the cutting edge of the HIV/AIDS epidemic and for addressing the problem of elevated rates of TB, STDs, hepatitis, and other infectious diseases. Inmate populations probably represent the largest concentration of persons infected with, or at high risk for, HIV due to drug use and unsafe sexual behaviors. Furthermore, short stays and high recidivism rates result in a substantial number of high-risk individuals who circulate between correctional facilities and their communities - most often, communities that are already beset by poverty, drug use, violence, and disease. It is imperative that correctional facilities offer effective programs for disease screening, treatment, and prevention.Correctional facilities offer significant opportunities for intervention. Their controlled settings allow efficient access to inmates for the delivery of prevention programs. However, the opportunity to provide comprehensive education and prevention programs in prison, jails, and juvenile facilities, has been missed to a great extent. CDC is working to improve community health through improved access to HIV, STD, and TB healthcare and prevention services within correctional settings and transitional programs in communities.
Continued efforts are needed for reducing occupational risks of healthcare workers
Physicians and many other health-related workers, such as nurses, physicians' assistants, phlebotomists, emergency first responders, and funeral services providers, are involved with procedures that might expose them to HIV. Some procedures are higher risk than others, but there is a theoretical risk of transmission whenever there is the potential for infected blood to come into contact with another person's blood or mucous membranes.
The use of universal precautions. Current recommendations for protecting healthcare workers and their patients from these exposures use universal precautions, as recommended by CDC's Hospital Infection Control Practices Advisory Committee. Under universal precautions, blood and body fluids (except sweat) of all patients are considered to pose a potential infectious hazard, and appropriate precautions are recommended for all healthcare personnel (not just physicians) to prevent contact with these fluids.
Safer medical devices. In addition, safer medical devices have been developed that will further reduce the risk of exposure to HIV and other infectious agents. Needle-less IV systems and safety needles are helping to further reduce transmission risks, and safer disposal containers are now available to prevent needle sticks or cuts when disposing of used medical devices.
Postexposure therapy. While the best protection clearly is to prevent HIV exposure, studies have found that administering antiretroviral therapy immediately following an exposure may reduce the risk of the worker developing HIV infection. CDC recently issued guidelines for the management of healthcare worker exposures to HIV and recommendations for postexposure therapy (PET). These guidelines outline a number of considerations in determining whether a healthcare worker should undergo PET and, if so, which regimen.
Confidentiality is one of the foundations of CDC programs, and it must be a requirement under any program enacted. Breaking trust with individuals and communities is highly detrimental to HIV prevention programs and can lead to illegal discrimination. It is essential that confidentiality protections be included as a provision of any HIV-related prevention activity.
Sound public health practice should be carefully considered in all policy and program discussions about HIV prevention and treatment programs. Decisions about implementing new or revised policies on either the state or national level must consider the public health implications of proposed policies. Program designers and policy makers must ensure that the approaches undertaken will be the most effective in reducing HIV transmission and saving lives.
Today's new medical treatments, while showing great success in keeping many HIV-infected people healthy longer, are still not working for everyone, often have serious side effects, are extremely expensive, and may be lulling people into a false sense of complacency about the need to keep themselves safe. Newer and even better treatments for HIV disease are anticipated and welcomed. In the meantime, we already know that prevention works. Preventing HIV infection must remain a high global priority, today and well into the next millennium.Source: Centers for Disease Control & Prevention National Center for HIV, STD, and TB Prevention
Since 1981, 688,200 cases of AIDS have been reported to the Centers for Disease Control and Prevention (CDC). It is estimated that 650,000 to 900,000 Americans are now living with HIV and that about 40,000 new infections occur every year. The figures on hepatitis are equally impressive: Between 1 and 1¼ million Americans have active hepatitis B; 130,000 to 320,000 new infections occur every year. Nearly 3 million Americans have active hepatitis C. Injection drug users (IDUs) are an important force in the continuing epidemics of these devastating diseases. IDUs become infected and transmit the viruses to others in two, often interconnected, ways:
| high-risk drug use–sharing blood-contaminated syringes and injection paraphernalia such as water, cookers, and cottons | |
| high-risk sex–unprotected sex, sex with many partners, failure to treat STDs |
Many health departments, community-based organizations, agencies, and providers are working hard to reach and work with IDUs to help them change their behaviors and reduce or eliminate their risk of acquiring or transmitting infection.
The problem of injection drug use and transmission of blood-borne disease persists, however. Solutions are hampered by society's pervasive negative attitudes toward IDUs, a lack of understanding of drug addiction as a treatable biomedical and psychological disease, limited funding for prevention and treatment, restrictive laws and regulations, and polarized philosophical viewpoints among various organizations and providers.If organizations and providers, public health staff, and prevention planners are to succeed in effectively reducing the transmission of HIV and other blood-borne infections, they must consider a comprehensive approach to working with IDUs. Such a comprehensive approach, now being advocated by the Centers for Disease Control and Prevention (CDC), incorporates a range of pragmatic strategies that take into account IDUs' various life circumstances, cultures and languages, behaviors, and readiness to change. It also incorporates several basic principles that serve as a framework for action.
Ensure coordination and collaboration. No single provider or institution can or does deliver all required services to IDUs, their sex partners, and their children. Coordination and collaboration are essential. Providers must work together, sharing their various expertises and outlooks, recognizing and overcoming their philosophical differences, building on existing relationships, and reaching out to groups with whom they may not have worked before.
Ensure coverage, access, and quality interventions will not be effective if they do not reach a critical mass of people, if IDUs cannot or will not use them, or if they are of poor quality. If they hope to truly reach and work with IDUs, agencies and providers must consider ways to effectively deal with these issues as they plan, deliver, and monitor programs and services.
Recognize and overcome stigma. Injection drug use is regarded with disapproval and fear, and a user's addiction is considered to be a moral failing. To successfully engage IDUs in prevention efforts and to advance public policy, these negative attitudes and misconceptions must be addressed. Addiction is now understood to be a treatable brain disease. This concept should be more widely known and accepted.
Tailor services and programs. IDUs are diverse populations with different languages, cultures, sexual preferences, life circumstances, behaviors, and requirements for services. Many, though not all, are poor and live high-risk lives on the margins of society. In planning and delivering interventions, programs and providers must take into account the factors that characterize IDUs–who they are, where they are, what they do, what motivates them, and with whom they socialize. Tailoring services and programs and involving IDUs in their planning, implementation, and monitoring will make them more effective.
