|
|
v Introduction
The Standards for Privacy of Individually Identifiable Health Information ("Privacy Rule") establishes, for the first time, a set of national standards for the protection of certain health information. The U.S. Department of Health and Human Services ("HHS") issued the Privacy Rule to implement the requirement of the Health Insurance Portability and Accountability Act of 1996 ("HIPAA").
1 The Privacy Rule standards address the use and disclosure of individuals’ health information–called "protected health information"–by organizations subject to the Privacy Rule–called "covered entities,"–as well as standards for individuals’ privacy rights to understand and control how their health information is used. Within HHS, the Office for Civil Rights ("OCR") has responsibility for implementing and enforcing the Privacy Rule with respect to voluntary compliance activities and civil money penalties.A major goal of the Privacy Rule is to assure that individuals’ health information is properly protected while allowing the flow of health information needed to provide and promote high quality health care and to protect the public’s health and well being. The Rule strikes a balance that permits important uses of information, while protecting the privacy of people who seek care and healing. Given that the health care marketplace is diverse, the Rule is designed to be flexible and comprehensive to cover the variety of uses and disclosures that need to be addressed.
This is a summary of key elements of the Privacy Rule and not a complete or comprehensive guide to compliance. Entities regulated by the Rule are obligated to comply with all of its applicable requirements and should not rely on this summary as a source of legal information or advice. To make it easier for entities to review the complete requirements of the Rule, provisions of the Rule referenced in this summary are cited in notes at the end of this document. To view the entire Rule, and for other additional helpful information about how it applies, see the OCR website: http://www.hhs.gov/ocr/hipaa. In the event of a conflict between this summary and the Rule, the Rule governs.
Links to the OCR Guidance Document are provided throughout this paper. Provisions of the Rule referenced in this summary are cited in endnotes at the end of this document. To review the entire Rule itself, and for other additional helpful information about how it applies, see the OCR website: http://www.hhs.gov/ocr/hipaa.
v Statutory & Regulatory Background
The Health Insurance Portability and Accountability Act of 1996 (HIPAA), Public Law 104-191, was enacted on August 21, 1996. Sections 261 through 264 of HIPAA require the Secretary of HHS to publicize standards for the electronic exchange, privacy and security of health information. Collectively these are known as the Administrative Simplification provisions.
HIPAA required the Secretary to issue privacy regulations governing individually identifiable health information, if Congress did not enact privacy legislation within three years of the passage of HIPAA. Because Congress did not enact privacy legislation, HHS developed a proposed rule and released it for public comment on November 3, 1999. The Department received over 52,000 public comments. The final regulation, the Privacy Rule, was published December 28, 2000.
2In March 2002, the Department proposed and released for public comment modifications to the Privacy Rule. The Department received over 11,000 comments. The final modifications were published in final form on August 14, 2002.
3 A text combining the final regulation and the modifications can be found at 45 CFR Part 160 and Part 164, Subparts A and E on the OCR website: http://www.hhs.gov/ocr/hipaav Who Is Covered by The Privacy Rule
The Privacy Rule, as well as all the Administrative Simplification rules, apply to health plans, health care clearinghouses, and to any health care provider who transmits health information in electronic form in connection with transactions for which the Secretary of HHS has adopted standards under HIPAA (the "covered entities"). For help in determining whether you are covered, use the decision tool at: http://www.cms.hhs.gov/hipaa/hipaa2/support/tools/decisionsupport/default.asp.
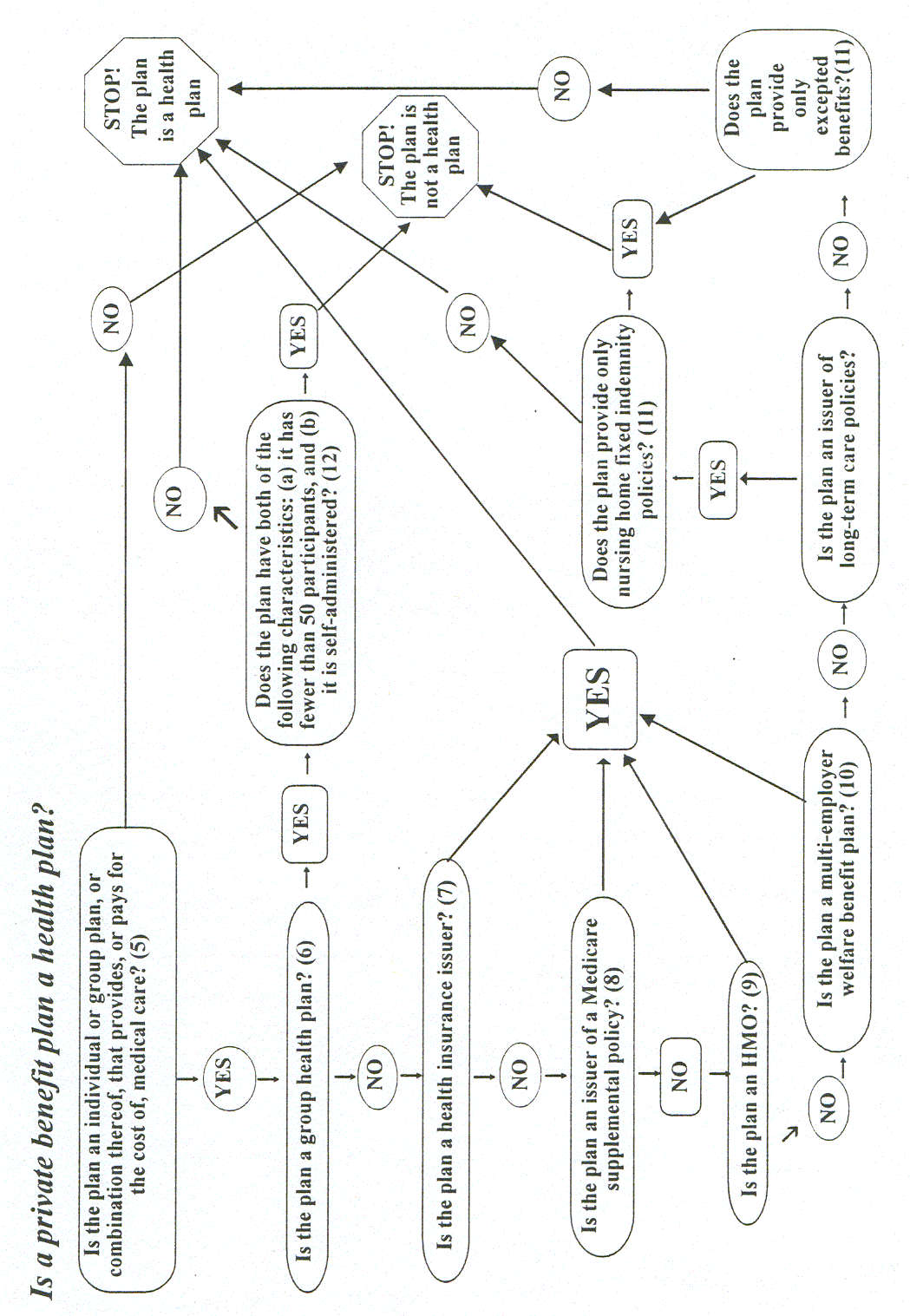
Health plans. Individual and group plans that provide or pay the cost of medical care are covered entities.
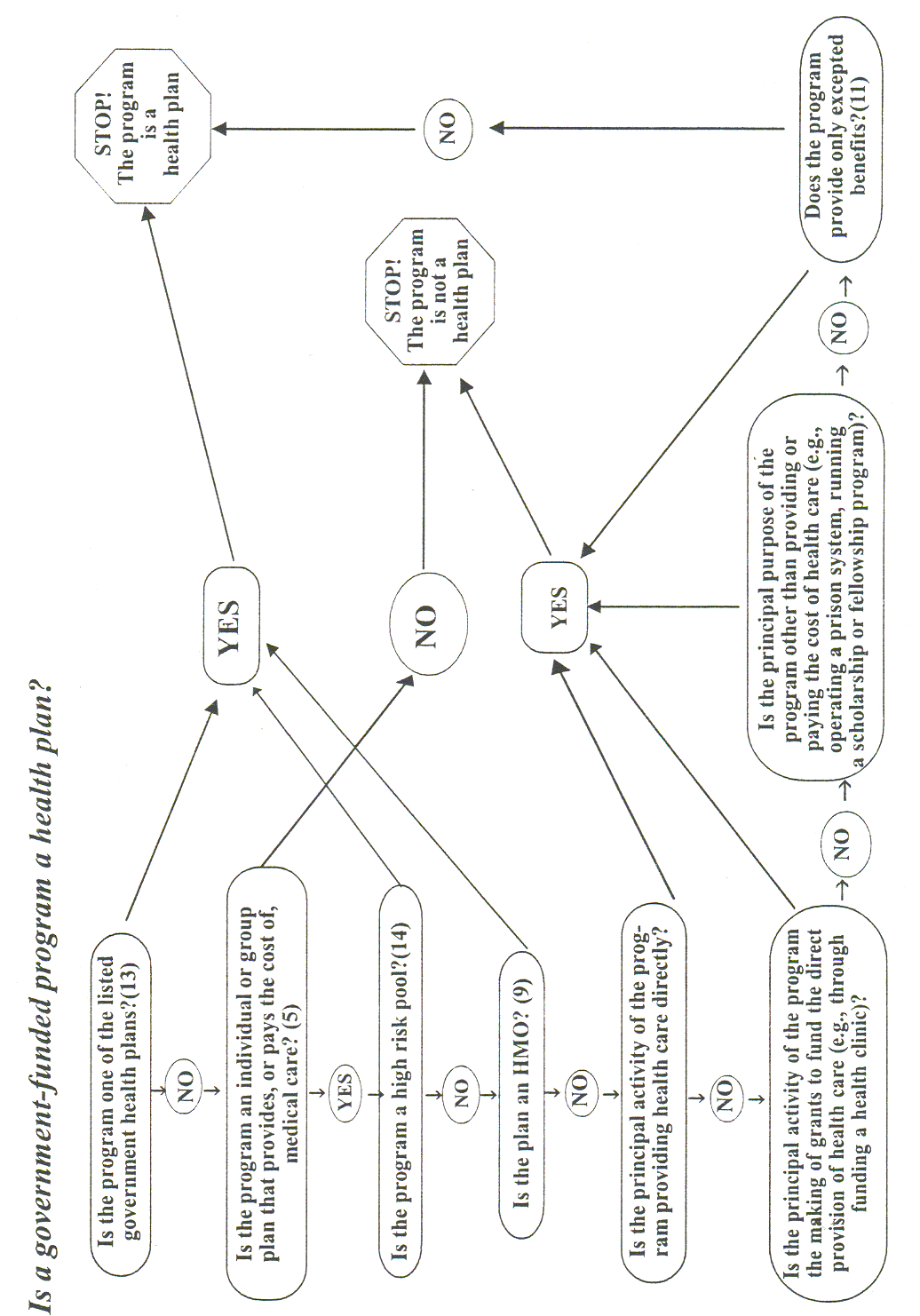
4 Health plans include health, dental, vision, and prescription drug insurers, health maintenance organizations ("HMOs"), Medicare, Medicaid, Medicare+Choice and Medicare supplement insurers, and long-term care insurers (excluding nursing home fixed-indemnity policies). Health plans also include employer-sponsored group health plans, government and church-sponsored health plans, and multi-employer health plans. There are exceptions—a group health plan with less than 50 participants that is administered solely by the employer that established and maintains the plan is not a covered entity. Two types of government funded programs are not health plans: (1) those whose principal purpose is not providing or paying the cost of health care, such as the food stamps program; and (2) those programs whose principal activity is directly providing health care, such as a community health center,5 or the making of grants to fund the direct provision of health care. Certain types of insurance entities are also not health plans, including entities providing only workers’ compensation, automobile insurance, and property and casualty insurance.Health care providers. Every health care provider, regardless of size, who electronically transmits health information in connection with certain transactions, is a covered entity. These transactions include claims, benefit eligibility inquiries, referral authorization requests, or other transactions for which HHS has established standards under the HIPAA Transactions Rule.
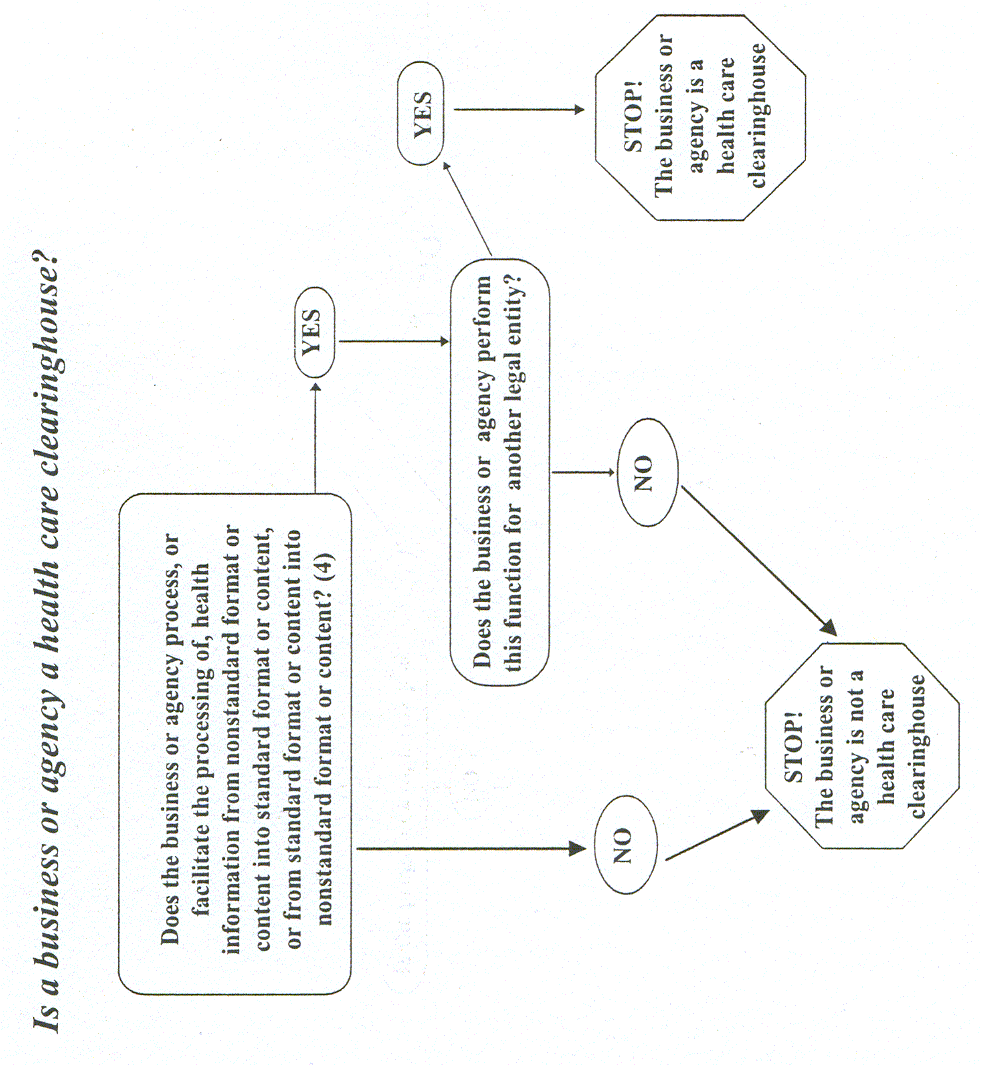
6 Using electronic technology, such as email, does not mean a health care provider is a covered entity; the transmission must be in connection with a standard transaction. The Privacy Rule covers a health care provider whether it electronically transmits these transactions directly or uses a billing service or other third party to do so on its behalf. Health care providers include all "providers of services" (e.g., institutional providers such as hospitals) and "providers of medical or health services" (e.g., noninstitutional providers such as physicians, dentists and other practitioners) as defined by Medicare, and any other person or organization that furnishes, bills, or is paid for health care.Health care clearinghouses. Health care clearinghouses are entities that process nonstandard information they receive from another entity into a standard (i.e., standard format or data content), or vice versa.