| most drug users cannot stop using without it | |
| treatment prevents transmission because it helps users reduce drug- and sex-related risk behaviors | |
| it has major positive effects on a user's life | |
| treatment is cost effective | |
| providers can reach IDUs with other messages and interventions during treatment | |
| society benefits from reduced drug use and associated crime |
| it reaches IDUs who don't participate in conventional service systems | |
| it provides services in settings that are familiar to IDUs | |
| outreach interventions help create a culture of risk reduction in the community, which helps to reinforce prevention messages | |
| peers, who are often used in community out-reach, are likely to be trusted by IDUs | |
| it's relatively low cost |
| the U.S. Public Health Service and other agencies and institutions recommend consistent, one-time only use of sterile syringes obtained from a reliable source as a central risk reduction strategy for IDUs who cannot or will not stop injecting | |
| the use of a sterile syringe every time helps ensure that IDUs who continue to inject will not acquire or transmit infection | |
| existing laws, regulations, and public and pharmacists' attitudes hamper IDUs' ability to btain and safely dispose of syringes and therefore promote multiperson use of syringes access to sterile syringes does not increase drug use or attract new people to drug use | |
| ensuring access to sterile syringes involves working with pharmacists; addressing existing syringe laws and regulations; and syringe exchange programs |
| many IDUs are in jail or prison because of their drug use | |
| inmates have disproportionately high rates of HIV infection, STDs, and hepatitis | |
| high-risk sex and drug-use behavior occurs in jails and prisons | |
| interventions benefit inmates and the communities to which almost all will return |
| IDUs are an important source of sexual transmission of HIV and hepatitis B | |
| high-risk drug use and sex behaviors are often linked |
| they allow IDUs to find out whether they are infected with HIV | |
| they allow infected IDUs access to counseling and medical care and other services | |
| they help infected IDUs inform sex and drug-use partners | |
| they help public health officials follow the chains of transmission and reach those at high risk | |
| they help uninfected but high-risk IDUs reduce their risk behaviors |
| they can help infected IDUs reduce high-risk drug and sex behaviors | |
| IDUs should have access to comprehensive and quality health care HIV disease management is complex and long-term, requiring close monitoring infected | |
| IDUs who receive substance abuse treatment and other health services are more likely to comply with medication regimens |
| preventing first use of alcohol, marijuana, inhalants, and other drugs among youth can | |
| reduce the risk that they will go on to use injection drugs | |
| preventing injection drug use eliminates injection-related blood-borne virus transmission | |
| preventing alcohol and drug use and associated crime and injuries benefits society |
In the United States, complacency about the need for HIV prevention may be among the strongest barriers communities face as they plan to meet the next century's prevention needs. The great success that many people, but not all, have had with new highly active antiretroviral therapies (HAART, also known as drug cocktails) and the resulting decline in the number of newly reported AIDS cases and deaths are indeed good news. The underlying reality, however, is that the HIV epidemic in our country is far from over. This is true not only for the nation, but for the continuing number of HIV-infected individuals who now must face years - perhaps a lifetime - of multiple daily medications possible unpleasant or severe side effects, and great expense associated with the medicines needed to suppress HIV and prevent opportunistic infections.
The success of HAART is good news for the people living longer, better lives because of it, but the availability of treatment may lull people into believing that preventing HIV infection is no longer important. This complacency about the need for prevention adds a new dimension of complexity for both program planners and individuals at risk.| While the number of AIDS cases is declining, the number of people living with HIV infection is growing. This increased prevalence of HIV in the population means that even more prevention efforts are needed, not fewer. For individuals at risk, increased prevalence means that each risk behavior carries an increased risk for infection. This makes the danger of relaxing preventive behaviors greater than ever. | |
| Past prevention efforts have resulted in behavior change for many individuals and have helped slow the epidemic overall. However, many studies find that high-risk behaviors, especially unprotected sex, are continuing at far too high a rate. This is true even for some people who have been counseled and tested for HIV, including those found to be infected. | |
| The long-term effectiveness of HAART is unknown. Further, HIV may develop resistance to these drugs. The powerful treatments are complicated and involve taking large numbers of pills. Even the most motivated patients may forget to take all their medications or skip doses. Some patients have been known to take drug holidays, completely stopping their medications for a number of days or weeks. These drug treatments are less effective when treatment schedules are not followed. Diversions from the prescribed treatment regimen increase the possibility of drug resistance developing, which would greatly narrow future treatment options for those infected with a drug-resistant strain of HIV. And, if the development of drug-resistance is coupled with a relaxation in preventive behaviors resistant strains could be transmitted to others and spread widely. | |
| Research among gay and bisexual men suggests that some individuals are less concerned about becoming infected than in the past and may be inclined to take more risks. This may be equally true in other groups at risk who might believe they no longer need to use condoms because protease inhibitors are so effective in treating HIV disease. The truth is, despite medical advances, HIV remains a serious and usually fatal disease that requires complex, costly, and difficult treatment regimens. These treatments don't work for everyone. Sometimes when they do work, they have unpleasant or intolerable side effects. Some people can't take them because the interaction with their other drugs causes serious problems. Still others find it extremely difficult to maintain the drug treatment schedules. As we continue working to develop better treatment options, we must not lose sight of the fact that preventing HIV infectionin the first place precludes the need for people to follow these difficult regimens. |
The challenge of monitoring the HIV/AIDS epidemic
The treatment effect on trends in the AIDS epidemic not only increases our need for combating complacency, but means that we have never been closer to losing our ability to monitor the epidemic.
| Until recently, AIDS cases provided a reliable picture of trends in the HIV epidemic. Before highly effective treatments were available, researchers could take into account the time between HIV infection and progression to AIDS and estimate where and how many new infections were occurring based on observed cases of disease. Today, trends in AIDS cases and deaths may provide a valuable measure of groups for whom highly effective treatment is not available or has not succeeded. However, they no longer tell us enough about where and how many new infections are occurring - information critical for addressing the increasing need for prevention and treatment services. To allow the U.S. to target programs and resources most effectively, we must be able to keep pace with where the epidemic is going. This means we need to improve our ability to track early HIV infections, before they progress to AIDS. |
Sustained, comprehensive prevention efforts begun in the 1980s have had a substantial impact on slowing the HIV/AIDS epidemic in our country. While it is difficult to measure prevention–or how many thousands of infections did not occur as a result of efforts to date– we know the epidemic was growing at rate of over 80% each year in the mid-1980s and has now stabilized. While the occurrence of approximately 40,000 new infections annually is deeply troubling, we have made tremendous progress. We also have more scientific evidence than ever before on which prevention programs are most effective. There is no question that prevention works and remains the best and most cost-effective approach for bringing the HIV/AIDS epidemic under control and saving lives.
| Many studies indicate that prevention programs can contribute to changes in personal behavior that reduce risks of infection, and these changes are sustained over time. A 1997 scientific consensus conference sponsored by the National Institutes of Health that reviewed existing data on the effectiveness of HIV behavioral interventions concluded that behavioral interventions to reduce risk for HIV/AIDS are effective and should be disseminated widely. | |
| Comprehensive school-based HIV and sex education programs have been shown to delay the initiation of sexual intercourse, reduce the frequency of intercourse, reduce the number of sex partners, or increase the use of condoms or other contraceptives. | |
| Efforts to reduce risks of injection drug users through policy changes also have been evaluated and found to be very effective. For example, both New York and Connecticut reported significant reductions in the sharing of drug injection equipment after implementation of programs and policies that increased access to sterile injection equipment. | |
| Perinatal prevention programs that identify and treat pregnant women who are HIV infected have shown dramatic success in reducing HIV transmission to their babies. | |
| Screening the blood supply for HIV and heat-treating blood products for the treatment of hemophilia have nearly eliminated HIV transmission through these early transmission routes. | |
| Postexposure prophylaxis for healthcare workers has shown some success in reducing HIV transmission rates among those with occupational exposure to HIV-infected blood. | |
| Numerous HIV prevention programs have been shown to be cost-effective when compared against the resources required to treat and deliver HIV medical care to a person over the remaining years of their life. With the rising costs of lifetime treatment of HIV, effective prevention has become even more cost effective. New CDC estimates find that if only 1,255 infections are prevented each year, CDC's federally funded HIV prevention efforts in the United States are cost effective. If only 3,995 infections are prevented, our nation's investment in HIV prevention has actually saved money. |
| People with HIV risk behaviors need an array of prevention messages, skills, and support to help them reduce sexual and drug-related risks. Drug injectors, for example, not only need strategies to help them stop using drugs or sharing needles, but also need to learn ways to protect themselves from sexual transmission if their partner has ever injected drugs and may have shared needles. | |
| Substance use is a major problem in this country, and the intersection of substance use and sexual HIV transmission cannot be overlooked. Ideally, everyone who abuses any drug (including alcohol) should be offered counseling and treatment to help them stop using drugs and prevent HIV infection. HIV prevention interventions for the vast majority of substance users who are not in treatment also must address the sexual risks that are common among people who use drugs, including crack cocaine, marijuana, and alcohol. | |
| Each and every generation of young people needs comprehensive, sustained health information and interventions that help them develop life-long skills for avoiding behaviors that could lead to HIV infection. Such comprehensive programs should include the involvement of parents as well as educators. The most effective programs start at an early age and are designed to encourage the adoption of healthy behaviors, such as exercising and eating a healthy diet, and to prevent the initiation of unhealthy ones, such as drug use, excessive alcohol consumption, smoking, and premature sexual activity, before they start. | |
| Scientific studies show that treatment of other sexually transmitted diseases can greatly reduce the risk of transmitting and acquiring HIV. |
| Primary HIVprevention means keeping people from becoming infected with HIV in the first place. Interventions must focus not only on uninfected populations - there also is a major role for preventing further infections by focusing on infected individuals and helping them develop skills for reducing the risk of infecting others. | |
| Secondary HIV prevention means keeping people who already are HIV-infected safe and healthy by helping them avoid opportunistic infections and stopping the infection from progressing to AIDS. | |
| In all prevention efforts, there is a growing need to address the link between HIV treatment and prevention. In some cases, such as preventing perinatal transmission to infants by providing antiretroviral drugs to the mother treatment is prevention. We also know that the treatment of other STDs can greatly reduce a person's risk for sexually acquired HIV infection. And, scientists even now are exploring the possibility that combination drug therapies may reduce infectivity. With the lines between prevention and treatment beginning to fade, ongoing services for people who are HIV positive must balance medical advances with the behavioral and social support needed to preserve their quality of life and prevent the spread of infection. | |
| We must maintain a focus on behavioral strategies. Even a vaccine doesn't stop a disease unless people use it – and in the case of HIV, a vaccine is unlikely to confer 100% lifelong immunity. Because no medical advance can succeed on its own, people must adapt their behaviors to work in tandem with it. To do this, they need several things: | |
| Access to prevention services and new medical treatments. For example, pregnant women who may not know they are infected with HIV cannot reduce the risk of transmission to their children unless they first get prenatal care that includes routine HIV counseling and voluntary testing. Those found to be infected then must have access to antiretroviral drugs. | |
| Assistance in developing skills to use new medical treatments. HAART, for example, involves complex treatment regimens and may require the development of compliance-related skills. For example, people may need to learn how to deal with side effects, what drug interactions might occur, how to lessen the risk of developing drug resistance or how to cope with complicated schedules. | |
| Support and encouragement from family, friends, care providers, and the community at large will help people make and sustain behavioral changes in their lives. |
Today, more than ever, we must recognize that medical advances do not negate the need for preventing disease-in fact the availability of newer and better treatments often increases the need for prevention. How well we continue our work to develop integrated approaches to prevention and treatment may well define the future course of the HIV pandemic.
The most effective methods for HIV prevention remain those that prevent exposure to HIV in the first place. Attempting to prevent HIV infection by taking antiretroviral therapy should never take the place of adopting and maintaining behaviors that prevent HIV exposure. These include sexual abstinence, having sex only with an uninfected partner, consistent and correct condom use, abstinence from injection drug use, and consistent use of clean equipment for those who are unable to cease injection drug use. In recent years, the Public Health Service (PHS) has recommended the use of antiretroviral drugs to reduce (1) HIV acquisition among those exposed in the workplace (e.g., accidental needle-sticks received by healthcare workers) and (2) perinatal HIV transmission. Questions have arisen about whether antiretroviral drugs should be offered to people with unanticipated sexual or drug injection-related exposures to HIV. However, no data currently exist about the effectiveness of such therapy for these types of exposures.
The following questions and answers provide insights, knowledge, and uncertainties about providing antiretroviral therapy to people with nonoccupational exposures to HIV.This refers to using antiretroviral drugs in an effort to reduce the chances of HIV infection after a person has been exposed to the virus. Clinicians also refer to this preventive therapy as post-exposure prophylaxis (PEP).
This refers to any exposure to HIV that occurs outside the performance of a health care-related job or occupation. It typically refers to exposure through unprotected sex or sharing needles with HIV-infected persons, or to infants' exposure from infected breast milk. While occupational exposures are primarily accidental, one-time events nonoccupational exposures may occur repeatedly.
In 1994, using surveillance data from healthcare workers in France, the United Kingdom, Italy, and the United States a case-control study documented that although failures occurred, the use of zidovudine (ZDV) for postexposure prophylaxis was associated with an approximately 81% decrease in the risk for HIV infection after percutaneous exposure to HIV-infected blood (Cardo 1997). The investigators have pointed out the study's limitations, including the facts that cases and controls were not matched and came from two separate populations; cases were often reported anecdotally; and details of the exposures were obtained retrospectively, whereas the information on controls was gathered prospectively. These and other factors may have introduced bias, possibly overestimating the magnitude of the protective effect. In addition, the validity of extrapolating those results to nonoccupational exposures was questioned.
In a clinical trial of ZDV administered to HIV-infected women during pregnancy and labor and to their infants for 6 weeks after birth, there was a 67% reduction in perinatal transmission. Since the reduction in maternal viral load did not correlate completely with protective efficacy, researchers hypothesized that other factors, including postnatal therapy for the infant, must have contributed to protection (Sperling 1996). Experts also considered the importance of early events in HIV infection and replication. These discussions suggested there is a period following exposure to HIV and prior to established infection during which HIV may be vulnerable to suppression with antiretroviral drugs so that infection does not occur. There are no human studies of antiretroviral drug therapy for sexual, drug use, or other non-occupational exposures to HIV. A variety of animal studies suggest potential benefits of the use of antiretroviral therapy after exposure to HIV. However, limitations of the animal data include lack of information regarding mucosal exposures, differences in drugs used, differences in drug metabolism between humans and animals, and the use of simian virus, which differs from HIV, in some experiments.Potential benefits have to be weighed against the significant health risks and costs associated with this therapy for nonoccupational exposures. First, these medications can have severe side effects (e.g., liver inflammation, kidney stones). Second, efficacy is unknown but is likely to be imperfect. If a person becomes infected despite taking antiretroviral medication, there is a theoretical risk that the viral strain will become resistant to the medications. And third, a 4-week course of these medications will cost $600-$1,000, which in many cases will not be covered by insurance.
This therapy should never be routine. It is a complicated medical therapy that should never be considered a desirable form of primary HIV prevention. This therapy is not a morning-after pill.Many issues are relevant concerning the possible appropriate use of such therapy, including the following: the likelihood that the source is infected with HIV the likelihood of transmission, including the many cofactors that might increase or decrease transmission risk characteristics (e.g., viral load, stage of infection) of an HIV-positive source isolated versus recurrent HIV exposures time delay between possible exposure and presentation for medical care the ability to adhere to the antiretroviral regimen
There is broad-based concern that people may initiate or resume risky behaviors because of the perception that using antiretroviral drugs for HIV exposures is a cure for HIV infection. Widespread use of antiretroviral drugs for HIV exposures, if accompanied by increased levels of risky behavior, may increase the number of new HIV infections if more people are exposed to HIV because of a misguided reliance on antiretroviral drugs as their primary protection strategy. On the other hand, many studies have shown that behavioral interventions are effective in preventing HIV exposure and infection.