7 In most instances, health care clearinghouses will receive individually identifiable health information only when they are providing these processing services to a health plan or health care provider as a business associate. In such instances, only certain provisions of the Privacy Rule are applicable to the health care clearinghouse’s uses and disclosures of protected health information.8 Health care clearinghouses include billing services, repricing companies, community health management information systems, and value-added networks and switches if these entities perform clearinghouse functions.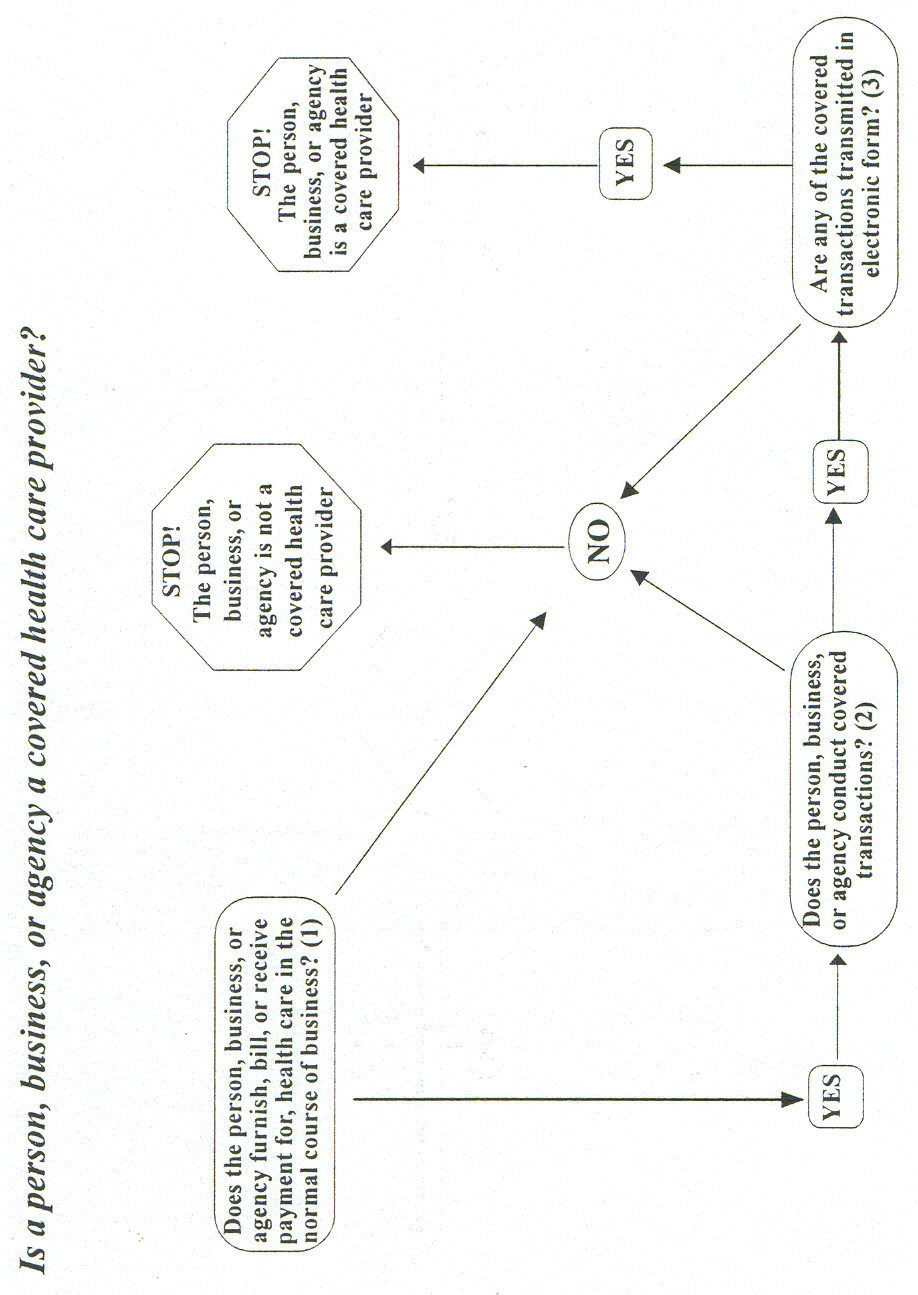
v Definitions
(1) Preventive, diagnostic, rehabilitative, maintenance, or palliative care, and counseling, service, assessment, or procedure with respect to the physical or mental condition, or functional status, of an individual or that affects the structure or function of the body; and
(2) Sale or dispensing of a drug, device, equipment, or other item in accordance with a prescription. See 45 C.F.R.160.103.
A transaction is a covered transaction if it meets the regulatory definition for the type of transaction. The regulatory definition for each type of covered transaction is as follows:
45 C.F.R.162.1101: Health care claims or equivalent encounter information transaction is either of the following:
(a) 12A request to obtain payment, and necessary accompanying information, from a health care provider to a health plan, for health care.
(b) If there is no direct claim, because the reimbursement contract is based on a mechanism other than charges or reimbursement rates for specific services, the transaction is the transmission of encounter information for the purpose of reporting health care.
45 C.F.R.162.1201: The eligibility for a health plan transaction is the transmission of either of the following:
(a) An inquiry from a health care provider to a health plan, or from one health plan to another health plan, to obtain any of the following information about a benefit plan for an enrollee:
(1) Eligibility to receive health care under the health plan.
(2) Coverage of health care under the health plan.
(3) Benefits associated with the benefit plan.
(b) A response from a health plan to a health care provider’s (or another health plan’s) inquiry described in paragraph (a) of this section.
45 C.F.R.162.1301: The referral certification and authorization transaction is any of the following transmissions:
(a) A request for the review of health care to obtain an authorization for the health care.
(b) A request to obtain authorization for referring an individual to another health care provider.
(c) A response to a request described in paragraph (a) or paragraph (b) of this section.
45 C.F.R.162.1401: A health care claim status transaction is the transmission of either of the following:
(a) An inquiry to determine the status of a health care claim.
(b) A response about the status of a health care claim.
45 C.F.R.162.1501: The enrollment and disenrollment in a health plan transaction is the transmission of subscriber enrollment information to a health plan to establish or terminate insurance coverage.
45 C.F.R.162.1601: The health care payment and remittance advice transaction is the transmission of either of the following for health care:
(a) The transmission of any of the following from a health plan to a health care provider’s financial institution:
(1) Payment.
(2) Information about the transfer of funds.
(3) Payment processing information.
(b) The transmission of either of the following from a health plan to a health care provider:
(1) Explanation of benefits.
(2) Remittance advice.
45 C.F.R.162.1701: The health plan premium payment transaction is the transmission of any of the following from the entity that is arranging for the provision of health care or is providing health care coverage payments for an individual to a health plan:
(a) Payment.
(b) Information about the transfer of funds.
(c) Detailed remittance information about individuals for whom premiums are being paid.
(d) Payment processing information to transmit health care premium payments including any of the following:
(1) Payroll deductions.
(2) Other group premium payments.
(3) Associated group premium payment information.
45 C.F.R.162.1801: The coordination of benefits transaction is the transmission from any entity to a health plan for the purpose of determining the relative payment responsibilities of the health plan, of either of the following for health care:
(a) Claims.
(b) Payment information.
In electronic form means: using electronic media, electronic storage media including memory devices in computers (hard drives) and any removable/transportable digital memory medium, such as magnetic tape or disk, optical disk, or digital memory card; or transmission media used to exchange information already in electronic storage media. Transmission media include, for example, the internet (wide-open), extranet (using internet technology to link a business with information accessible only to collaborating parties), leased lines, dial-up lines, private networks, and the physical movement of removable/transportable electronic storage media. Certain transmissions, including of paper, via facsimile, and of voice, via telephone, are not considered to be transmissions via electronic media, because the information being exchanged did not exist in electronic form before the transmission.
As pertinent here, a health care clearinghouse is a "public or private entity that does either of the following functions:
(1) Processes or facilitates the processing of health information ... in a nonstandard format or containing nonstandard data content into standard data elements or a standard transaction.
(2) Receives a standard transaction ... and processes or facilitates the processing of health information [in the standard transaction] into nonstandard format or nonstandard data content for the receiving entity". See 45 C.F.R. 160.103
A "standard transaction," for the purpose of this definition, is a transaction that complies with the standard for that transaction that the Secretary adopted in 45 CFR Part 162. See 45 C.F.R. 162.103. See the list of covered transactions in endnote 2.
A group health plan is: an employee welfare benefit plan (as defined in section 3 (1) of the Employee Retirement Income and Security Act of 1974 (ERISA), 29 U.S.C. 1002 (1)), including insured and self-insured plans, to the extent that the plan provides medical care (see endnote 5), including items and services paid for as medical care, to employees or their dependants directly or through insurance, reimbursement, or otherwise, that: (1) has 50 or more participants (see endnote 12); or (2) is administered by an entity other than the employer that established and maintains the plan. See 45 C.F.R. 160.103.
A health insurance insurer is: an insurance company, insurance service or insurance organization (including an HMO) that is licensed to engage in the business of insurance in a state and is subject to state law that regulates insurance. (This term does not include a group health plan). See 45 C.F.R. 160.103.
An issuer of a Medicare supplemental policy is: a private entity that offers a health insurance policy or other health benefit plan, to individuals who are entitled to have payments made under Medicare, which provides reimbursement for expenses incurred for services and items for which payment may be made under Medicare, but which are not reimbursable by reason of the applicability of deductibles, coinsurance amounts, or other limitations imposed pursuant to or other limitations imposed by Medicare. A Medicare supplemental policy does not include policies or plans excluded under section 1882(g)(1) of the Social Security Act. See 42 U.S.C. 1395ss (g)(1).
A health maintenance organization is: a federally qualified health maintenance organization, an organization recognized as a health maintenance organization under state law, or a similar organization regulated for solvency under state law in the same manner and to the same extent as a health maintenance organization as previously described. See 45 C.F.R. 160.103.
A multi-employer welfare program is: an employee welfare benefit plan or any other arrangement that is established or maintained for the purpose of offering and providing health benefits to the employees of two or more employers. See 45 C.F.R. 160.103.
Excepted benefits are: coverage for accident, or disability income insurance, or any combination thereof; coverage issued as a supplement to liability insurance; liability insurance, including general liability insurance and automotive liability insurance; workers’ compensation or similar insurance; automobile medical payment insurance; credit only insurance; coverage for onsite medical clinics; other similar insurance coverage, specified in regulations, under which benefits for medical care are secondary or incidental to other insurance benefits. See 42 U.S.C. 300gg-91(c)(1).
A participant means: any employee or former employee of an employer, or any member or former member of an employee organization, who is or may become eligible to receive a benefit of any type from an employee benefit plan which covers employees of such employer or member of such organization, or whose beneficiaries may be eligible to receive any such benefit.
The listed government-funded health plans are: the Medicare program under Title XVIII of the Social Security Act (Parts A, B and C) (42 U.S.C. 1395, et seq.); the Medicaid program under Title XIX of the Social Security Act (42 U.S.C. 1396, et seq.); the health care program for active military personnel (10 U.S.C. 1074, et seq.); the veterans health care program (38 U.S.C. Ch.17); the Civilian Health and Medical Program of the Uniformed Services (CHAMPUS) (10 U.S.C. 1061, et seq.); the Indian Health Service program under the Indian Health Care Improvement Act (25 U.S.C. 1601); the Federal Employees Health Benefit Program (5 U.S.C. Ch. 89); and approved state child health programs under Title XXI of the Social Security Act (42 U.S.C. 1397, et seq.) (SCHIP).
A high risk pool is a mechanism established under State law to provide health insurance coverage or comparable coverage to eligible individuals.
v Business Associates
Business associate defined.
In general, a business associate is a person or organization, other than a member of a covered entity’s workforce, that performs certain functions or activities on behalf of, or provides certain services to, a covered entity that involve the use or disclosure of individually identifiable health information. Business associate functions or activities on behalf of a covered entity include claims processing, data analysis, utilization review, and billing. 9 Business associate services to a covered entity are limited to legal, actuarial, accounting, and consulting, data aggregation, management, administrative, accreditation, or financial services. However, persons or organizations are not considered business associates if their functions or services do not involve the use or disclosure of protected health information, and where any access to protected health information by such persons would be incidental, if at all. A covered entity can be the business associate of another covered entity.Business associate contract. When a covered entity uses a contractor or other nonworkforce member to perform "business associate" services or activities, the Rule requires that the covered entity include certain protections for the information in a business associate agreement (in certain circumstances governmental entities may use alternative means to achieve the same protections). In the business associate contract, a covered entity must impose specified written safeguards on the individually identifiable health information used or disclosed by its business associates.
10 Moreover, a covered entity may not contractually authorize its business associate to make any use or disclosure of protected health information that would violate the Rule. Covered entities that have an existing written contract or agreement with business associates prior to October 15, 2002, which is not renewed or modified prior to April 14, 2003, are permitted to continue to operate under that contract until they renew the contract or April 14, 2004, whichever is first.11 Sample business associate contract language is available on the OCR website at: http://www.hhs.gov/ocr/hipaa/contractprov.html. Also see OCR "Business Associate" Guidance.v What Information Is Protected
Protected health information. The Privacy Rule protects all "individually identifiable health information" held or transmitted by a covered entity or its business associate, in any form or media, whether electronic, paper, or oral. The Privacy Rule calls this information "protected health information (PHI)."
12 "Individually identifiable health information" is information, including demographic data, that relates to:|
the individual’s past, present or future physical or mental health or condition; | |
|
the provision of health care to the individual; or | |
|
the past, present, or future payment for the provision of health care to the individual. |
and that identifies the individual or for which there is a reasonable basis to believe can be used to identify the individual.
13 Individually identifiable health information includes many common identifiers (e.g., name, address, birth date, Social Security Number). The Privacy Rule excludes from protected health information employment records that a covered entity maintains in its capacity as an employer, and education and certain other records subject to, or defined in, the Family Educational Rights and Privacy Act, 20 U.S.C. §1232g.De-identified health information. There are no restrictions on the use or disclosure of de-identified health information.