Yes. Antiretroviral drugs currently are recommended for occupational exposure to HIV.
Zidovudine is recommended for women during pregnancy, labor, and delivery, and for infants for a few weeks after exposure to HIV at birth. However, it is not known whether treating infants alone will reduce the risk of infection in infants born to HIV-infected mothers. There are no existing recommendations for the use of antiretroviral drugs after nonoccupational exposure to HIV.In addition to the above issues, consultants considered what factors in specific scenarios would most influence their decision to use antiretroviral drugs for potential nonoccupational exposures to HIV. These include how often and to whom potential exposures occur, the potential risks of using antiretroviral drugs after nonoccupational HIV exposures, and rates of adherence to and side effects from these medications following occupational exposures. Cost-benefit analysis of the use of antiretroviral drugs following nonoccupational exposures to HIV and implementation, ethics, and public policy issues need to be considered.
CDC affirms that efficacy data and additional epidemiological information are needed (e.g., the number of infections that could be averted by antiretroviral drugs, the number of people who would need to be treated to avert one infection effects of antiretroviral drug availability on risk behavior, physician practices in prescribing antiretroviral drugs) before public policy recommendations can be formulated. Currently, the use of antiretroviral drugs for nonoccupational exposures to HIV should be considered a clinical intervention of unproven efficacy. Individual practitioners and their patients may opt to consider its use in specific circumstances following exposures to HIV that carry a particularly high risk of infection, but only after careful consideration of the potential risks and benefits and with a full awareness of the many gaps in our current knowledge. ReferencesCardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in healthcare workers after percutaneous exposure. N Engl J Med 1997;337:1485-90.
CDC. Update: provisional Public Health Service recommendations for chemoprophylaxis after occupational exposure to HIV. MMWR 1996;45:468-72. CDC. PHS statement on management of occupational exposures to HIV, including considerations regarding zidovudine postexposure use. MMWR 1990;39 (RR-1):1-14. CDC. U.S. Public Health Service Task Force recommendations for use of antiretroviral drugs during pregnancy for maternal health and reduction of perinatal transmission of human immunodeficiency virus type 1 in the United States. MMWR in press Katz MH and Gerberding JL. Postexposure treatment of people exposed to the human immunodeficiency virus through sexual contact or injection drug use. N Engl J Med 1997;336:1097-1099. Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N Engl J Med1996;335:1621-29.It was spring of 1996 when Beth Bye says she returned from the dead. The Wisconsin woman hadn't actually died, but with her body ravaged in the late stages of AIDS infection, she had run out of options, and death was, indeed, near. AIDS-related dementia and blindness had crept in—signs that her doctor told her meant time was short. She made funeral arrangements and considered moving to a hospice for her remaining days.
Then, as if to say not so fast, medical science handed her another option. New drugs called protease inhibitors, first approved in 1995, were about to revolutionize the treatment of patients infected with the AIDS virus. These drugs usually are taken with two other drugs called reverse transcriptase inhibitors. The combined drug cocktail has helped change AIDS in the last three years from being an automatic death sentence to what is now often a chronic, but manageable, disease. Within two months of beginning the triple cocktail treatment, also known as highly active antiretroviral therapy (HAART), Bye's viral load—a measure of new AIDS virus produced in the body—dropped to undetectable levels. Her red and white blood cell counts normalized, an important sign that the immune system was starting to work again. Suddenly she could do simple things she had long given up, such as walk the dog for 2 miles. Bye, now 40, was even able to return to her teaching job and currently works 30 hours a week.My recovery was like being on death row and getting that last minute pardon from the governor, she says. This so-called Lazarus Effect, named for the biblical figure who was raised from the dead, has occurred with many AIDS patients who take the triple therapy. It returns many who were debilitated and dying to relatively healthy and productive life, says Richard Klein, HIV/AIDS coordinator for the Food and Drug Administration's Office of Special Health Issues. Many health experts, in fact, credit the powerful HAART therapy with helping the domestic AIDS death rate to drop by 47 percent in 1997, the last year for which figures are available. Other factors have contributed as well, says Anthony Fauci, M.D., director of the National Institute of Allergy and Infectious Diseases. It is also likely that increased access to care, our growing expertise and experience in caring for HIV-infected people, and the decrease in new HIV infections in the late 1980s due to prevention efforts are partly responsible for the reduction in HIV-related deaths we are seeing today. In 1997, for the first time since 1990, AIDS fell out of the top 10 causes of death in the United States, dropping from 8th to 14th place, according to the national Centers for Disease Control and Prevention. By 1998, about 16,000 people were still alive who would have died the previous year if AIDS mortality had continued at its former rate. Still, about 40,000 new infections occur yearly.
So far, the combination HAART treatment is the closest thing medical science has to an effective therapy. The key to its success in some patients lies in the drug combination's ability to disrupt HIV at different stages in its replication. Reverse transcriptase inhibitors, which usually make up two drugs in the HAART regimen, restrain an enzyme crucial to an early stage of HIV duplication. Protease inhibitors hold back another enzyme that functions near the end of the HIV replication process. The combination can be prescribed to those newly infected with the virus, as well as AIDS patients.
FDA approved the first drug specifically to combat HIV and AIDS in 1987. Commonly known as AZT (zidovudine), it is in the family of reverse transcriptase inhibitors called nucleoside analogs. Others in this class include ddi (didanosine) ddc (zalcitabine), D4T (stavudine), 3TC (lamivudine), and most recently Ziagen (abacavir). In 1997, FDA approved Combivir, a mixture of AZT and 3TC that allows patients to reduce the number of pills needed, which can be upwards of 20 a day for certain drug combinations. Viramune (nevirapine), the first reverse transcriptase inhibitor in a class called non-nucleoside analogs, was approved in 1996. The following year, FDA approved a related drug, Rescriptor (delavirdine). In 1998, a third drug in this class, Sustiva (efavirenz) was approved. Protease inhibitors, the last part of the triple cocktail, have only been on the market about three years. FDA approved the first one, Invirase (saquinavir), in late 1995. Others approved since include Norvir (ritonavir), Crixivan (indinavir), Viracept (nelfinavir), and Agenerase (amprenivir). Viracept was the first of its class to be labeled for use in children and adults. Norvir and Agenerase are now approved for children as well. FDA also has approved Fortovase, a new formulation of saquinavir that comes in a soft gelatin capsule that allows more drug to be absorbed into the body than the earlier version.Though the use of protease inhibitors with other AIDS drugs has had a drastic impact on the health of HIV and AIDS patients, there are drawbacks. For example, the HAART treatment is not an AIDS cure, says FDA's Klein. Though HIV, the virus that causes AIDS, may not be detectable in the blood following successful HAART treatment, experts generally feel that the virus is still present, lurking in hiding spots such as the lymph nodes, the brain testes, and the retina.