14 De-identified health information neither identifies nor provides a reasonable basis to identify an individual. There are two ways to de-identify information; either: 1) a formal determination by a qualified statistician; or 2) the removal of specified identifiers of the individual and of the individual’s relatives, household members, and employers. This is required, and is adequate only if the covered entity has no actual knowledge that the remaining information could be used to identify the individual.15v General Principle for Uses and Disclosures
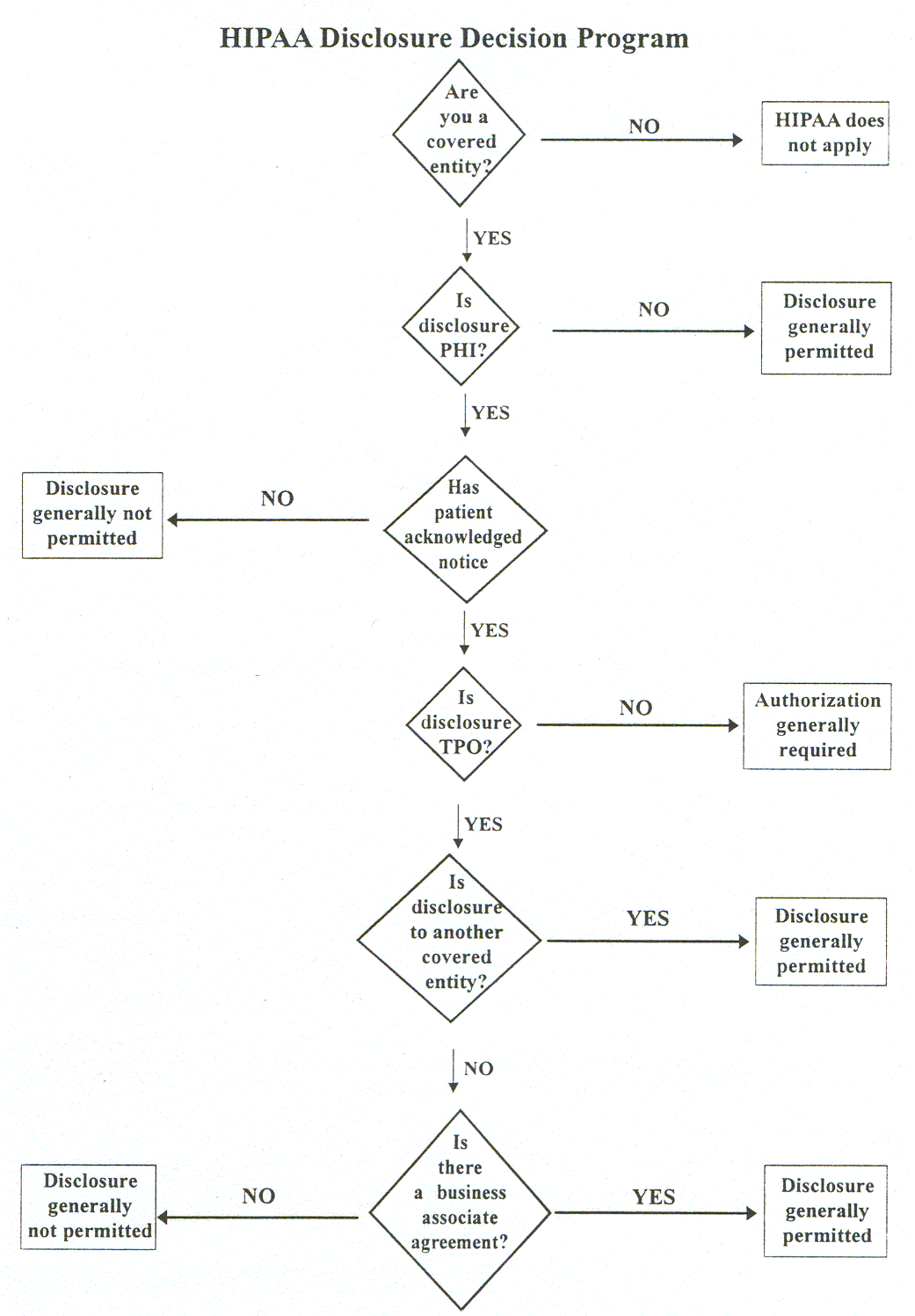
Basic principle. A major purpose of the Privacy Rule is to define and limit the circumstances in which an individual’s protected heath information may be used or disclosed by covered entities. A covered entity may not use or disclose protected health information, except either: (1) as the Privacy Rule permits or requires; or (2) as the individual who is the subject of the information (or the individual’s personal representative) authorizes in writing.
16Required disclosures. A covered entity must disclose protected health information in only two situations: (a) to individuals (or their personal representatives) specifically when they request access to, or an accounting of disclosures of, their protected health information; and (b) to HHS when it is undertaking a compliance investigation or review or enforcement action. See OCR "Government Access" Guidance.
v Permitted Uses and Disclosures
Permitted uses and disclosures. A covered entity is permitted, but not required, to use and disclose protected health information, without an individual’s authorization, for the following purposes or situations: (1) To the Individual (unless required for access or accounting of disclosures); (2) Treatment, Payment, and Health Care Operations; (3) Opportunity to Agree or Object; (4) Incident to an otherwise permitted use and disclosure; (5) Public Interest and Benefit Activities; and (6) Limited Data Set for the purposes of research, public health or health care operations.
18 Covered entities may rely on professional ethics and best judgments in deciding which of these permissive uses and disclosures to make.To the individual. A covered entity may disclose protected health information to the individual who is the subject of the information.
Treatment, payment, health care operations. A covered entity may use and disclose protected health information for its own treatment, payment, and health care operations activities.
Treatment is the provision, coordination, or management of health
care and related services for an individual by one or more health care
providers, including consultation between providers regarding a patient and
referral of a patient by one provider to another.20
Payment encompasses activities of a health plan to obtain
premiums, determine or fulfill responsibilities for coverage and provision
of benefits, and furnish or obtain reimbursement for health care delivered
to an individual
Health care operations are any of the following activities: (a) quality assessment and improvement activities, including case management and care coordination; (b) competency assurance activities, including provider or health plan performance evaluation, credentialing, and accreditation; (c) conducting or arranging for medical reviews, audits, or legal services, including fraud and abuse detection and compliance programs; (d) specified insurance functions, such as underwriting, risk rating, and reinsuring risk; (e) business planning, development, management, and administration; and (f) business management and general administrative activities of the entity, including but not limited to: de-identifying protected health information, creating a limited data set, and certain fundraising for the benefit of the covered entity.
22Most uses and disclosures of psychotherapy notes for treatment, payment, and health care operations purposes require an authorization as described below.
23Obtaining "consent" (written permission from individuals to use and disclose their protected health information for treatment, payment, and health care operations) is optional under the Privacy Rule for all covered entities.
24 The content of a consent form, and the process for obtaining consent, are at the discretion of the covered entity electing to seek consent.Facility directories. It is a common practice in many health care facilities, such as hospitals, to maintain a directory of patient contact information. A covered health care provider may rely on an individual’s informal permission to list in its facility directory the individual’s name, general condition, religious affiliation, and location in the provider’s facility.
25 The provider may then disclose the individual’s condition and location in the facility to anyone asking for the individual by name, and also may disclose religious affiliation to clergy. Members of the clergy are not required to ask for the individual by name when inquiring about patient religious affiliation.For notification and other purposes. A covered entity also may rely on an individual’s informal permission to disclose to the individual’s family, relatives, or friends, or to other persons whom the individual identifies, protected health information directly relevant to that person’s involvement in the individual’s care or payment for care.
26 This provision, for example, allows a pharmacist to dispense filled prescriptions to a person acting on behalf of the patient. Similarly, a covered entity may rely on an individual’s informal permission to use or disclose protected health information for the purpose of notifying (including identifying or locating) family members, personal representatives, or others responsible for the individual’s care of the individual’s location, general condition, or death. In addition, protected health information may be disclosed for notification purposes to public or private entities authorized by law or charter to assist in disaster relief efforts.Incidental use and disclosure. The Privacy Rule does not require that every risk of an incidental use or disclosure of protected health information be eliminated. A use or disclosure of this information that occurs as a result of, or as "incident to," an otherwise permitted use or disclosure is permitted as long as the covered entity has adopted reasonable safeguards as required by the Privacy Rule, and the information being shared was limited to the "minimum necessary," as required by the Privacy Rule.
Public interest and benefit activities. The Privacy Rule permits use and disclosure of protected health information, without an individual’s authorization or permission, for 12 national priority purposes.
Required by law. Covered entities may use and disclose protected health information without individual authorization as required by law (including by statute, regulation, or court orders).
Public health activities. Covered entities may disclose protected health information to: (1) public health authorities authorized by law to collect or receive such information for preventing or controlling disease, injury, or disability and to public health or other government authorities authorized to receive reports of child abuse and neglect; (2) entities subject to FDA regulation regarding FDA regulated products or activities for purposes such as adverse event reporting, tracking of products, product recalls, and post marketing surveillance; (3) individuals who may have contracted or been exposed to a communicable disease when notification is authorized by law; and (4) employers, regarding employees, when requested by employers, for information concerning a work-related illness or injury or workplace- related medical surveillance, because such information is needed by the employer to comply with the Occupational Safety and Health Administration (OHSA), the Mine Safety and Health Administration (MHSA), or similar state law. See OCR "Public Health" Guidance.
Victims of abuse, neglect, or domestic violence.
In certain circumstances, covered entities may disclose protected health information to appropriate government authorities regarding victims of abuse, neglect, or domestic violence.Health oversight activities. Covered entities may disclose protected health information to health oversight agencies (as defined in the Rule) for purposes of legally authorized health oversight activities, such as audits and investigations necessary for oversight of the health care system and government benefit programs.
Judicial and administrative proceedings. Covered entities may disclose protected health information in a judicial or administrative proceeding if the request for the information is through an order from a court or administrative tribunal. Such information may also be disclosed in response to a subpoena or other lawful process if certain assurances regarding notice to the individual or a protective order are provided.
Law enforcement purposes. Covered entities may disclose protected health information to law enforcement officials for law enforcement purposes under the following six circumstances, and subject to specified conditions: (1) as required by law (including court orders, court-ordered warrants, subpoenas) and administrative requests; (2) to identify or locate a suspect, fugitive, material witness, or missing person; (3) in response to a law enforcement official’s request for information about a victim or suspected victim of a crime; (4) to alert law enforcement of a person’s death, if the covered entity suspects that criminal activity caused the death; (5) when a covered entity believes that protected health information is evidence of a crime that occurred on its premises; and (6) by a covered health care provider in a medical emergency not occurring on its premises, when necessary to inform law enforcement about the commission and nature of a crime, the location of the crime or crime victims, and the perpetrator of the crime.
Decedents. Covered entities may disclose protected health information to funeral directors as needed, and to coroners or medical examiners to identify a deceased person, determine the cause of death, and perform other functions authorized by law.
Cadaveric organ, eye, or tissue donation. Covered entities may use or disclose protected health information to facilitate the donation and transplantation of cadaveric organs, eyes, and tissue.
Research.
"Research" is any systematic investigation designed to develop or contribute to generalizable knowledge.29 The Privacy Rule permits a covered entity to use and disclose protected health information for research purposes, without an individual’s authorization, provided the covered entity obtains either: (1) documentation that an alteration or waiver of individuals’ authorization for the use or disclosure of protected health information about them for research purposes has been approved by an Institutional Review Board or Privacy Board; (2) representations from the researcher that the use or disclosure of the protected health information is solely to prepare a research protocol or for similar purpose preparatory to research, that the researcher will not remove any protected health information from the covered entity, and that protected health information for which access is sought is necessary for the research; or (3) representations from the researcher that the use or disclosure sought is solely for research on the protected health information of decedents, that the protected health information sought is necessary for the research, and, at the request of the covered entity, provides documentation of the death of the individuals about whom information is sought.30 A covered entity also may use or disclose, without an individual’s authorization, a limited data set of protected health information for research purposes (see discussion below).31 See OCR "Research" Guidance.Serious threat to health or safety.
Covered entities may disclose protected health information that they believe is necessary to prevent or lessen a serious and imminent threat to a person or the public, when such disclosure is made to someone they believe can prevent or lessen the threat (including the target of the threat). Covered entities may also disclose to law enforcement if the information is needed to identify or apprehend an escapee or violent criminal.Essential government functions. An authorization is not required to use or disclose protected health information for certain essential government functions. Such functions include: assuring proper execution of a military mission, conducting intelligence and national security activities that are authorized by law, providing protective services to the President, making medical suitability determinations for U.S. State Department employees, protecting the health and safety of inmates or employees in a correctional institution, and determining eligibility for or conducting enrollment in certain government benefit programs.
Workers’ compensation. Covered entities may disclose protected health information as authorized by, and to comply with, workers’ compensation laws and other similar programs providing benefits for work-related injuries or illnesses. See OCR "Workers’ Compensation" Guidance.
v Authorized Uses and Disclosures
Authorization.
A covered entity must obtain the individual’s written authorization for any use or disclosure of protected health information that is not for treatment, payment or health care operations or otherwise permitted or required by the Privacy Rule.33 A covered entity may not condition treatment, payment, enrollment, or benefits eligibility on an individual granting an authorization, except in limited circumstances. 34An authorization must be written in specific terms. It may allow use and disclosure of protected health information by the covered entity seeking the authorization, or by a third party. Examples of disclosures that would require an individual’s authorization include disclosures to a life insurer for coverage purposes, disclosures to an employer of the results of a pre-employment physical or lab test, or disclosures to a pharmaceutical firm for their own marketing purposes.
All authorizations must be in plain language, and contain specific information regarding the information to be disclosed or used, the person(s) disclosing and receiving the information, expiration, right to revoke in writing, and other data. The Privacy Rule contains transition provisions applicable to authorizations and other express legal permissions obtained prior to April 14, 2003.
35Psychotherapy notes
36.7 A covered entity must obtain an individual’s authorization to use or disclose psychotherapy notes with the following exceptions:37|
The covered entity who originated the notes may use them for treatment. | |
|
A covered entity may use or disclose, without an individual’s authorization, the psychotherapy notes, for its own training, and to defend itself in legal proceedings brought by the individual, for HHS to investigate or determine the covered entity’s compliance with the Privacy Rules, to avert a serious and imminent threat to public health or safety, to a health oversight agency for lawful oversight of the originator of the psychotherapy notes, for the lawful activities of a coroner or medical examiner or as required by law. |
Marketing. Marketing is any communication about a product or service that encourages recipients to purchase or use the product or service.
38 The Privacy Rule carves out the following health-related activities from this definition of marketing:|
Communications to describe health-related products or services, or payment for them, provided by or included in a benefit plan of the covered entity making the communication; | |
|
Communications about participating providers in a provider or health plan network, replacement of or enhancements to a health plan, and health-related products or services available only to a health plan’s enrollees that add value to, but are not part of, the benefits plan; | |
|
Communications for treatment of the individual; and | |
|
Communications for case management or care coordination for the individual, or to direct or recommend alternative treatments, therapies, health care providers, or care settings to the individual. |
Marketing also is an arrangement between a covered entity and any other entity whereby the covered entity discloses protected health information, in exchange for direct or indirect remuneration, for the other entity to communicate about its own products or services, encouraging the use or purchase of those products or services. A covered entity must obtain an authorization to use or disclose protected health information for marketing, except for face-to-face marketing communications between a covered entity and an individual, and for a covered entity’s provision of promotional gifts of nominal value. No authorization is needed, however, to make a communication that falls within one of the exceptions to the marketing definition. An authorization for marketing that involves the covered entity’s receipt of direct or indirect remuneration from a third party must reveal that fact. See OCR Marketing Guidance.
v Limiting Uses and Disclosures to the Minimum Necessary
Minimum necessary.
A central aspect of the Privacy Rule is the principle of "minimum necessary" use and disclosure. A covered entity must make reasonable efforts to use, disclose, and request only the minimum amount of protected health information needed to accomplish the intended purpose of the use, disclosure, or request.39 A covered entity must develop and implement policies and procedures to reasonably limit uses and disclosures to the minimum necessary. When the minimum necessary standard applies to a use or disclosure, a covered entity may not use, disclose, or request the entire medical record for a particular purpose, unless it can specifically justify the whole record as the amount reasonably needed for the purpose. See OCR "Minimum Necessary" Guidance.The minimum necessary requirement is not imposed in any of the following circumstances: (a) disclosure to or a request by a health care provider for treatment; (b) disclosure to an individual who is the subject of the information, or the individual’s personal representative; (c) use or disclosure made pursuant to an authorization; (d) disclosure to HHS for complaint investigation, compliance review or enforcement; (e) use or disclosure that is required by law; or (f) use or disclosure required for compliance with the HIPAA Transactions Rule or other HIPAA Administrative Simplification Rules.
Access and uses. For internal uses, a covered entity must develop and implement policies and procedures that restrict access and uses of protected health information based on the specific roles of the members of their workforce. These policies and procedures must identify the persons, or classes of persons, in the workforce who need access to protected health information to carry out their duties, the categories of protected health information to which access is needed, and any conditions under which they need the information to do their jobs.