The improved sense of well-being, and the belief that lower viral load means they will not transmit the virus has translated, in some communities, to a lapse in certain prevention practices, Klein says. He adds that this is dangerous because infected people, even with diminished viral counts, can spread the virus. Another concern is that the combination therapy, besides being very expensive, requires a much more complicated treatment regimen. Patients need to stay aware of and adhere to their dosing schedule, says Klein. If not taken on a strict regimen, protease inhibitors can result in the emergence of HIV strains that are resistant to treatment. Numerous studies also have shown that viral load can rapidly rebound to high levels if patients discontinue part or all of the triple therapy regimen. AIDS treatments may interact with many commonly prescribed drugs. For example, Pfizer Inc. plans to label its impotence drug Viagra to warn of possible interactions with certain protease inhibitors, which appear to raise levels of Viagra in the blood. AIDS drugs also may prompt onset of diabetes or a worsening of existing diabetes and hyperglycemia (high blood sugar), along with increased bleeding in people with hemophilia types A or B. Some patients on triple therapy have experienced a type of weight redistribution where face and limbs become thin while breasts, stomach or neck enlarges. Some have nicknamed the appearance of fat deposits at the back of the shoulders buffalo hump. Fat deposits in the midsection are sometimes called Crix belly, after the drug Crixivan, although it has been seen in people taking all approved protease inhibitors, says Klein. Research is currently under way to determine if protease inhibitors cause a permanent change in fat metabolism. There is considerable concern over the long-term effects for patients, says Klein, including the possibility that the cholesterol increases in some patients who experience fat redistribution could increase the risk for cardiovascular complications such as strokes or heart attacks. FDA has asked each of the makers of protease inhibitors to study these abnormalities.Because AIDS patients have suppressed immune systems, they can fall prey to certain illnesses that people with healthy immune responses don't get, or get only very rarely. One common such illness is Pneumocystis carinii pneumonia (PCP), which can be life-threatening. Treatments to prevent PCP are NebuPent (aerosolized pentamidine), a fine mist inhaler, and drugs such as Bactrim and Septra that contain both trimethoprim and sulfa. Mepron (atovaquone) is approved for treating mild-to-moderate PCP in pregnant women and patients who cannot tolerate standard treatment. Neutrexin (trimexetrate glucoronate) also is approved for pregnant women and for moderate-to-severe PCP when given with Leucovorin (folinic acid).
Cytomegalovirus retinitis is a potentially severe AIDS-related eye infection that can lead to blindness. Approved treatments include ganciclovir, marketed as Cytovene in oral dosage and as Vitrosert as an implant, Foscavir (foscarnet), and Vistide (cidovir). For mycobacterium avium, an infection that before AIDS was almost always confined to patients with severe chronic lung diseases such as emphysema, FDA has approved Biaxin (clarithromycin), Mycobutin (rifabutin), and Zithromax (azithromycin). Kaposi's sarcoma (KS) is a type of AIDS-related cancer that causes characteristic purple or pink skin tumors that are flat or slightly raised. Intron A (human interferon-alpha), doxorubicin liposome injection, or daunorubicin citrate liposome injection can be used to treat KS. Panretin, a topical gel, also is approved for treating certain types of KS lesions. AIDS wasting syndrome involves major weight loss, chronic diarrhea or weakness, and constant or intermittent fever for at least 30 days. Approved treatments include Marinol (dronabinol), Megace (megestrol), and Serostim (somatropin rDNA for injection).In 1998 recommendations, the Public Health Service Task Force stated that the decision to take anti-HIV drugs during pregnancy should be made by the pregnant woman after her healthcare provider has explained benefits and risks. There are some compelling reasons to take the drugs. For example, an HIV-positive pregnant woman who takes AZT after the first trimester decreases the chance of the baby being born with HIV. Studies show that AZT taken according to a strict regimen decreases by nearly 66 percent the odds of infecting the newborn.
The task force says women should consider delaying therapy until after the 10th to 12th week of pregnancy, after the fetus's organs have gone through their most rapid development. This delay may minimize any adverse effects of AZT on fetal development, but it needs to be balanced with the health of the mother and possible transmission of HIV to the fetus. Most children with HIV became infected from their mothers near the time of birth. This means that for many babies treatment can be started soon after birth. Federal guidelines recommend that all HIV-infected children younger than 1 year and all HIV-infected children of any age with symptoms of HIV infection or evidence of immune suppression be treated with anti-HIV drugs. For HIV-infected children with no symptoms, therapy can be deferred if risk of disease is considered low based on viral load and immune status. Triple combination therapy can be used for all HIV-infected infants, children and adolescents treated with HIV drugs. Infants during the first six weeks of life who have been exposed to HIV but whose HIV status is unknown can be treated with AZT as sole therapy. Infants diagnosed with HIV while receiving AZT alone should be switched to combination therapy.Though the AIDS death rate has dropped drastically, and educational efforts aimed at curbing the number of new HIV infections have had a small impact, experts say the next hurdles are to develop an AIDS-preventive vaccine and to create new therapies, such as ones that would effectively treat AIDS patients when drug-resistant strains of HIV develop. On both fronts, promising efforts are in progress.
For example, NIAID is conducting trials of three novel HIV vaccine approaches. One trial is testing a vaccine applied to spots such as the moist tissues lining the urinary and reproductive tracts. This is because most HIV infections, such as those acquired through sexual exposure, are transmitted across these mucosal sites. Researchers theorize that a vaccine that prompts the body to produce antibodies at these sites may have a protective effect against the AIDS virus. Another vaccine approach is using common Salmonella bacteria to deliver HIV proteins in a way that may trigger the body to produce a better immune response. A third study is examining a cancer drug, GM-CSF, to determine its effect on stimulating immunity. NIAID also is experimenting with a vaccine approach that neutralizes antibodies to HIV, which then bind to the virus in a way that may prevent it from infecting cells. A new class of drugs called fusion inhibitors has been shown in early trials to block HIV's entry into cells, which may keep the virus from reproducing. These drugs hold particular promise for patients whose HIV viral loads have rebounded to elevated levels because the virus strains they carry have become resistant to triple combination therapy. Researchers reported at the 6th Conference on Retroviruses and Opportunistic Infections in February 1999 that one fusion inhibitor, T-20, significantly lowered virus amounts in a group of patients with drug-resistant viral strains. Other therapies aimed at eradicating the virus that remains after successful combination treatment include drugs targeted at bolstering the immune system such as IL-2 (Interleukin-2) and G-CSF (Neupogen). Though these and other potential treatments may individually or in combination help wipe out AIDS sometime in the future, what's really needed, says NIAID's Fauci, are types of drugs that don't yet exist. These agents would ideally be potent, inexpensive, relatively nontoxic even after prolonged periods, active against viral strains resistant to currently available agents, and easy to administer. Source: John Henkel. FDA Consumer, July-Aug 1999.In the United States, opportunistic infections continue to produce morbidity and mortality among the estimated 650,000-900,000 persons who are infected with HIV, especially among the estimated 200,000-250,000 persons who are severely immunosuppressed (i.e., persons who have a CD4+ T-lymphocyte count of less than 200 cells/uL). However surveillance data indicate that the incidence of opportunistic infections has been changing in the United States.
In HIV-infected men who have sex with men, Pneumocystis carinii pneumonia (PCP), toxoplasmic encephalitis, fungal infections, and disseminated Mycobacterium avium complex (MAC) disease have decreased in incidence. Prophylactic regimens against opportunistic pathogens and more potent antiretroviral drugs appear to be important factors influencing this decline in incidence. However, these decreases have not been observed among HIV-infected injecting-drug users suggesting that more emphasis should be placed on providing currently recommended chemoprophylactic agents to all persons who have HIV infection and who meet appropriate criteria for prophylaxis for opportunistic infections. The surveillance data also indicate that the incidence of some opportunistic infections is not decreasing among either men who have sex with men or injecting-drug users, indicating that preventive strategies need to be developed and applied to a wider spectrum of opportunistic infections. An important issue in opportunistic-infection prophylaxis is whether to offer or continue prophylaxis on the basis of the lowest CD4+ T-lymphocyte count or of a more recent count that has been elevated as a result of antiretroviral therapy. This issue is particularly pertinent because of the administration of potent drug combinations that include protease inhibitors, which may increase CD4+ counts by 100-250 cells/uL. It is currently unknown whether such increases in CD4+ counts provide anti-infective protection comparable with that afforded to patients whose counts never declined below the current level. Until data assessing these risks are available, most experts recommend that prophylaxis be initiated or continued on the basis of the lowest CD4+ count.| Tuberculosis (TB) is caused by a germ called Mycobacterium tuberculosis. | |
| TB is spread through the air. You need to have close contact with a person who has TB to get it. | |
| Get tested for TB as soon as possible after learning you have HIV. Go to your doctor or your health department for a skin test for TB. n You can take medicines to prevent and to treat TB. |
Tuberculosis (TB) is a disease caused by a germ called Mycobacterium (my-ko-bak-TEER-I-um) tuberculosis. TB most often affects the lungs, but TB germs can infect any part of the body. TB may be latent or active TB. Latent means that the germs are in the person's body but are not causing illness. If you have latent TB you will not have symptoms and cannot spread TB. However, if HIV has made your immune system too weak to stop the TB germs from growing, they can multiply and cause active TB (also called TB disease).
In people with HIV, TB in the lungs or anywhere else in the body is called an AIDS-defining condition. In other words, a person with both HIV and active TB has AIDS.TB is spread from one person to another through the air. When a person who has TB disease of the lung or throat coughs, sneezes, or sings, tiny, moist drops that contain TB germs are sent into the air. A person who breathes air that contains these drops may get TB. People with TB disease are most likely to spread it to people they spend time with every day, such as family members, friends, or coworkers.
You can't get TB from shaking hands, sitting on a toilet seat, or sharing dishes or utensils.Some activities and jobs may increase your chances of spending time with people who have TB and getting TB. These include working in a healthcare setting (a hospital, a clinic, a doctor's office), in jails and prisons, and in shelters for homeless people. You and your doctor should decide whether you should be working in such a place. If you do things that may increase your chances of getting TB, you and your doctor may decide that you need to be tested for TB more often than once a year.
If you can, avoid spending time with someone who has active TB but is not taking medicine or has just started taking medicine. A person who has been taking medicine for a few weeks can normally no longer spread TB to you. That person's doctor will say when it's safe for other people to spend time with him or her. If you are exposed to a person with active TB, you should ask your doctor about getting treatment, even if your skin test was negative for TB.Your symptoms depend on where in your body the TB germs are growing. TB germs usually grow in the lungs. TB in the lungs may cause:
| bad cough that lasts longer than 3 weeks | |
| pain in the chest | |
| coughing up blood or phlegm from deep inside the lungs |
| weakness or fatigue | |
| weight loss | |
| no appetite | |
| chills | |
| fever | |
| sweating at night |
No. Active TB most often affects the lungs. But it can also affect almost any other body
organ, such as the kidneys or the spine. A person whose TB is not in the lungs or throat
HIV and Tuberculosis usually cannot give TB to other people.
Yes. Latent TB is much more likely to become active TB in someone with HIV. This is because HIV weakens the immune system, which makes it harder for the body to fight off diseases like TB.
Yes. If you have not already had TB or if you had a positive result from a skin test for TB in the past, get a tuberculin skin test, or TST at the health department or your doctor's office.
When you have the test, a healthcare worker will inject a small amount of testing fluid (called tuberculin) just under the skin on the lower part of your arm. After 2 or 3 days, the healthcare worker will check your arm to see whether you had a positive reaction to the test. If you have a positive test result (which usually means you have latent TB), you may need other tests to see whether you have TB disease (active TB). These tests usually include a chest x-ray and a test of the phlegm you cough up. Because TB can grow somewhere else in your body, other tests may be done. If you have a negative test result you should be tested again at least once a year, depending on your chances of getting TB. Discuss your chances of getting TB with your doctor. If you are an HIV-infected mother whose baby was born after you got HIV, have your baby tested for TB when the baby is 9 to 12 months old.Yes. The drug isoniazid can help prevent latent TB from becoming active TB. People with HIV infection who need to take isoniazid are also given a vitamin called pyridoxine to prevent peripheral neuropathy (a disorder of the nervous system).
Get tested for latent TB, with a TST, as soon as possible after you learn you have HIV. If your skin test result is positive (but you do not have active TB), you will most likely be given 12 months of treatment with isoniazid to prevent active TB. You need to take your medicine for the full 12 months because TB germs die very slowly. Take your medicine exactly as your doctor or nurse tells you. If you are a woman who is pregnant, you may still take isoniazid to fight TB. However, your doctor may tell you not to take the medicine until after the first 3 months of your pregnancy. The germs that caused your latent TB might not be killed by isoniazid. In that case, you will be given another drug (probably rifampin) that is used to prevent TB.Yes. The drugs that fight TB work as well as in people with HIV as they do in people who do not have HIV.
Several drugs are used to treat active TB. You will need to take more than one drug for several weeks. Your symptoms may go away within a few weeks after you start taking the medicine. TB germs die very slowly, so you need to keep taking your medicine exactly as your doctor or nurse tells you (the right amount at the right time for the right length of time).Yes. If you have TB disease of the lungs or throat, you can probably spread TB to other people. You may need to stay home from work or school or other activities for a few weeks. After you've taken your medicine for a few weeks, you will probably no longer be able to spread TB to others, but you need to continue taking your medicine for 6 to 9 months to be totally cured. Your doctor or nurse will tell you when you can return to work or school or other activities. The medicine should not affect your strength, your sexual function, or your ability to work. Taking the medicine as prescribed will keep you from again becoming sick with TB disease.
Yes. But you should know that medicines for TB and the protease inhibitors affect each other. Your doctor will decide which combination of medicines will work best for you.
When TB germs are not killed by a certain drug, that TB is called drug-resistant. TB germs may become resistant when patients do not take their medicine long enough or in the right amount at the right times. Follow your doctor's advice when taking medicines.
People who have drug-resistant TB can transmit it to others. Drug-resistant TB is found often in people who come from areas where TB is common (for example, Africa, Southeast Asia, Latin America) but it also occurs in parts of the United States. When several different drugs can't kill TB germs, the TB is called multidrug-resistant TB (MDR TB). A patient with MDR TB may need to see a doctor who is an expert on drug-resistant TB and who can recommend the best combination of drugs to fight the germs.This information is for people who are infected with the human immunodeficiency virus (HIV). HIV is the virus that causes the disease acquired immunodeficiency syndrome (AIDS). You might also want to share this chapter with your friends and family. It will help you, and them, understand more about HIV.
Although HIV is a serious infection, people with HIV and AIDS are living longer, healthier lives today, thanks to new and effective treatments. This chapter will help you understand how you can live with HIV and how you can keep yourself healthy. You probably have many questions about HIV, such as:| What is HIV and how did I get it? | |
| What is the difference between HIV and AIDS?/li> | |
| How can I stay healthy longer?/li> | |
| How do I protect other people from my HIV?/li> | |
| Where can I find help in fighting HIV? |
This chapter will give you answers to many of your questions. You should feel free to ask your doctor any question about HIV. Other sources of information about HIV are listed at the back of this chapter.