Disclosures and requests for disclosures.
. Covered entities must establish and implement policies and procedures (which may be standard protocols) for routine, recurring disclosures, or requests for disclosures, that limits the protected health information disclosed to that which is the minimum amount reasonably necessary to achieve the purpose of the disclosure. Individual review of each disclosure is not required. For non-routine, nonrecurring disclosures, or requests for disclosures that it makes, covered entities must develop criteria designed to limit disclosures to the information reasonably necessary to accomplish the purpose of the disclosure and review each of these requests individually in accordance with the established criteria.Reasonable reliance. If another covered entity makes a request for protected health information, a covered entity may rely, if reasonable under the circumstances, on the request as complying with this minimum necessary standard. Similarly, a covered entity may rely upon requests as being the minimum necessary protected health information from: (a) a public official, (b) a professional (such as an attorney or accountant) who is the covered entity’s business associate, seeking the information to provide services to or for the covered entity; or (c) a researcher who provides the documentation or representation required by the Privacy Rule for research.
v Notice and Other Individual Rights
Privacy practices notice
. Each covered entity, with certain exceptions, must provide a notice of its privacy practices.40 The Privacy Rule requires that the notice contain certain elements. The notice must describe the ways in which the covered entity may use and disclose protected health information. The notice must state the covered entity’s duties to protect privacy, provide a notice of privacy practices, and abide by the terms of the current notice. The notice must describe individuals’ rights, including the right to complain to HHS and to the covered entity if they believe their privacy rights have been violated. The notice must include a point of contact for further information and for making complaints to the covered entity. Covered entities must act in accordance with their notices. The Rule also contains specific distribution requirements for direct treatment providers, all other health care providers, and health plans. See OCR "Notice" Guidance.¨
Notice distribution. A covered health care provider with a direct treatment relationship with individuals must deliver a privacy practices notice to patients starting April 14, 2003 as follows:|
Not later than the first service encounter by personal delivery (for patient visits), by automatic and contemporaneous electronic response (for electronic service delivery), and by prompt mailing (for telephonic service delivery); | |
|
By posting the notice at each service delivery site in a clear and prominent place where people seeking service may reasonably be expected to be able to read the notice; and | |
|
In emergency treatment situations, the provider must furnish its notice as soon as practicable after the emergency abates. |
Covered entities, whether direct treatment providers or indirect treatment providers (such as laboratories) or health plans must supply notice to anyone
41 on request.41 A covered entity must also make its notice electronically available on any web site it maintains for customer service or benefits information.The covered entities in an organized health care arrangement may use a joint privacy practices notice, as long as each agrees to abide by the notice content with respect to the protected health information created or received in connection with participation in the arrangement.
42 Distribution of a joint notice by any covered entity participating in the organized health care arrangement at the first point that an OHCA member has an obligation to provide notice satisfies the distribution obligation of the other participants in the organized health care arrangement.A health plan must distribute its privacy practices notice to each of its enrollees by its Privacy Rule compliance date. Thereafter, the health plan must give its notice to each new enrollee at enrollment, and send a reminder to every enrollee at least once every three years that the notice is available upon request. A health plan satisfies its distribution obligation by furnishing the notice to the "named insured," that is, the subscriber for coverage that also applies to spouses and dependents. *
¨
Acknowledgment of notice receipt. A covered health care provider with a direct treatment relationship with individuals must make a good faith effort to obtain written acknowledgments from patients of receipt of the privacy practices notice.43 The Privacy Rule does not prescribe any particular content for the acknowledgments. The provider must document the reason for any failure to obtain the patient’s written acknowledgments. The provider is relieved of the need to request acknowledgments in an emergency treatment situation.Access. Except in certain circumstances, individuals have the right to review and obtain a copy of their protected health information in a covered entity’s designated record set.
44 The "designated record set" is that group of records maintained by or for a covered entity that is used, in whole or part, to make decisions about individuals, or that is a provider’s medical and billing records about individuals, or a health plan’s enrollment, payment, claims adjudication, and case or medical management record systems.45 The Rule exempts from the right of access the following protected health information: psychotherapy notes, information compiled for legal proceedings, laboratory results to which the Clinical Laboratory Improvement Act (CLIA) prohibits access, or information held by certain research laboratories. For information included within the right of access, covered entities may deny an individual access in certain specified situations, such as when a health care professional believes access could cause harm to the individual or another. In such situations, the individual must be given the right to have such denials reviewed by a licensed health care professional for a second opinion. 46 Covered entities may impose reasonable, cost-based fees for the cost of copying and postage. See OCR "Miscellaneous" Guidance.Amendment. The Rule gives individuals the right to have covered entities amend their protected health information in a designated record set when that information is inaccurate or incomplete.
47 If a covered entity accepts an amendment request, it must make reasonable efforts to provide the amendment to persons that the individual has identified as needing it, and to persons that the covered entity knows might rely on the information to the individual’s detriment.48 If the request is denied, covered entities must provide the individual with a written denial and allow the individual to submit a statement of disagreement for inclusion in the record. The Rule specifies processes for requesting and responding to a request for amendment. A covered entity must amend protected health information in its designated record set upon receipt of notice to amend from another covered entity.Disclosure accounting. Individuals have a right to an accounting of the disclosures of their protected health information by a covered entity or the covered entity’s business associates.
49 The maximum disclosure accounting period is the six years immediately preceding the accounting request, except a covered entity is not obligated to account for any disclosure made before its Privacy Rule compliance date.The Privacy Rule does not require accounting for disclosures: (a) for treatment, payment, or health care operations; (b) to the individual or the individual’s personal representative; (c) for notification of or to persons involved in an individual’s health care or payment for health care, for disaster relief, or for facility directories; (d) pursuant to an authorization; (e) of a limited data set; (f) for national security or intelligence purposes; (g) to correctional institutions or law enforcement officials for certain purposes regarding inmates or individuals in lawful custody; or (h) incident to otherwise permitted or required uses or disclosures. Accounting for disclosures to health oversight agencies and law enforcement officials must be temporarily suspended on their written representation that an accounting would likely impede their activities.
Restriction request. Individuals have the right to request that a covered entity restrict use or disclosure of protected health information for treatment, payment or health care operations, disclosure to persons involved in the individual’s health care or payment for health care, or disclosure to notify family members or others about the individual’s general condition, location, or death.
50 A covered entity is under no obligation to agree to requests for restrictions. A covered entity that does agree must comply with the agreed restrictions, except for purposes of treating the individual in a medical emergency.51Confidential communications requirements. Health plans and covered health care providers must permit individuals to request an alternative means or location for receiving communications of protected health information by means other than those that the covered entity typically employs.
52 For example, an individual may request that the provider communicate with the individual through a designated address or phone number. Similarly, an individual may request that the provider send communications in a closed envelope rather than a post card. Health plans must accommodate reasonable requests if the individual indicates that the disclosure of all or part of the protected health information could endanger the individual. The health plan may not question the individual’s statement of endangerment. Any covered entity may condition compliance with a confidential communication request on the individual specifying an alternative address or method of contact and explaining how any payment will be handled.v Administrative Requirements
HHS recognizes that covered entities range from the smallest provider to the largest, multi-state health plan. Therefore the flexibility and stability of the Rule are intended to allow covered entities to analyze their own needs and implement solutions appropriate for their own environment. What is appropriate for a particular covered entity will depend on the nature of the covered entity’s business, as well as the covered entity’s size and resources.
Privacy policies and procedures. A covered entity must develop and implement written privacy policies and procedures that are consistent with the Privacy Rule.53
Privacy personnel. A covered entity must designate a privacy official responsible for developing and implementing its privacy policies and procedures, and a contact person or contact office responsible for receiving complaints and providing individuals with information on the covered entity’s privacy practices.54
Workforce training and management. A covered entity must train all workforce members on its privacy policies and procedures, as necessary and appropriate for them to carry out their functions.55 A covered entity must have and apply appropriate sanctions against workforce members who violate its privacy policies and procedures or the Privacy Rule.56
Mitigation. A covered entity must mitigate, to the extent practicable, any harmful effect it learns was caused by use or disclosure of protected health information by its workforce or its business associates in violation of its privacy policies and procedures or the Privacy Rule.57
Data safeguards. A covered entity must maintain reasonable and appropriate administrative, technical, and physical safeguards to prevent intentional or unintentional use or disclosure of protected health information in violation of the Privacy Rule and to limit its incidental use and disclosure pursuant to otherwise permitted or required use or disclosure.58 For example, such safeguards might include shredding documents containing protected health information before discarding them, securing medical records with lock and key or pass code, and limiting access to keys or pass codes. See OCR "Incidental Uses and Disclosures" Guidance.
Complaints.
A covered entity must have procedures for individuals to complain about its compliance with its privacy policies and procedures and the Privacy Rule.59 The covered entity must explain those procedures in its privacy practices notice.60 Among other things, the covered entity must identify to whom individuals can submit complaints to at the covered entity and advise that complaints also can be submitted to the Secretary of HHS.Retaliation and waiver. A covered entity may not retaliate against a person for exercising rights provided by the Privacy Rule, for assisting in an investigation by HHS or another appropriate authority, or for opposing an act or practice that the person believes in good faith violates the Privacy Rule.
61 A covered entity may not require an individual to waive any right under the Privacy Rule as a condition for obtaining treatment, payment, enrollment or benefits eligibility. 62Documentation and record retention. A covered entity must maintain, until six years after the latter of the date of their creation or last effective date, its privacy policies and procedures, its privacy practices notices, disposition of complaints, and other actions, activities, and designations that the Privacy Rule requires to be documented.
63Fully-insured group health plan exception. The only administrative obligations with which a fully-insured group health plan that has no more than enrollment data and summary health information is required to comply are the (1) ban on retaliatory acts and waiver of individual rights, and (2) documentation requirements with respect to plan documents if such documents are amended to provide for the disclosure of protected health information to the plan sponsor by a health insurance issuer or HMO that services the group health plan.
64v
Organizational OptionsThe Rule contains provisions that address a variety of organizational issues that may affect the operation of the privacy protections.
Hybrid entity. The Privacy Rule permits a covered entity that is a single legal entity and that conducts both covered and non-covered functions to elect to be a "hybrid entity."
65 (The activities that make a person or organization a covered entity are its "covered functions."66) To be a hybrid entity, the covered entity must designate in writing its operations that perform covered functions as one or more "health care components." After making this designation, most of the requirements of the Privacy Rule will apply only to the health care components. A covered entity that does not make this designation is subject in its entirety to the Privacy Rule.Affiliated covered entity. Legally separate covered entities that are affiliated by common ownership or control may designate themselves (including their health care components) as a single covered entity for Privacy Rule compliance.
67 The designation must be in writing. An affiliated covered entity that performs multiple covered functions must operate its different covered functions in compliance with the Privacy Rule provisions applicable to those covered functions.Organized health care arrangement. The Privacy Rule identifies relationships in which participating covered entities share protected health information to manage and benefit their common enterprise as "organized health care arrangements."
68 Covered entities in an organized health care arrangement can share protected health information with each other for the arrangement’s joint health care operations.69Covered entities with multiple covered functions. A covered entity that performs multiple covered functions must operate its different covered functions in compliance with the Privacy Rule provisions applicable to those covered functions.
70 The covered entity may not use or disclose the protected health information of an individual who receives services from one covered function (e.g., health care provider) for another covered function (e.g., health plan) if the individual is not involved with the other function.Group health plan disclosures to plan sponsors. A group health plan and the health insurer or HMO offered by the plan may disclose the following protected health information to the "plan sponsor"—the employer, union, or other employee organization that sponsors and maintains the group health plan
71:|
Enrollment or disenrollment information with respect to the group health plan or a health insurer or HMO offered by the plan. | |
|
If requested by the plan sponsor, summary health information for the plan sponsor to use to obtain premium bids for providing health insurance coverage through the group health plan, or to modify, amend, or terminate the group health plan. "Summary health information" is information that summarizes claims history, claims expenses, or types of claims experience of the individuals for whom the plan sponsor has provided health benefits through the group health plan, and that is stripped of all individual identifiers other than five digit zip code (though it need not qualify as de-identified protected health information). | |
| Protected health information of the group health plan’s enrollees for the plan sponsor to perform plan administration functions. The plan must receive certification from the plan sponsor that the group health plan document has been amended to impose restrictions on the plan sponsor’s use and disclosure of the protected health information. These restrictions must include the representation that the plan sponsor will not use or disclose the protected health information for any employment-related action or decision or in connection with any other benefit plan. |
v Other Provisions: Personal Representatives and Minors' Personal Representatives
Personal representatives. The Privacy Rule requires a covered entity to treat a "personal representative" the same as the individual, with respect to uses and disclosures of the individual’s protected health information, as well as the individual’s rights under the Rule.
72 A personal representative is a person legally authorized to make health care decisions on an individual’s behalf or to act for a deceased individual or the estate. The Privacy Rule permits an exception when a covered entity has a reasonable belief that the personal representative may be abusing or neglecting the individual, or that treating the person as the personal representative could otherwise endanger the individual.Special case: minors. In most cases, parents are the personal representatives for their minor children. Therefore, in most cases, parents can exercise individual rights, such as access to the medical record, on behalf of their minor children. In certain exceptional cases, the parent is not considered the personal representative. In these situations, the Privacy Rule defers to State and other law to determine the rights of parents to access and control the protected health information of their minor children. If State and other law is silent concerning parental access to the minor’s protected health information, a covered entity has discretion to provide or deny a parent access to the minor’s health information, provided the decision is made by a licensed health care professional in the exercise of professional judgment. See OCR "Personal Representatives" Guidance.
v State Laws
Preemption. In general, State laws that are contrary to the Privacy Rule are preempted by the federal requirements, which means that the federal requirements will apply.
73 "Contrary" means that it would be impossible for a covered entity to comply with both the State and federal requirements, or that the provision of State law is an obstacle to accomplishing the full purposes and objectives of the Administrative Simplification provisions of HIPAA.74 The Privacy Rule provides exceptions to the general rule of federal preemption for contrary State laws that (1) relate to the privacy of individually identifiable health information and provide greater privacy protections or privacy rights with respect to such information; (2) provide for the reporting of disease or injury, child abuse, birth, or death, or for public health surveillance, investigation, or intervention; or (3) require certain health plan reporting, such as for management or financial audits.Exception determination. In addition, preemption of a contrary State law will not occur if HHS determines, in response to a request from a State or other entity or person, that the State law:
|
Is necessary to prevent fraud and abuse related to the provision of or payment for health care; | |
|
Is necessary to ensure appropriate State regulation of insurance and health plans to the extent expressly authorized by statute or regulation; | |
|
Is necessary for State reporting on health care delivery or costs; | |
|
Is necessary for purposes of serving a compelling public health, safety, or welfare need, and, if a Privacy Rule provision is at issue, if the Secretary determines that the intrusion into privacy is warranted when balanced against the need to be served; or | |
| Has as its principal purpose the regulation of the manufacture, registration, distribution, dispensing, or other control of any controlled substances (as defined in 21 U.S.C. 802), or that is deemed a controlled substance by State law. |
v Enforcement and Penalties for Noncompliance
Compliance. Consistent with the principles for achieving compliance provided in the Rule, HHS will seek the cooperation of covered entities and may provide technical assistance to help them comply voluntarily with the Rule.75 The Rule provides processes for persons to file complaints with HHS, describes the responsibilities of covered entities to provide records and compliance reports and to cooperate with, and permit access to information for, investigations and compliance reviews.
Civil money penalties. HHS may impose civil money penalties on a covered entity of $100 per failure to comply with a Privacy Rule requirement.76 That penalty may not exceed $25,000 per year for multiple violations of the identical Privacy Rule requirement in a calendar year. HHS may not impose a civil money penalty under specific circumstances, such as when a violation is due to reasonable cause and did not involve willful neglect and the covered entity corrected the violation within 30 days of when it knew or should have known of the violation.
Criminal penalties. A person who knowingly obtains or discloses individually identifiable health information in violation of HIPAA faces a fine of $50,000 and up to one-year imprisonment.77 The criminal penalties increase to $100,000 and up to five years imprisonment if the wrongful conduct involves false pretenses, and to $250,000 and up to ten years imprisonment if the wrongful conduct involves the intent to sell, transfer, or use individually identifiable health information for commercial advantage, personal gain, or malicious harm. Criminal sanctions will be enforced by the Department of Justice.
v Compliance Dates
Compliance schedule. All covered entities, except "small health plans," must be compliant with the Privacy Rule by April 14, 2003.
78 Small health plans, however, have until April 14, 2004 to comply.Small health plans.
A health plan with annual receipts of not more than $5 million is a small health plan.79 Health plans that file certain federal tax returns and report receipts on those returns should use the guidance provided by the Small Business Administration at 13 Code of Federal Regulations (CFR) 121.104 to calculate annual receipts. Health plans that do not report receipts to the Internal Revenue Service (IRS), for example, group health plans regulated by the Employee Retirement Income Security Act 1974 (ERISA) that are exempt from filing income tax returns, should use proxy measures to determine their annual receipts.80 See What constitutes a small health plan?:"v Copies of the Rule & Related Materials
The entire Privacy Rule, as well as guidance and additional materials, may be found on our website, http://www.hhs.gov/ocr/hipaa
End Notes
1
Pub. L. 104-191.2
65 FR 82462.3
67 FR 531827.4
45 C.F.R. §§ 160.102, 160.103.5
Even if an entity, such as a community health center, does not meet the definition of a health plan, it may, nonetheless, meet the definition of a health care provider, and, if it transmits health information in electronic form in connection with the transactions for which the Secretary of HHS has adopted standards under HIPAA, may still be a covered entity.6
45 C.F.R. §§ 160.102, 160.103; see Social Security Act § 1172(a)(3), 42 U.S.C. § 1320d -1(a)(3). The transaction standards are established by the HIPAA Transactions Rule at 45 C.F.R. Part 1627
45 C.F.R. § 160.103.8
45 C.F.R. § 164.500(b).9
45 C.F.R. § 160.103.10
45 C.F.R. §§ 164.502(e), 164.504(e).11
45 C.F.R. § 164.53212
45 C.F.R. § 160.103.13
45 C.F.R. § 160.10314
45 C.F.R. §§ 164.502(d)(2), 164.514(a) and (b).15
The following identifiers of the individual or of relatives, employers, or household members of the individual must be removed to achieve the "safe harbor" method of de-identification: (A) Names; (B) All geographic subdivisions smaller than a State, including street address, city, county, precinct, zip code, and their equivalent geocodes, except for the initial three digits of a zip code if, according to the current publicly available data from the Bureau of Census (1) the geographic units formed by combining all zip codes with the same three initial digits contains more than 20,000 people; and (2) the initial three digits of a zip code for all such geographic units containing 20,000 or fewer people is changed to 000; (C) All elements of dates (except year) for dates directly related to the individual, including birth date, admission date, discharge date, date of death; and all ages over 89 and all elements of dates (including year) indicative of such age, except that such ages and elements may be aggregated into a single category of age 90 or older; (D) Telephone numbers; (E) Fax numbers; (F) Electronic mail addresses: (G) Social security numbers; (H) Medical record numbers; (I) Health plan beneficiary numbers; (J) Account numbers; (K) Certificate/license numbers; (L) Vehicle identifiers and serial numbers, including license plate numbers; (M) Device identifiers and serial numbers; (N) Web Universal Resource Locators (URLs); (O) Internet Protocol (IP) address numbers; (P) Biometric identifiers, including finger and voice prints; (Q) Full face photographic images and any comparable images; and ® any other unique identifying number, characteristic, or code, except as permitted for re-identification purposes provided certain conditions are met. In addition to the removal of the above-stated identifiers, the covered entity may not have actual knowledge that the remaining information could be used alone or in combination with any other information to identify an individual who is subject of the information. 45 C.F.R. § 164.514(b).16
45 C.F.R. § 164.502(a).17
45 C.F.R. § 164.502(a)(2).18
45 C.F.R. § 164.502(a)(1).19
45 C.F.R. § 164.506(c).20
45 C.F.R. § 164.501.21
45 C.F.R. § 164.501.22
45 C.F.R. § 164.501.23
45 C.F.R. § 164.508(a)(2)24
45 C.F.R. § 164.506(b).25
45 C.F.R. § 164.510(a).26
45 C.F.R. § 164.510(b).27
45 C.F.R. §§ 164.502(a)(1)(iii).28
See 45 C.F.R. § 164.512.29
The Privacy Rule defines research as, "a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge." 45 C.F.R. § 164.501.30
45 C.F.R. § 164.512(i).31
45 CFR § 164.514(e).32
45 C.F.R. § 164.514(e). A limited data set is protected health information that excludes the following direct identifiers of the individual or of relatives, employers, or household members of the individual: (i) Names; (ii) Postal address information, other than town or city, State and zip code; (iii) Telephone numbers; (iv) Fax numbers; (v) Electronic mail addresses: (vi) Social security numbers; (vii) Medical record numbers; (viii) Health plan beneficiary numbers; (ix) Account numbers; (x) Certificate/license numbers; (xi) Vehicle identifiers and serial numbers, including license plate numbers; (xii) Device identifiers and serial numbers; (xiii) Web Universal Resource Locators (URLs); (xiv) Internet Protocol (IP) address numbers; (xv) Biometric identifiers, including finger and voice prints; (xvi) Full face photographic images and any comparable images. 45 C.F.R. § 164.514(e)(2).33
45 C.F.R. § 164.508. 34 A covered entity may condition the provision of health care solely to generate protected health information for disclosure to a third party on the individual giving authorization to disclose the information to the third party. For example, a covered entity physician may condition the provision of a physical examination to be paid for by a life insurance issuer on an individual’s authorization to disclose the results of that examination to the life insurance issuer. A health plan may condition enrollment or benefits eligibility on the individual giving authorization, requested before the individual’s enrollment, to obtain protected health information (other than psychotherapy notes) to determine the individual’s eligibility or enrollment or for underwriting or risk rating. A covered health care provider may condition treatment related to research (e.g., clinical trials) on the individual giving authorization to use or disclose the individual’s protected health information for the research. 45 C.F.R. 508(b)(4).35
45 CFR § 164.532.36
"Psychotherapy notes" means notes recorded (in any medium) by a health care provider who is a mental health professional documenting or analyzing the contents of conversation during a private counseling session or a group, joint, or family counseling session and that are separated from the rest of the of the individual’s medical record. Psychotherapy notes excludes medication prescription and monitoring, counseling session start and stop times, the modalities and frequencies of treatment furnished, results of clinical tests, and any summary of the following items: diagnosis, functional status, the treatment plan, symptoms, prognosis, and progress to date. 45 C.F.R. § 164.501.37 45 C.F.R. § 164.508(a)(2).
38
45 C.F.R. §§ 164.501 and 164.508(a)(3).39
45 C.F.R. §§ 164.502(b) and 164.514 (d).40
45 C.F.R. §§ 164.520(a) and (b). A group health plan, or a health insurer or HMO with respect to the group health plan, that intends to disclose protected health information (including enrollment data or summary health information) to the plan sponsor, must state that fact in the notice. Special statements are also required in the notice if a covered entity intends to contact individuals about health-related benefits or services, treatment alternatives, or appointment reminders, or for the covered entity’s own fundraising.41
45 C.F.R. § 164.520(c).42
45 C.F.R. § 164.520(d).43
45 C.F.R. § 164.520(c).44
45 C.F.R. § 164.524.45
45 C.F.R. § 164.501. 46 A covered entity may deny an individual access, provided that the individual is given a right to have such denials reviewed by a licensed health care professional (who is designated by the covered entity and who did not participate in the original decision to deny), when a licensed health care professional has determined, in the exercise of professional judgment, that: (a) the access requested is reasonably likely to endanger the life or physical safety of the individual or another person; (b) the protected health information makes reference to another person (unless such other person is a health care provider) and the access requested is reasonably likely to cause substantial harm to such other person; or (c) the request for access is made by the individual’s personal representative and the provision of access to such personal representative is reasonably likely to cause substantial harm to the individual or another person. A covered entity may deny access to individuals, without providing the individual an opportunity for review, in the following protected situations: (a) the protected health information falls under an exception to the right of access; (b) an inmate request for protected health information under certain circumstances; (c) information that a provider creates or obtains in the course of research that includes treatment for which the individual has agreed not to have access as part of consenting to participate in the research (as long as access to the information is restored upon completion of the research); (d) for records subject to the Privacy Act, information to which access may be denied under the Privacy Act, 5 U.S.C. § 552a; and (e) information obtained under a promise of confidentiality from a source other than a health care provider, if granting access would likely reveal the source. 45 C.F.R. § 164.524.47
45 C.F.R. § 164.526.48
Covered entities may deny an individual’s request for amendment only under specified circumstances. A covered entity may deny the request if it: (a) may exclude the information from access by the individual; (b) did not create the information (unless the individual provides a reasonable basis to believe the originator is no longer available); (c) determines that the information is accurate and complete; or (d) does not hold the information in its designated record set. 164.526(a)(2).49
45 C.F.R. § 164.528.50
45 C.F.R. § 164.522(a).51
45 C.F.R. § 164.522(a). In addition, a restriction agreed to by a covered entity is not effective under this subpart to prevent uses or disclosures permitted or required under §§ 164.502(a)(2)(ii), 164.510(a) or 164.512.52
45 C.F.R. § 164.522(b).53
45 C.F.R. § 164.530(i).54
45 C.F.R. § 164.530(a).55
45 C.F.R. § 164.530(b).56
45 C.F.R. § 164.530(e).57
45 C.F.R. § 164.530(f).58
45 C.F.R. § 164.530(c).59
45 C.F.R. § 164.530(d).60
45 C.F.R. § 164.520(b)(1)(vi).61
45 C.F.R. § 164.530(g).62
45 C.F.R. § 164.530(h).63
45 C.F.R. § 164.530(j).64
45 C.F.R. § 164.530(k).65
45 C.F.R. §§ 164.103, 164.105.66
45 C.F.R. § 164.103.67
45 C.F.R. §164.105. Common ownership exists if an entity possesses an ownership or equity interest of five percent or more in another entity; common control exists if an entity has the direct or indirect power significantly to influence or direct the actions or policies of another entity. 45 C.F.R. §§ 164.103.68
The Privacy Rule at 45 C.F.R. § 1640.103 identifies five types of organized health care arrangements:|
A clinically-integrated setting where individuals typically receive health care from more than one provider. | |
|
An organized system of health care in which the participating covered entities hold themselves out to the public as part of a joint arrangement and jointly engage in utilization review, quality assessment and improvement activities, or risk-sharing payment activities. | |
|
A group health plan and the health insurer or HMO that insures the plan’s benefits, with respect to protected health information created or received by the insurer or HMO that relates to individuals who are or have been participants or beneficiaries of the group health plan. | |
|
All group health plans maintained by the same plan sponsor. | |
|
All group health plans maintained by the same plan sponsor and all health insurers and HMOs that insure the plans’ benefits, with respect to protected health information created or received by the insurers or HMOs that relates to individuals who are or have been participants or beneficiaries in the group health plans. |
69
45 C.F.R. § 164.506(c)(5).70
45 C.F.R. § 164.504(g).71
45 C.F.R. § 164.504(f).72
45 C.F.R. § 164.502(g).73
45 C.F.R. §160.203.74
45 C.F.R. § 160.202.75
45 C.F.R.§ 160.30476
42 U.S.C. § 1320d-5.77
42 U.S.C. §1320d-6.78
45 C.F.R. § 164.534.79
45 C.F.R. § 160.103.80
Fully insured health plans should use the amount of total premiums that they paid for health insurance benefits during the plan’s last full fiscal year. Self-insured plans, both funded and unfunded, should use the total amount paid for health care claims by the employer, plan sponsor or benefit fund, as applicable to their circumstances, on behalf of the plan during the plan’s last full fiscal year. Those plans that provide health benefits through a mix of purchased insurance and self-insurance should combine proxy measures to determine their total annual receipts.v Incidental Uses Disclosures [ 45 CFR 164.502(a)(1)(iii)]
Background
Many customary health care communications and practices play an important or even essential role in ensuring that individuals receive prompt and effective health care. Due to the nature of these communications and practices, as well as the various environments in which individuals receive health care or other services from covered entities, the potential exists for an individual’s health information to be disclosed incidentally.
For example, a hospital visitor may overhear a provider’s confidential conversation with another provider or a patient, or may glimpse a patient’s information on a sign-in sheet or nursing station white board.
The HIPAA Privacy Rule is not intended to impede these customary and essential communications and practices and, thus, does not require that all risk of incidental use or disclosure be eliminated to satisfy its standards. Rather, the Privacy Rule permits certain incidental uses and disclosures of protected health information to occur when the covered entity has in place reasonable safeguards and minimum necessary policies and procedures to protect an individual’s privacy.
How the Rule Works
General provision. The Privacy Rule permits certain incidental uses and disclosures that occur as a by-product of another permissible or required use or disclosure, as long as the covered entity has applied reasonable safeguards and implemented the minimum necessary standard, where applicable, with respect to the primary use or disclosure. See 45 CFR 164.502(a)(1)(iii). An incidental use or disclosure is a secondary use or disclosure that cannot reasonably be prevented, is limited in nature, and that occurs as a result of another use or disclosure that is permitted by the Rule. However, an incidental use or disclosure is not permitted if it is a by product of an underlying use or disclosure which violates the Privacy Rule.
Reasonable safeguards. A covered entity must have in place appropriate administrative, technical, and physical safeguards that protect against uses and disclosures not permitted by the Privacy Rule, as well as that limit incidental uses or disclosures. See 45 CFR 164.530(c). It is not expected that a covered entity’s safeguards guarantee the privacy of protected health information from any and all potential risks. Reasonable safeguards will vary from covered entity to covered entity depending on factors, such as the size of the covered entity and the nature of its business. In implementing reasonable safeguards, covered entities should analyze their own needs and circumstances, such as the nature of the protected health information it holds, and assess the potential risks to patients’ privacy. Covered entities should also take into account the potential effects on patient care and may consider other issues, such as the financial and administrative burden of implementing particular safeguards.
Many health care providers and professionals have long made it a practice to ensure reasonable safeguards for individuals’ health information – for instance:
|
By speaking quietly when discussing a patient’s condition with family members in a waiting room or other public area; | |
|
By avoiding using patients’ names in public hallways and elevators, and posting signs to remind employees to protect patient confidentiality; | |
|
By isolating or locking file cabinets or records rooms; or | |
|
By providing additional security, such as passwords, on computers maintaining personal information. |
Protection of patient confidentiality is an important practice for many health care and health information management professionals; covered entities can build upon those codes of conduct to develop the reasonable safeguards required by the Privacy Rule.
Minimum necessary. Covered entities also must implement reasonable minimum necessary policies and procedures that limit how much protected health information is used, disclosed, and requested for certain purposes. These minimum necessary policies and procedures also reasonably must limit who within the entity has access to protected health information, and under what conditions, based on job responsibilities and the nature of the business. The minimum necessary standard does not apply to disclosures, including oral disclosures, among health care providers for treatment purposes. For example, a physician is not required to apply the minimum necessary standard when discussing a patient’s medical chart information with a specialist at another hospital. See 45 CFR 164.502(b) and 164.514(d), and the fact sheet and frequently asked questions on this web site about the minimum necessary standard, for more information.