When HIV enters your body, it infects your CD4 cells and kills them. CD4 cells sometimes called T-helper cells) help your body fight off infection and disease. Usually, CD4 cell counts in someone with a healthy immune system range from 500 to 1800. When you lose CD4 cells, your immune system breaks down and you can't fight infections and diseases as well. When your CD4 cell count goes under 200, doctors say you have AIDS. Doctors also say you have AIDS if you have HIV and certain diseases, such as tuberculosis or Pneumocystis carinii [NEW-mo-SIS-tis CA-RIN-nee-eye] pneumonia (PCP), even if your CD4 cell count is over 200.
| Make sure you have a doctor who knows how to treat HIV. | |
| Follow your doctor's instructions. Keep your appointments. Your doctor may prescribe medicine for you. Take the medicine just the way he or she tells you to because taking only some of your medicine gives your HIV infection more chance to fight back. If you get sick from your medicine, call your doctor for advice–don't change how you take your medicine on your own or because of advice from friends. | |
| Get immunizations (shots) to prevent infections such as pneumonia and flu.Your doctor will tell you when to get these shots. | |
| If you smoke or if you use drugs not prescribed by your doctor, quit. | |
| Eat healthy foods. This will help keep you strong, keep your energy and weight up, and help your body protect itself. | |
| Exercise regularly to stay strong and fit. |
| Get enough sleep and rest. | |
| Take time to relax. Many people find prayer or meditation, along with exercise and rest, helps them cope with the stress of having HIV infection or AIDS. |
At your first appointment your doctor will ask you questions, do a checkup, draw blood, and do a tuberculosis skin test and other tests. Your doctor also may give you some immunizations (shots). Tell your doctor about any health problems you are having so that you can get treatment. You also should ask your doctor any questions you have about HIV or AIDS such as what to do if your medicine makes you sick, where to get help in quitting smoking or drug use, or how to eat healthy foods.
When your doctor draws blood, it is used for many tests, including the CD4 cell count and viral load testing. Viral load testing measures the amount of HIV in your blood. Viral load tests help predict what will happen next with your HIV infection if you don't get treatment. They are used with CD4 cell counts to decide when to start and when to change your drug therapies.
Keep your follow-up appointments with your doctor. At follow-up appointments you and your doctor will talk about your test results, and he or she may prescribe medicine for you.
| MAC (mycobacterium avium [my-ko-bak-TEER-i-um a-VEE-i-um] complex) | |
| CMV (cytomegalovirus [si-to-MEG-eh-lo-vi-res]) | |
| TB (tuberculosis [too-burr-qu-LO-sis]) | |
| toxo (toxoplasmosis [tok-so-plaz-MO-sis]) | |
| crypto (cryptosporidiosis [krip-to-spo-rid-e-O-sis]) |
| breathing problems | |
| mouth problems, such as thrush (white spots), sores, change in taste, | |
| dryness, trouble swallowing, or loose teeth | |
| fever for more than two days | |
| weight loss | |
| poor vision or floaters(moving lines or spots in your vision) | |
| diarrhea |
| Don't have unprotected sex–sex without a condom. Abstinence–not having sex–is the best way to protect other people. If you have sex, use a new latex condom (rubber) each and every time. | |
| If you use a lubricant, use a water-based lubricant. You should not use new petroleum-based jelly, cold cream baby oil, or other oils because they can weaken a condom and it may break. | |
| If you are allergic to latex, you can use polyurethane (a type of plastic) condoms. | |
| If male condoms are not available, use female condoms. | |
| If you choose to use a spermicide (a cream, foam, or gel used to kill sperm) use it as the instructions say. You can use condoms with or without spermicide. | |
| For oral sex, use protection such as a condom, dental dam (a square piece of latex used by dentists), or plastic food wrap. Do not reuse these items. | |
| Keep sex toys for your own use only and don't use someone else's sex toys. | |
| Don't share drug needles or drug works. In many places there are needle exchange programs. Use them. Better yet seek help if you inject drugs.You can fight HIV much better if you don't have a drug habit. | |
| Tell people you've had sex with that you have HIV. This will not be easy, but it will help them get the help they need. Your local public health department may help you find these people and tell them they have been exposed to HIV. If they have HIV, this may help them get care and avoid spreading HIV to others. | |
| If a woman you had sex with is pregnant, even if you are not the father, it is very important that you tell her you have HIV. If she has HIV, she needs to get early medical care for her own health and to protect her baby. | |
| Don't donate blood, plasma, or organs. | |
| Keep razors or toothbrushes for your own use only and don't use someone else's razor or toothbrush. HIV can be spread through fresh blood on such items. |
| What birth control methods are best for me? | |
| Will HIV cause problems for me during pregnancy or delivery? | |
| Will my baby have HIV? | |
| Will treatment for my HIV infection cause problems for my baby? | |
| If I am pregnant and want an abortion, where can I go for it? What if they won't help me because I have HIV? | |
| If I choose to get pregnant, what medical and community programs and support groups can help me and my baby? |
| answers to your questions about HIV and AIDS | |
| doctors, insurance, and help in making healthcare decisions | |
| food, housing, and transportation | |
| planning to meet financial and daily needs | |
| support groups for you and your loved ones | |
| home nursing care | |
| help in legal matters, including Americans with Disabilities Act (ADA) claims | |
| confidential help in applying for Social Security disability benefits |
You also can get information on these things from the CDC National AIDS Hotline at (800) 342-2437. Many people living with HIV feel better if they can talk with other people who also have HIV. Here are some ways to find others with HIV:
| Contact your local AIDS service organization. Look under AIDS or Social Service Organizations in the yellow pages of your telephone book. | |
| Contact a local hospital, church, or American Red Cross chapter for referrals. | |
| Read HIV newsletters or magazines. | |
| Join support groups or Internet forums. | |
| Volunteer to help others with HIV. | |
| Be an HIV educator or public speaker, or work on a newsletter. | |
| Attend social events to meet other people who have HIV. |
n You can protect yourself from many infections by preparing food and drinks properly.
n Meat, poultry (such as chicken or turkey), and fish can make you sick if they are raw, undercooked, or spoiled. n Raw fruits and vegetables are safe to eat if you wash them carefully first. n Don't drink water straight from lakes, rivers, streams, or springs.Food and water can carry germs that cause illness. Germs in food or water may cause serious infections in people with HIV. You can protect yourself from many infections by preparing food and drinks properly.
Germs in food and water that can make someone with HIV ill include Salmonella, Campylobacter, Listeria and Cryptosporidium. They can cause diarrhea, upset stomach, vomiting, stomach cramps, fever, headache, muscle pain bloodstream infection, meningitis, or encephalitis.
No, they can occur in anyone. However, these illnesses are much more common in people with HIV.
No. The diarrhea and nausea are often much worse and more difficult to treat in people with HIV. These illnesses are also more likely to cause serious problems in people with HIV, such as bloodstream infections and meningitis. People with HIV also have a harder time recovering fully from these illnesses.
Yes. Meat, poultry (such as chicken or turkey), and fish can make you sick only if they are raw, undercooked, or spoiled. To avoid illness:
| Cook all meat and poultry until they are no longer pink in the middle. If you use a meat thermometer, the temperature inside the meat or poultry should be over 165° F. Fish should be cooked until it is flaky, not rubbery. | |
| After handling raw meat, poultry, and fish, wash your hands well with soap and water before you touch any other food. | |
| Thoroughly wash cutting boards, cooking utensils, and countertops with soap and hot water after they have had contact with raw meat, poultry, or fish. | |
| Do not let uncooked meat, poultry, or fish or their juices touch other food or each other. | |
| Do not let meat, poultry, or fish sit at room temperature for more than a few minutes. Keep them in the refrigerator until you are ready to cook them. | |
| Eat or drink only pasteurized milk or dairy products. |
Yes. Eggs are safe to eat if they are well cooked. Cook eggs until the yolk and white are solid, not runny. Do not eat foods that may contain raw eggs, such as hollandaise sauce, cookie dough, homemade mayonnaise, and Caesar salad dressing. If you prepare these foods at home, use pasteurized eggs instead of eggs in the shell. You can find pasteurized eggs in the dairy case at your supermarket.