An incidental use or disclosure that occurs as a result of a failure to apply reasonable safeguards or the minimum necessary standard, where required, is not permitted under the Privacy Rule.
For example:
| The minimum necessary standard requires that a covered entity limit who within the entity has access to protected health information, based on who needs access to perform their job duties. If a hospital employee is allowed to have routine, unimpeded access to patients’ medical records, where such access is not necessary for the hospital employee to do his job, the hospital is not applying the minimum necessary standard. Therefore, any incidental use or disclosure that results from this practice, such as another worker overhearing the hospital employee’s conversation about a patient’s condition, would be an unlawful use or disclosure under the Privacy Rule. |
v Minimum Necessary [ 45 CFR 164.502(b), 164.514(d)]
Background
The minimum necessary standard, a key protection of the HIPAA Privacy Rule, is derived from confidentiality codes and practices in common use today. It is based on sound current practice that protected health information should not be used or disclosed when it is not necessary to satisfy a particular purpose or carry out a function. The minimum necessary standard requires covered entities to evaluate their practices and enhance safeguards as needed to limit unnecessary or inappropriate access to and disclosure of protected health information. The Privacy Rule’s requirements for minimum necessary are designed to be sufficiently flexible to accommodate the various circumstances of any covered entity.
How the Rule Works
The Privacy Rule generally requires covered entities to take reasonable steps to limit the use or disclosure of, and requests for, protected health information to the minimum necessary to accomplish the intended purpose. The minimum necessary standard does not apply to the following:
|
Disclosures to or requests by a health care provider for treatment purposes. | |
|
Disclosures to the individual who is the subject of the information. | |
|
Uses or disclosures made pursuant to an individual’s authorization. | |
|
Uses or disclosures required for compliance with the Health Insurance Portability and Accountability Act (HIPAA) Administrative Simplification Rules. | |
|
Disclosures to the Department of Health and Human Services (HHS) when disclosure of information is required under the Privacy Rule for enforcement purposes. | |
|
Uses or disclosures that are required by other law. |
The implementation specifications for this provision require a covered entity to develop and implement policies and procedures appropriate for its own organization, reflecting the entity’s business practices and work force. While guidance cannot anticipate every question or factual application of the minimum necessary standard to each specific industry context, where it would be generally helpful we will seek to provide additional clarification on this issue in the future. In addition, the Department will continue to monitor the workability of the minimum necessary standard and consider proposing revisions, where appropriate, to ensure that the Rule does not hinder timely access to quality health care.
Uses and disclosures of, and requests for, protected health information. For uses of protected health information, the covered entity’s policies and procedures must identify the persons or classes of persons within the covered entity who need access to the information to carry out their job duties, the categories or types of protected health information needed, and conditions appropriate to such access. For example, hospitals may implement policies that permit doctors, nurses, or others involved in treatment to have access to the entire medical record, as needed. Case-by-case review of each use is not required. Where the entire medical record is necessary, the covered entity’s policies and procedures must state so explicitly and include a justification.
For routine or recurring requests and disclosures, the policies and procedures may be standard protocols and must limit the protected health information disclosed or requested to that which is the minimum necessary for that particular type of disclosure or request. Individual review of each disclosure or request is not required.
For non-routine disclosures and requests, covered entities must develop reasonable criteria for determining and limiting the disclosure or request to only the minimum amount of protected health information necessary to accomplish the purpose of a non-routine disclosure or request. Non-routine disclosures and requests must be reviewed on an individual basis in accordance with these criteria and limited accordingly.
Of course, where protected health information is disclosed to, or requested by, health care providers for treatment purposes, the minimum necessary standard does not apply.
Reasonable reliance
. In certain circumstances, the Privacy Rule permits a covered entity to rely on the judgment of the party requesting the disclosure as to the minimum amount of information that is needed. Such reliance must be reasonable under the particular circumstances of the request. This reliance is permitted when the request is made by:|
A public official or agency who states that the information requested is the minimum necessary for a purpose permitted under 45 CFR 164.512 of the Rule, such as for public health purposes (45 CFR 164.512(b)). | |
|
Another covered entity. | |
|
A professional who is a workforce member or business associate of the covered entity holding the information and who states that the information requested is the minimum necessary for the stated purpose. | |
|
A researcher with appropriate documentation from an Institutional Review Board (IRB) or Privacy Board. |
The Rule does not require such reliance, however, and the covered entity always retains discretion to make its own minimum necessary determination for disclosures to which the standard applies.
v Personal Representatives [ 45 CFR 164.502(g)]
Background
The HIPAA Privacy Rule establishes a foundation of Federally-protected rights which permit individuals to control certain uses and disclosures of their protected health information. Along with these rights, the Privacy Rule provides individuals with the ability to access and amend this information, and the right to an accounting of certain disclosures. The Department recognizes that there may be times when individuals are legally or otherwise incapable of exercising their rights, or simply choose to designate another to act on their behalf with respect to these rights. Under the Rule, a person authorized (under State or other applicable law, e.g., tribal or military law) to act on behalf of the individual in making health care related decisions is the individual’s "personal representative." Section 164.502(g) provides when, and to what extent, the personal representative must be treated as the individual for purposes of the Rule. In addition to these formal designations of a personal representative, the Rule at 45 CFR 164.510(b) addresses situations in which persons are involved in the individual’s health care but are not expressly authorized to act on the individual’s behalf.
How the Rule Works
General provisions. Except as otherwise provided in 45 CFR 164.502(g), the Privacy Rule requires covered entities to treat an individual’s personal representative as the individual with respect to uses and disclosures of the individual’s protected health information, as well as the individual’s rights under the Rule.
The personal representative stands in the shoes of the individual and has the ability to act for the individual and exercise the individual’s rights. For instance, covered entities must provide the individual’s personal representative with an accounting of disclosures in accordance with 45 CFR 164.528, as well as provide the personal representative access to the individual’s protected health information in accordance with 45 CFR 164.524 to the extent such information is relevant to such representation. In addition to exercising the individual’s rights under the Rule, a personal representative may also authorize disclosures of the individual’s protected health information.
In general, the scope of the personal representative’s authority to act for the individual under the Privacy Rule derives from his or her authority under applicable law to make health care decisions for the individual. Where the person has broad authority to act on behalf of a living individual in making decisions related to health care, such as a parent with respect to a minor child or a legal guardian of a mentally incompetent adult, the covered entity must treat the personal representative as the individual for all purposes under the Rule, unless an exception applies. (See below with respect to abuse, neglect or endangerment situations, and the application of State law in the context of parents and minors). Where the authority to act for the individual is limited or specific to particular health care decisions, the personal representative is to be treated as the individual only with respect to protected health information that is relevant to the representation. For example, a person with an individual’s limited health care power of attorney regarding only a specific treatment, such as use of artificial life support, is that individual’s personal representative only with respect to protected health information that relates to that health care decision. The covered entity should not treat that person as the individual for other purposes, such as to sign an authorization for the disclosure of protected health information for marketing purposes. Finally, where the person has authority to act on behalf of a deceased individual or his estate, which does not have to include the authority to make decisions related to health care, the covered entity must treat the personal representative as the individual for all purposes under the Rule. State or other law should be consulted to determine the authority of the personal representative to receive or access the individual’s protected health information.
Who must be recognized as the individual’s personal representative. The following chart displays who must be recognized as the personal representative for a category of individuals:
| If the individual is: | The personal representative is: |
| An adult or an emancipated minor |
A person with legal authority to make health care decisions on behalf of the individual Examples: Health care power of attorney Court-appointed legal guardian General power of attorney |
| An unemancipated minor | A parent, guardian, or other person acting
in loco parentis with legal authority to make health
care decisions on behalf of the minor child
Exceptions: See parents and minors discussion below. |
| Deceased |
A person with legal authority to act on behalf of the decedent or the estate (not restricted to health care decisions) Examples: Executor of the estate Next of kin or other family member Durable power of attorney |
Parents and unemancipated minors. The Privacy Rule defers to State or other applicable laws that address the ability of a parent, guardian, or other person acting in loco parentis (collectively, "parent") to obtain health information about a minor child. In most cases under the Rule, the parent is the personal representative of the minor child and can exercise the minor’s rights with respect to protected health information, because the parent usually has the authority to make health care decisions about his or her minor child. Regardless of whether a parent is the personal representative, the Privacy Rule permits a covered entity to disclose to a parent, or provide the parent with access to, a minor child’s protected health information when and to the extent it is expressly permitted or required by State or other laws (including relevant case law). Likewise, the Privacy Rule prohibits a covered entity from disclosing a minor child’s protected health information to a parent, or providing a parent with access to, such information when and to the extent it is expressly prohibited under State or other laws (including relevant case law). Thus, State and other applicable law governs when such law explicitly requires, permits, or prohibits the disclosure of, or access to, the health information about a minor child. The Privacy Rule specifies three circumstances in which the parent is not the "personal representative" with respect to certain health information about his or her minor child.
The Privacy Rule specifies three circumstances in which the parent is not the "personal representative" with respect to certain health information about his or her minor child. These exceptions generally track the ability of certain minors to obtain specified health care without parental consent under State or other laws, or standards of professional practice. In these situations, the parent does not control the minor’s health care decisions, and thus under the Rule, does not control the protected health information related to that care. The three exceptional circumstances when a parent is not the minor’s personal representative are:
| When State or other law does not require the consent of a parent or other person before a minor can obtain a particular health care service, and the minor consents to the health care service; |
Example: A State law provides an adolescent the right to obtain mental health treatment without the consent of his or her parent, and the adolescent consents to such treatment without the parent’s consent.
| When a court determines or other law authorizes someone other than the parent to make treatment decisions for a minor; |
Example: A court may grant authority to make health care decisions for the minor to an adult other than the parent, to the minor, or the court may make the decision(s) itself.
| When a parent agrees to a confidential relationship between the minor and the physician. |
Example: A physician asks the parent of a 16-year-old if the physician can talk with the child confidentially about a medical condition and the parent agrees.
Even in these exceptional circumstances, where the parent is not the "personal representative" of the minor, the Privacy Rule defers to State or other laws that require, permit, or prohibit the covered entity to disclose to a parent, or provide the parent access to, a minor child’s protected health information. Further, in these situations, if State or other law is silent or unclear concerning parental access to the minor’s protected health information, a covered entity has discretion to provide or deny a parent with access to the minor’s health information, if doing so is consistent with State or other applicable law, and provided the decision is made by a licensed health care professional in the exercise of professional judgment.
Abuse, neglect, and endangerment situations. When a physician or other covered entity reasonably believes that an individual, including an unemancipated minor, has been or may be subjected to domestic violence, abuse or neglect by the personal representative, or that treating a person as an individual’s personal representative could endanger the individual, the covered entity may choose not to treat that person as the individual’s personal representative, if in the exercise of professional judgment, doing so would not be in the best interests of the individual. For example, if a physician reasonably believes that disclosing information about an incompetent elderly individual to the individual’s personal representative would endanger that individual, the Privacy Rule permits the physician to decline to make such disclosure.
v Business Associates [ 45 CFR 164.502(e), 164.504(e), 164.532(d) and (e)]
Background
By law, the HIPAA Privacy Rule applies only to covered entities– health plans, health care clearinghouses, and certain health care providers. However, most health care providers and health plans do not carry out all of their health care activities and functions by themselves. Instead, they often use the services of a variety of other persons or businesses. The Privacy Rule allows covered providers and health plans to disclose protected health information to these "business associates" if the providers or plans obtain satisfactory assurances that the business associate will use the information only for the purposes for which it was engaged by the covered entity, will safeguard the information from misuse, and will help the covered entity comply with some of the covered entity’s duties under the Privacy Rule. Covered entities may disclose protected health information to an entity in its role as a business associate only to help the covered entity carry out its health care functions – not for the business associate’s independent use or purposes, except as needed for the proper management and administration of the business associate.
How the Rule Works
General provision. The Privacy Rule requires that a covered entity obtain satisfactory assurances from its business associate that the business associate will appropriately safeguard the protected health information it receives or creates on behalf of the covered entity. The satisfactory assurances must be in writing, whether in the form of a contract or other agreement between the covered entity and the business associate.
What is a "business associate?" A "business associate" is a person or entity that performs certain functions or activities that involve the use or disclosure of protected health information on behalf of, or provides services to, a covered entity.
|
A member of the covered entity’s workforce is not a business associate. | |
|
A covered health care provider, health plan, or health care clearinghouse can be a business associate of another covered entity. |
The Privacy Rule lists some of the functions or activities, as well as the particular services, that make a person or entity a business associate, if the activity or service involves the use or disclosure of protected health information. The types of functions or activities that may make a person or entity a business associate include payment or health care operations activities, as well as other functions or activities regulated by the Administrative Simplification Rules.
|
Business associate functions and activities include: claims processing or administration; data analysis, processing or administration; utilization review; quality assurance; billing; benefit management; practice management; and repricing. | |
|
Business associate services are: legal; actuarial; accounting; consulting; data aggregation; management; administrative; accreditation; and financial. |
See the definition of "business associate" at 45 CFR 160.103.
Examples of business associates.
|
A third party administrator that assists a health plan with claims processing. | |
|
A CPA firm whose accounting services to a health care provider involve access to protected health information. | |
|
An attorney whose legal services to a health plan involve access to protected health information. | |
|
A consultant that performs utilization reviews for a hospital. | |
|
A health care clearinghouse that translates a claim from a nonstandard format into a standard transaction on behalf of a health care provider and forwards the processed transaction to a payer. | |
|
An independent medical transcriptionist that provides transcription services to a physician. | |
|
A pharmacy benefits manager that manages a health plan’s pharmacist network. |
Business associate contracts
. A covered entity’s contract or other written arrangement with its business associate must contain the elements specified at 45 CFR 164.504(e). For example, the contract must:|
Describe the permitted and required uses of protected health information by the business associate; | |
|
Provide that the business associate will not use or further disclose the protected health information other than as permitted or required by the contract or as required by law; and | |
|
Require the business associate to use appropriate safeguards to prevent a use or disclosure of the protected health information other than as provided for by the contract. |
Where a covered entity knows of a material breach or violation by the business associate of the contract or agreement, the covered entity is required to take reasonable steps to cure the breach or end the violation, and if such steps are unsuccessful, to terminate the contract or arrangement. If termination of the contract or agreement is not feasible, a covered entity is required to report the problem to the Department of Health and Human Services (HHS) Office for Civil Rights (OCR).
Sample business associate contract language is available on the HHS OCR Privacy of Health Information website at http://www.hhs.gov/ocr/hipaa/contractprov.html.
Transition provisions for existing contracts. Covered entities (other than small health plans) that have an existing contract (or other written agreement) with a business associate prior to October 15, 2002, are permitted to continue to operate under that contract for up to one additional year beyond the April 14, 2003 compliance date, provided that the contract is not renewed or modified prior to April 14, 2003. This transition period applies only to written contracts or other written arrangements. Oral contracts or other arrangements
are not eligible for the transition period. Covered entities with contracts that qualify are permitted to continue to operate under those contracts with their business associates until April 14, 2004, or until the contract is renewed or modified, whichever is sooner, regardless of whether the contract meets the Rule’s applicable contract requirements at 45 CFR 164.502(e) and 164.504(e). A covered entity must otherwise comply with the Privacy Rule, such as making only permissible disclosures to the business associate and permitting individuals to exercise their rights under the Rule.See 45 CFR 164.532(d) and (e).
Exceptions to the business associate standard. The Privacy Rule includes the following exceptions to the business associate standard. See 45 CFR 164.502(e). In these situations, a covered entity is not required to have a business associate contract or other written agreement in place before protected health information may be disclosed to the person or entity.
| Disclosures by a covered entity to a health care provider for treatment of the individual. |
For example:
o
A hospital is not required to have a business associate contract with the specialist to whom it refers a patient and transmits the patient’s medical chart for treatment purposes.o
A physician is not required to have a business associate contract with a laboratory as a condition of disclosing protected health information for the treatment of an individual.o
A hospital laboratory is not required to have a business associate contract to disclose protected health information to a reference laboratory for treatment of the individual.|
Disclosures to a health plan sponsor, such as an employer, by a group health plan, or by the health insurance issuer or HMO that provides the health insurance benefits or coverage for the group health plan, provided that the group health plan’s documents have been amended to limit the disclosures or one of the exceptions at 45 CFR 164.504(f) have been met. | |
|
The collection and sharing of protected health information by a health plan that is a public benefits program, such as Medicare, and an agency other than the agency administering the health plan, such as the Social Security Administration, that collects protected health information to determine eligibility or enrollment, or determines eligibility or enrollment, for the government program, where the joint activities are authorized by law. |
Other situations in which a business associate contract is NOT required.
|
When a health care provider discloses protected health information to a health plan for payment purposes, or when the health care provider simply accepts a discounted rate to participate in the health plan’s network. A provider that submits a claim to a health plan and a health plan that assesses and pays the claim are each acting on its own behalf as a covered entity, and not as the "business associate" of the other. | |
|
With persons or organizations (e.g., janitorial service or electrician) whose functions or services do not involve the use or disclosure of protected health information, and where any access to protected health information by such persons would be incidental, if at all. | |
|
With a person or organization that acts merely as a conduit for protected health information, for example, the US Postal Service, certain private couriers, and their electronic equivalents. | |
|
Among covered entities who participate in an organized health care arrangement (OHCA) to make disclosures that relate to the joint health care activities of the OHCA. | |
|
Where a group health plan purchases insurance from a health insurance issuer or HMO. The relationship between the group health plan and the health insurance issuer or HMO is defined by the Privacy Rule as an OHCA, with respect to the individuals they jointly serve or have served. Thus, these covered entities are permitted to share protected health information that relates to the joint health care activities of the OHCA. | |
|
Where one covered entity purchases a health plan product or other insurance, for example, reinsurance, from an insurer. Each entity is acting on its own behalf when the covered entity purchases the insurance benefits, and when the covered entity submits a claim to the insurer and the insurer pays the claim. | |
|
To disclose protected health information to a researcher for research purposes, either with patient authorization, pursuant to a waiver under 45 CFR 164.512(i), or as a limited data set pursuant to 45 CFR 164.514(e). Because the researcher is not conducting a function or activity regulated by the Administrative Simplification Rules, such as payment or health care operations, or providing one of the services listed in the definition of "business associate" at 45 CFR 160.103, the researcher is not a business associate of the covered entity, and no business associate agreement is required. | |
|
When a financial institution processes consumer-conducted financial transactions by debit, credit, or other payment card, clears checks, initiates or processes electronic funds transfers, or conducts any other activity that directly facilitates or effects the transfer of funds for payment for health care or health plan premiums. When it conducts these activities, the financial institution is providing its normal banking or other financial transaction services to its customers; it is not performing a function or activity for, or on behalf of, the covered entity. |
v Uses and Disclosures for Treatment, Payment, and Health Care Operations
Background
The HIPAA Privacy Rule establishes a foundation of Federal protection for personal health information, carefully balanced to avoid creating unnecessary barriers to the delivery of quality health care. As such, the Rule generally prohibits a covered entity from using or disclosing protected health information unless authorized by patients, except where this prohibition would result in unnecessary interference with access to quality health care or with certain other important public benefits or national priorities.
Ready access to treatment and efficient payment for health care, both of which require use and disclosure of protected health information, are essential to the effective operation of the health care system. In addition, certain health care operations—such as administrative, financial, legal, and quality improvement activities—conducted by or for health care providers and health plans, are essential to support treatment and payment. Many individuals expect that their health information will be used and disclosed as necessary to treat them, bill for treatment, and, to some extent, operate the covered entity’s health care business. To avoid interfering with an individual’s access to quality health care or the efficient payment for such health care, the Privacy Rule permits a covered entity to use and disclose protected health information, with certain limits and protections, for treatment, payment, and health care operations activities.
How the Rule Works
What are treatment, payment, and health care operations? The core health care activities of "Treatment," "Payment," and "Health Care Operations" are defined in the Privacy Rule at 45 CFR 164.501.
|
"Treatment" generally means the provision, coordination, or management of health care and related services among health care providers or by a health care provider with a third party, consultation between health care providers regarding a patient, or the referral of a patient from one health care provider to another. | |
|
"Payment" encompasses the various activities of health care providers to obtain payment or be reimbursed for their services and of a health plan to obtain premiums, to fulfill their coverage responsibilities and provide benefits under the plan, and to obtain or provide reimbursement for the provision of health care. |
In addition to the general definition, the Privacy Rule provides examples of common payment activities which include, but are not limited to:
o Determining eligibility or coverage under a plan and adjudicating claims; o Risk adjustments; o Billing and collection activities; o Reviewing health care services for medical necessity, coverage, justification of charges, and the like;o Utilization review activities; and
o Disclosures to consumer reporting agencies (limited to specified identifying information about the individual, his or her payment history, and identifying information about the covered entity).
| "Health care operations" are certain administrative, financial, legal, and quality improvement activities of a covered entity that are necessary to run its business and to support the core functions of treatment and payment. These activities, which are limited to the activities listed in the definition of "health care operations" at 45 CFR 164.501, include: |
o
Conducting quality assessment and improvement activities, population- based activities relating to improving health or reducing health care costs, and case management and care coordination;o Reviewing the competence or qualifications of health care professionals, evaluating provider and health plan performance, training health care and non-health care professionals, accreditation, certification, licensing, or credentialing activities;
o Underwriting and other activities relating to the creation, renewal, or replacement of a contract of health insurance or health benefits, and ceding, securing, or placing a contract for reinsurance of risk relating to health care claims;
o Conducting or arranging for medical review, legal, and auditing services, including fraud and abuse detection and compliance programs;
o Business planning and development, such as conducting cost-management and planning analyses related to managing and operating the entity; and
o Business management and general administrative activities, including those related to implementing and complying with the Privacy Rule and other Administrative Simplification Rules, customer service, resolution of internal grievances, sale or transfer of assets, creating de-identified health information or a limited data set, and fundraising for the benefit of the covered entity.
General provisions at 45 CFR 164.506. A covered entity may, without the individual’s authorization:
| Use or disclose protected health information for its own treatment, payment, and health care operations activities. |
For example:
o
A hospital may use protected health information about an individual to provide health care to the individual and may consult with other health care providers about the individual’s treatment.o
A health care provider may disclose protected health information about an individual as part of a claim for payment to a health plan.o
A health plan may use protected health information to provide customer service to its enrollees.| A covered entity may disclose protected health information for the treatment activities of any health care provider (including providers not covered by the Privacy Rule). |
For example:
o
A primary care provider may send a copy of an individual’s medical record to a specialist who needs the information to treat the individual.o
A hospital may send a patient’s health care instructions to a nursing home to which the patient is transferred.| A covered entity may disclose protected health information to another covered entity or a health care provider (including providers not covered by the Privacy Rule) for the payment activities of the entity that receives the information. |
For example:
o
A physician may send an individual’s health plan coverage information to a laboratory who needs the information to bill for services it provided to the physician with respect to the individual.o
A hospital emergency department may give a patient’s payment information to an ambulance service provider that transported the patient to the hospital in order for the ambulance provider to bill for its treatment services.| A covered entity may disclose protected health information to another covered entity for certain health care operation activities of the entity that receives the information if: |
- Each entity either has or had a relationship with the individual who is the subject of the information, and the protected health information pertains to the relationship; and
- The disclosure is for a quality-related health care operations activity (i.e., the activities listed in paragraphs (1) and (2) of the definition of "health care operations" at 45 CFR 164.501) or for the purpose of health care fraud and abuse detection or compliance.
For example:
o
A health care provider may disclose protected health information to a health plan for the plan’s Health Plan Employer Data and Information Set (HEDIS) purposes, provided that the health plan has or had a relationship with the individual who is the subject of the information.| A covered entity that participates in an organized health care arrangement (OHCA) may disclose protected health information about an individual to another covered entity that participates in the OHCA for any joint health care operations of the OHCA. |
For example:
o
The physicians with staff privileges at a hospital may participate in the hospital’s training of medical students.Uses and disclosures of psychotherapy notes. Except when psychotherapy notes are used by the originator to carry out treatment, or by the covered entity for certain other limited health care operations, uses and disclosures of psychotherapy notes for treatment, payment, and health care operations require the individual’s authorization. See 45 CFR 164.508(a)(2).
Minimum necessary. A covered entity must develop policies and procedures that reasonably limit its disclosures of, and requests for, protected health information for payment and health care operations to the minimum necessary. A covered entity also is required to develop role-based access policies and procedures that limit which members of its work-force may have access to protected health information for treatment, payment, and health care operations, based on those who need access to the information to do their jobs.
However, covered entities are not required to apply the minimum necessary standard to disclosures to or requests by a health care provider for treatment purposes. See the fact sheet and frequently asked questions on this web site about the minimum necessary standard for more information.
Consent. A covered entity may voluntarily choose, but is not required, to obtain the individual’s consent for it to use and disclose information about him or her for treatment, payment, and health care operations. A covered entity that chooses to have a consent process has complete discretion under the Privacy Rule to design a process that works best for its business and consumers.
A "consent" document is not a valid permission to use or disclose protected health information for a purpose that requires an "authorization" under the Privacy Rule (see 45 CFR 164.508), or where other requirements or conditions exist under the Rule for the use or disclosure of protected health information.
Right to request privacy protection. Individuals have the right to request restrictions on how a covered entity will use and disclose protected health information about them for treatment, payment, and health care operations. A covered entity is not required to agree to an individual’s request for a restriction, but is bound by any restrictions to which it agrees. See 45 CFR 164.522(a).
Individuals also may request to receive confidential communications from the covered entity, either at alternative locations or by alternative means. For example, an individual may request that her health care provider call her at her office, rather than her home. A health care provider must accommodate an individual’s reasonable request for such confidential communications. A health plan must accommodate an individual’s reasonable request for confidential communications, if the individual clearly states that not doing so could endanger him or her. See 45 CFR 164.522(b).
Notice. Any use or disclosure of protected health information for treatment, payment, or health care operations must be consistent with the covered entity’s notice of privacy practices. A covered entity is required to provide the individual with adequate notice of its privacy practices, including the uses or disclosures the covered entity may make of the individual’s information and the individual’s rights with respect to that information. See the fact sheet and frequently asked questions on this web site about the notice standard for more information.
v Marketing [45 CFR 164.501, 164.508(a)(3)]
Background
The HIPAA Privacy Rule gives individuals important controls over whether and how their protected health information is used and disclosed for marketing purposes. With limited exceptions, the Rule requires an individual’s written authorization before a use or disclosure of his or her protected health information can be made for marketing. So as not to interfere with core health care functions, the Rule distinguishes marketing communications from those communications about goods and services that are essential for quality health care.
How the Rule Works
The Privacy Rule addresses the use and disclosure of protected health information for marketing purposes by:
|
Defining what is "marketing" under the Rule; | |
|
Excepting from that definition certain treatment or health care operations activities; | |
|
Requiring individual authorization for all uses or disclosures of protected health information for marketing purposes with limited exceptions. |
What is "marketing"? The Privacy Rule defines "marketing" as making "a communication about a product or service that encourages recipients of the communication to purchase or use the product or service." Generally, if the communication is "marketing," then the communication can occur only if the covered entity first obtains an individual’s "authorization." This definition of marketing has certain exceptions, as discussed below.
Examples of "marketing" communications requiring prior authorization are:
|
A communication from a hospital informing former patients about a cardiac facility, that is not part of the hospital, that can provide a baseline EKG for $39, when the communication is not for the purpose of providing treatment advice. | |
|
A communication from a health insurer promoting a home and casualty insurance product offered by the same company. |
What else is "Marketing"? Marketing also means: "An arrangement between a covered entity and any other entity whereby the covered entity discloses protected health information to the other entity, in exchange for direct or indirect remuneration, for the other entity or its affiliate to make a communication about its own product or service that encourages recipients of the communication to purchase or use that product or service." This part of the definition to marketing has no exceptions. The individual must authorize these marketing communications before they can occur.
Simply put, a covered entity may not sell protected health information to a business associate or any other third party for that party’s own purposes. Moreover, covered entities may not sell lists of patients or enrollees to third parties without obtaining authorization from each person on the list.
For example, it is "marketing" when:
|
A health plan sells a list of its members to a company that sells blood glucose monitors, which intends to send the plan’s members brochures on the benefits of purchasing and using the monitors. | |
|
A drug manufacturer receives a list of patients from a covered health care provider and provides remuneration, then uses that list to send discount coupons for a new antidepressant medication directly to the patients. |
What is NOT "marketing"? The Privacy Rule carves out exceptions to the definition of marketing under the following three categories:
A communication is not "marketing" if it is made to describe a health-related product or service (or payment for such product or service) that is provided by, or included in a plan of benefits of, the covered entity making the communication, including communications about:
|
The entities participating in a health care provider network or health plan network; | |
|
Replacement of, or enhancements to, a health plan; and | |
|
Health-related products or services available only to a health plan enrollee that add value to, but are not part of, a plan of benefits. |
This exception to the marketing definition permits communications by a covered entity about its own products or services.
For example, under this exception, it is not "marketing" when:
|
A hospital uses its patient list to announce the arrival of a new specialty group (e.g., orthopedic) or the acquisition of new equipment (e.g., x-ray machine or magnetic resonance image machine) through a general mailing or publication. | |
|
A health plan sends a mailing to subscribers approaching Medicare eligible age with materials describing its Medicare supplemental plan and an application form. |
A communication is not "marketing" if it is made for treatment of the individual.
For example, under this exception, it is not "marketing" when:
|
A pharmacy or other health care provider mails prescription refill reminders to patients, or contracts with a mail house to do so. | |
|
A primary care physician refers an individual to a specialist for a follow-up test or provides free samples of a prescription drug to a patient. |
A communication is not "marketing" if it is made for case management or care coordination for the individual, or to direct or recommend alternative treatments, therapies, health care providers, or settings of care to the individual.
For example, under this exception, it is not "marketing" when:
|
An endocrinologist shares a patient’s medical record with several behavior management programs to determine which program best suits the ongoing needs of the individual patient. | |
|
A hospital social worker shares medical record information with various nursing homes in the course of recommending that the patient be transferred from a hospital bed to a nursing home. |
For any of the three exceptions to the definition of marketing, the activity must otherwise be permissible under the Privacy Rule, and a covered entity may use a business associate to make the communication. As with any disclosure to a business associate, the covered entity must obtain the business associate’s agreement to use the protected health information only for the communication activities of the covered entity.
Marketing authorizations and when authorizations are NOT necessary. Except as discussed below, any communication that meets the definition of marketing is not permitted, unless the covered entity obtains an individual’s authorization. To determine what constitutes an acceptable "authorization," see 45 CFR 164.508. If the marketing involves direct or indirect remuneration to the covered entity from a third party, the authorization must state that such remuneration is involved. See 45 CFR 164.508(a)(3)
.A communication does not require an authorization, even if it is marketing, if it is in the form of a face-to-face communication made by a covered entity to an individual, or a promotional gift of nominal value is provided by the covered entity.