Yes. Raw fruits and vegetables are safe to eat if you wash them carefully first. Wash, then peel fruit that you will eat raw. Eating raw alfalfa sprouts and tomatoes can cause illness, but washing them well can reduce your risk of illness.
| Don't drink water straight from lakes, rivers, streams, or springs. | |
| Because you cannot be sure if your tap water is safe, you may wish to avoid tap water, including water or ice from a refrigerator ice-maker, which is made with tap water. Always check with the local health department and water utility to see if they have issued any special notices for people with HIV about tap water. | |
| You may also wish to boil or filter your water, or to drink bottled water. Processed carbonated (bubbly) drinks in cans or bottles should be safe, but drinks made at a fountain might not be because they are made with tap water. If you choose to boil or filter your water or to drink only bottled water, do this all the time, not just at home. Boiling is the best way to kill germs in your water. Heat your water at a rolling boil for 1 minute. After the boiled water cools, put it in a clean bottle or pitcher with a lid and store it in the refrigerator. Use the water for drinking, cooking, or making ice. Water bottles and ice trays should be cleaned with soap and water before use. Don't touch the inside of them after cleaning. If you can, clean your water bottles and ice trays yourself. |
| Read food labels carefully. Be sure that all dairy products that you purchase have been pasteurized. Do not buy any food that contains raw or undercooked meat or eggs if it is meant to be eaten raw. Be sure that the sell by date has not passed. | |
| Put packaged meat, poultry, or fish in separate plastic bags to prevent their juices from dripping onto other groceries or each other. | |
| Check the package that the food comes in to make sure that it isn't damaged. | |
| Do not buy food that has been displayed in unsafe or unclean conditions.Examples include meat that is allowed to sit without refrigeration or cooked shrimp that is displayed with raw shrimp. | |
| After shopping, put all cold and frozen foods into your refrigerator or freezer as soon as you can. Do not leave food sitting in the car. Keeping cold or frozen food out of refrigeration for even a couple of hours can give germs a chance to grow. |
Yes. Like grocery stores, restaurants follow guidelines for cleanliness and good hygiene set by the health department. However, you should follow these general rules in restaurants:
| Order all food well done. If meat is served pink or bloody, send it back to the kitchen for more cooking. Fish should be flaky, not rubbery, when you cut it. | |
| Order fried eggs cooked on both sides. Avoid eggs that are sunny-side up. Scrambled eggs should be cooked until they are not runny. Do not order foods that may contain raw eggs, such as Caesar salad or hollandaise sauce. If you aren't sure about the ingredients in a dish, ask your waiter before you order. | |
| Do not order any raw or lightly steamed fish or shellfish, such as oysters, clams, mussels, sushi, or sashimi. All fish should be cooked until done. |
Yes. Not all countries have high standards of food hygiene. You need to take special care abroad, particularly in developing countries. Follow these rules when in other countries:
| Do not eat uncooked fruits and vegetables unless you can peel them. Avoid salads. | |
| Eat cooked foods while they are still hot. | |
| Boil all water before drinking it. Use only ice made from boiled water. Drink only canned or bottled drinks or beverages made with boiled water. | |
| Steaming-hot foods, fruits you peel yourself, bottled and canned processed drinks, and hot coffee or tea should be safe. |
If you are going to be caring for someone with HIV infection, you need to understand the basic facts about HIV and AIDS. AIDS (acquired immunodeficiency syndrome) is caused by HIV (human immunodeficiency virus). People who are infected with HIV can look and feel healthy and may not know for years that they are infected. However, they can infect other people no matter how healthy they seem. HIV slowly wipes out parts of the body's immune system; then the HIV-infected person gets sick because the body can't fight off diseases. Some of these diseases can kill them.
Signs of HIV infection are like those of many other common illnesses, such as swollen glands, tiring easily, losing weight, fever, or diarrhea. Different people have different symptoms. HIV is in people's blood, semen, vaginal fluid, and breast milk. The only way to tell if someone is infected with HIV is with a blood test. There is no vaccine to prevent HIV infection and no cure for AIDS. There are treatments that can keep infected people healthy longer and prevent diseases that people with AIDS often get. Research is ongoing. HIV slowly makes an infected person sicker and sicker. Diseases and infections will cause serious illness, but people often get better–until the next illness. Sometimes, HIV can damage the brain and cause changes in feelings and moods even make it hard to think clearly. Someone with AIDS can feel fine in the morning and be very sick in the afternoon. It can seem like riding a roller coaster, slowly climbing up to feeling good, then plunging down into another illness.The most common ways HIV is spread are:
| By having unprotected anal, vaginal, or oral sex with one who is infected with HIV | |
| By sharing needles or syringes (works) with someone who is infected with HIV | |
| From mothers to their babies before the baby is born, during birth, or through breast-feeding. Taking the drug AZT during pregnancy can reduce the chances of infecting the baby by two-thirds, but will not prevent all babies from becoming infected with HIV. |
You don't get HIV from the air, food, water, insects, animals, dishes, knives, forks, spoons, toilet seats, or anything else that doesn't involve blood, semen, vaginal fluids, or breast milk. You don't get HIV from feces, nasal fluid, saliva, sweat, tears, urine, or vomit, unless these have blood mixed in them. You can help people with HIV eat dress, even bathe, without becoming infected yourself, as long as you follow the steps described later in the section on Protecting Yourself. You do get other germs from many of the things listed above, so do use common sense.
Infants and children with HIV infection or AIDS need the same things as other children–lots of love and affection. Small children need to be held, played with, kissed, hugged, fed, and rocked to sleep. As they grow, they need to play have friends, and go to school, just like other kids. Kids with HIV are still kids, and need to be treated like any other kids in the family.
Kids with AIDS need much of the same care that grown-ups with AIDS need, but there are a few extra things to look out for.| Watch for any changes in health or the way the child acts. If you notice anything unusual for that child, let
the doctor know. For a child with AIDS, little problems can become big problems very quickly. Watch for breathing
problems, fever, unusual sleepiness, diarrhea, or changes in how much they eat. Talk to the child's doctor about what
else to look for and when to report it.
|
| Talk to the doctor before the child gets any immunizations (including oral polio vaccine) or booster shots. Some vaccines could make the child sick. No child with HIV or anyone in the household should ever take oral polio vaccine. | |
| Stuffed and furry toys can hold dirt and might hide germs that can make the child sick. Plastic and washable toys are better. If the child has any stuffed toys, wash them in a washing machine often and keep them as clean as possible. | |
| Keep the child away from litter boxes and sandboxes that a pet or other animal might have been in. | |
| Ask the child's doctor what to do about pets that might be in the house. | |
| Try to keep the child from getting infectious diseases, especially chickenpox. If the child with HIV infection gets near somebody with chickenpox, tell the child's doctor right away. Chickenpox can kill a child with AIDS. | |
| Bandage any cuts or scrapes quickly and completely after washing with soap and warm water. Use gloves if the child is bleeding. | |
| Taking care of a child who is sick is very hard for people who love that child. You will need help and emotional support. You are not alone. There are people who can help you get through this. |