For example, no prior authorization is necessary when:
|
A hospital provides a free package of formula and other baby products to new mothers as they leave the maternity ward. | |
|
An insurance agent sells a health insurance policy in person to a customer and proceeds to also market a casualty and life insurance policy as well. |
v Disclosure For Public Health Activities [45 CFR 164.512(b)]
Background
The HIPAA Privacy Rule recognizes the legitimate need for public health authorities and others responsible for ensuring public health and safety to have access to protected health information to carry out their public health mission. The Rule also recognizes that public health reports made by covered entities are an important means of identifying threats to the health and safety of the public at large, as well as individuals. Accordingly, the Rule permits covered entities to disclose protected health information without authorization for specified public health purposes.
How the Rule Works
General public health activities. The Privacy Rule permits covered entities to disclose protected health information, without authorization, to public health authorities who are legally authorized to receive such reports for the purpose of preventing or controlling disease, injury, or disability. This would include, for example, the reporting of a disease or injury; reporting vital events, such as births or deaths; and conducting public health surveillance, investigations, or interventions. See 45 CFR 164.512(b)(1)(i). Also, covered entities may, at the direction of a public health authority, disclose protected health information to a foreign government agency that is acting in collaboration with a public health authority. See 45 CFR 164.512(b)(1)(i). Covered entities who are also a public health authority may use, as well as disclose, protected health information for these public health purposes. See 45 CFR 164.512(b)(2).
A "public health authority" is an agency or authority of the United States government, a State, a territory, a political subdivision of a State or territory, or Indian tribe that is responsible for public health matters as part of its official mandate, as well as a person or entity acting under a grant of authority from, or under a contract with, a public health agency. See 45 CFR 164.501. Examples of a public health authority include State and local health departments, the Food and Drug Administration (FDA), the Centers for Disease Control and Prevention, and the Occupational Safety and Health Administration (OSHA).
Generally, covered entities are required reasonably to limit the protected health information disclosed for public health purposes to the minimum amount necessary to accomplish the public health purpose. However, covered entities are not required to make a minimum necessary determination for public health disclosures that are made pursuant to an individual’s authorization, or for disclosures that are required by other law. See 45 CFR 164.502(b). For disclosures to a public health authority, covered entities may reasonably rely on a minimum necessary determination made by the public health authority in requesting the protected health information. See 45 CFR 164.514(d)(3)( iii)(A). For routine and recurring public health disclosures, covered entities may develop standard protocols, as part of their minimum necessary policies and procedures, that address the types and amount of protected health information that may be disclosed for such purposes. See 45 CFR 164.514(d)(3)(i).
Other public health activities. The Privacy Rule recognizes the important role that persons or entities other than public health authorities play in certain essential public health activities. Accordingly, the Rule permits covered entities to disclose protected health information, without authorization, to such persons or entities for the public health activities discussed below.
|
Child abuse or neglect. Covered entities may disclose protected health information to report known or suspected child abuse or neglect, if the report is made to a public health authority or other appropriate government authority that is authorized by law to receive such reports. For instance, the social services department of a local government might have legal authority to receive reports of child abuse or neglect, in which case, the Privacy Rule would permit a covered entity to report such cases to that authority without obtaining individual authorization. Likewise, a covered entity could report such cases to the police department when the police department is authorized by law to receive such reports. See 45 CFR 164.512(b)(1)(ii). See also 45 CFR 512(c) for information regarding disclosures about adult victims of abuse, neglect, or domestic violence. | |
|
Quality, safety or effectiveness of a product or activity regulated by the FDA. Covered entities may disclose protected health information to a person subject to FDA jurisdiction, for public health purposes related to the quality, safety or effectiveness of an FDA-regulated product or activity for which that person has responsibility. Examples of purposes or activities for which such disclosures may be made include, but are not limited to: |
o
Collecting or reporting adverse events (including similar reports regarding food and dietary supplements), product defects or problems (including problems regarding use or labeling), or biological product deviations;o
Tracking FDA-regulated products;o
Enabling product recalls, repairs, replacement or look back (which includes locating and notifying individuals who received recalled or withdrawn products or products that are the subject of look back); ando
Conducting postmarketing surveillance.See 45 CFR 164.512(b)(1)(iii). The "person" subject to the jurisdiction of the FDA does not have to be a specific individual. Rather, it can be an individual or an entity, such as a partnership, corporation, or association. Covered entities may identify the party or parties responsible for an FDA-regulated product from the product label, from written material that accompanies the product (know as labeling), or from sources of labeling, such as the Physician’s Desk Reference.
|
Persons at risk of contracting or spreading a disease. A covered entity may disclose protected health information to a person who is at risk of contracting or spreading a disease or condition if other law authorizes the covered entity to notify such individuals as necessary to carry out public health interventions or investigations. For example, a covered health care provider may disclose protected health information as needed to notify a person that (s)he has been exposed to a communicable disease if the covered entity is legally authorized to do so to prevent or control the spread of the disease. See 45 CFR 164.512(b)(1)(iv). | |
|
Workplace medical surveillance. A covered health care provider who provides a health care service to an individual at the request of the individual’s employer, or provides the service in the capacity of a member of the employer’s work-force, may disclose the individual’s protected health information to the employer for the purposes of workplace medical surveillance or the evaluation of work-related illness and injuries to the extent the employer needs that information to comply with OSHA, the Mine Safety and Health Administration (MSHA), or the requirements of State laws having a similar purpose. The information disclosed must be limited to the provider’s findings regarding such medical surveillance or work-related illness or injury. The covered health care provider must provide the individual with written notice that the information will be disclosed to his or her employer (or the notice may be posted at the work-site if that is where the service is provided). See 45 CFR 164.512(b)(1)(v). |
v Research
[45 CFR 164.501, 164.508, 164.512(i)] [ See also 45 CFR 164.514(e), 164.528, 164.532]
Background
The HIPAA Privacy Rule establishes the conditions under which protected health information may be used or disclosed by covered entities for research purposes. Research is defined in the Privacy Rule as, "a systematic investigation, including research, development, testing, and evaluation, designed to develop or contribute to generalizable knowledge." See 45 CFR 164.501. A covered entity may always use or disclose for research purposes health information which has been de-identified (in accordance with 45 CFR 164.502(d), and 164.514(a)-(c) of the Rule) without regard to the provisions below.
The Privacy Rule also defines the means by which individuals will be informed of uses and disclosures of their medical information for research purposes, and their rights to access information about them held by covered entities. Where research is concerned, the Privacy Rule protects the privacy of individually identifiable health information, while at the same time ensuring that researchers continue to have access to medical information necessary to conduct vital research. Currently, most research involving human subjects operates under the Common Rule (45 CFR Part 46, Subpart A) and/or the Food and Drug Administration’s (FDA) human subject protection regulations (21 CFR Parts 50 and 56), which have some provisions that are similar to, but separate from, the Privacy Rule’s provisions for research. These human subject protection regulations, which apply to most Federally-funded and to some privately funded research, include protections to help ensure the privacy of subjects and the confidentiality of information. The Privacy Rule builds upon these existing Federal protections. More importantly, the Privacy Rule creates equal standards of privacy protection for research governed by the existing Federal human subject regulations and research that is not.
How the Rule Works
In the course of conducting research, researchers may obtain, create, use, and/or disclose individually identifiable health information. Under the Privacy
Rule, covered entities are permitted to use and disclose protected health information for research with individual authorization, or without individual authorization under limited circumstances set forth in the Privacy Rule.
Research use/disclosure without authorization.
To use or disclose protected health information without authorization by the research participant, a covered entity must obtain one of the following:| Documented Institutional Review Board (IRB) or Privacy Board approval. Documentation that an alteration or waiver of research participants’ authorization for use/disclosure of information about them for research purposes has been approved by an IRB or a Privacy Board. See 45 CFR 164.512(i)(1)(i). This provision of the Privacy Rule might be used, for example, to conduct records research, when researchers are unable to use de-identified information, and the research could not practicably be conducted if research participants’ authorization were required. |
A covered entity may use or disclose protected health information for research purposes pursuant to a waiver of authorization by an IRB or Privacy Board, provided it has obtained documentation of all of the following:
o Identification of the IRB or Privacy Board and the date on which the alteration or waiver of authorization was approved;
o A statement that the IRB or Privacy Board has determined that the alteration or waiver of authorization, in whole or in part, satisfies the three criteria in the Rule;
o A brief description of the protected health information for which use or access has been determined to be necessary by the IRB or Privacy Board;
o A statement that the alteration or waiver of authorization has been reviewed and approved under either normal or expedited review procedures; and
o The signature of the chair or other member, as designated by the chair, of the IRB or the Privacy Board, as applicable.
The following three criteria must be satisfied for an IRB or Privacy Board to approve a waiver of authorization under the Privacy Rule:
o The use or disclosure of protected health information involves no more than a minimal risk to the privacy of individuals, based on, at least, the presence of the following elements:
an adequate plan to protect the identifiers from improper use and disclosure;
an adequate plan to destroy the identifiers at the earliest opportunity consistent with conduct of the research, unless there is a health or research justification for retaining the identifiers or such retention is otherwise required by law; and
adequate written assurances that the protected health information will not be reused or disclosed to any other person or entity, except as required by law, for authorized oversight of the research project, or for other research for which the use or disclosure of protected health information would be permitted by this subpart;
o
The research could not practicably be conducted without the waiver or alteration; ando
The research could not practicably be conducted without access to and use of the protected health information.|
Preparatory to research. Representations from the researcher, either in writing or orally, that the use or disclosure of the protected health information is solely to prepare a research protocol or for similar purposes preparatory to research, that the researcher will not remove any protected health information from the covered entity, and representation that protected health information for which access is sought is necessary for the research purpose. See 45 CFR 164.512(i)(1)(ii). This provision might be used, for example, to design a research study or to assess the feasibility of conducting a study. | |
|
Research on protected health information of decedents. Representations from the researcher, either in writing or orally, that the use or disclosure being sought is solely for research on the protected health information of decedents, that the protected health information being sought is necessary for the research, and, at the request of the covered entity, documentation of the death of the individuals about whom information is being sought. See 45 CFR 164.512(i)(1)(iii). |
o
Establish the permitted uses and disclosures of the limited data set by the recipient, consistent with the purposes of the research, and which may not include any use or disclosure that would violate the Rule if done by the covered entity;o
Limit who can use or receive the data; ando
Require the recipient to agree to the following:Not to use or disclose the information other than as permitted by the data use agreement or as otherwise required by law;
Use appropriate safeguards to prevent the use or disclosure of the information other than as provided for in the data use agreement;
Report to the covered entity any use or disclosure of the information not provided for by the data use agreement of which the recipient becomes aware;
Ensure that any agents, including a subcontractor, to whom the recipient provides the limited data set agrees to the same restrictions and conditions that apply to the recipient with respect to the limited data set; and
Not to identify the information or contact the individual.
Research use/disclosure with individual authorization.
The Privacy Rule also permits covered entities to use or disclose protected health information for research purposes when a research participant authorizes the use or disclosure of information about him or herself. Today, for example, a research participant’s authorization will typically be sought for most clinical trials and some records research. In this case, documentation of IRB or Privacy Board approval of a waiver of authorization is not required for the use or disclosure of protected health information.To use or disclose protected health information with authorization by the research participant, the covered entity must obtain an authorization that satisfies the requirements of 45 CFR 164.508. The Privacy Rule has a general set of authorization requirements that apply to all uses and disclosures, including those for research purposes. However, several special provisions apply to research authorizations:
|
Unlike other authorizations, an authorization for a research purpose may state that the authorization does not expire, that there is no expiration date or event, or that the authorization continues until the "end of the research study;" and | |
|
An authorization for the use or disclosure of protected health information for research may be combined with a consent to participate in the research, or with any other legal permission related to the research study. |
Accounting for research disclosures.
In general, the Privacy Rule gives individuals the right to receive an accounting of certain disclosures of protected health information made by a covered entity. See 45 CFR 164.528. This accounting must include disclosures of protected health information that occurred during the six years prior to the individual’s request for an accounting, or since the applicable compliance date (whichever is sooner), and must include specified information regarding each disclosure. A more general accounting is permitted for subsequent multiple disclosures to the same person or entity for a single purpose. See 45 CFR 36 164.528(b)(3). Among the types of disclosures that are exempt from this accounting requirement are:|
Research disclosures made pursuant to an individual’s authorization; | |
|
Disclosures of the limited data set to researchers with a data use agreement under 45 CFR 164.514(e). |
In addition, for disclosures of protected health information for research purposes without the individual’s authorization pursuant to 45 CFR164.512(i), and that involve at least 50 records, the Privacy Rule allows for a simplified accounting of such disclosures by covered entities. Under this simplified accounting provision, covered entities may provide individuals with a list of all protocols for which the patient’s protected health information may have been disclosed under 45 CFR 164.512(i), as well as the researcher’s name and contact information. Other requirements related to this simplified accounting provision are found in 45 CFR 164.528(b)(4).
Transition provisions
. Under the Privacy Rule, a covered entity may use and disclose protected health information that was created or received for research, either before or after the compliance date, if the covered entity obtained any one of the following prior to the compliance date:|
An authorization or other express legal permission from an individual to use or disclose protected health information for the research; | |
|
The informed consent of the individual to participate in the research; or | |
|
A waiver of informed consent by an IRB in accordance with the Common Rule or an exception under FDA’s human subject protection regulations at 21 CFR 50.24. |
However, if a waiver of informed consent was obtained prior to the compliance date, but informed consent is subsequently sought after the compliance date, the covered entity must obtain the individual’s authorization as required at 45 CFR 164.508. For example, if there was a temporary waiver of informed consent for emergency research under the FDA’s human subject protection regulations, and informed consent was later sought after the compliance date, individual authorization would be required before the covered entity could use or disclose protected health information for the research after the waiver of informed consent was no longer valid.
The Privacy Rule allows covered entities to rely on such express legal permission, informed consent, or IRB-approved waiver of informed consent, which they create or receive before the applicable compliance date, to use and disclose protected health information for specific research studies, as well as for future unspecified research that may be included in such permission.
v Disclosures For Workers' Compensation Purposes [45 CFR 164.512(1)
Background
The HIPAA Privacy Rule does not apply to entities that are either workers’ compensation insurers, workers’ compensation administrative agencies, or employers, except to the extent they may otherwise be covered entities. However, these entities need access to the health information of individuals who are injured on the job or who have a work-related illness to process or adjudicate claims, or to coordinate care under workers’ compensation systems. Generally, this health information is obtained from health care providers who treat these individuals and who may be covered by the Privacy Rule. The Privacy Rule recognizes the legitimate need of insurers and other entities involved in the workers’ compensation systems to have access to individuals’ health information as authorized by State or other law. Due to the significant variability among such laws, the Privacy Rule permits disclosures of health information for workers’ compensation purposes in a number of different ways.
How the Rule Works
Disclosures without individual authorization. The Privacy Rule permits covered entities to disclose protected health information to workers’ compensation insurers, State administrators, employers, and other persons or entities involved in workers’ compensation systems, without the individual’s authorization:
|
As authorized by and to the extent necessary to comply with laws relating to workers’ compensation or similar programs established by law that provide benefits for work-related injuries or illness without regard to fault. This includes programs established by the Black Lung Benefits Act, the Federal Employees’ Compensation Act, the Longshore and Harbor Workers’ Compensation Act, and the Energy Employees’ Occupational Illness Compensation Program Act. See 45 CFR 164.512(l). | |
|
To the extent the disclosure is required by State or other law. The disclosure must comply with and be limited to what the law requires. See 45 CFR 164.512(a). | |
|
For purposes of obtaining payment for any health care provided to the injured or ill worker. See 45 CFR 164.502(a)(1)(ii) and the definition of "payment" at 45 CFR 164.501. |
Disclosures with individual authorization
. In addition, covered entities may disclose protected health information to workers’ compensation insurers and others involved in workers’ compensation systems where the individual has provided his or her authorization for the release of the information to the entity. The authorization must contain the elements and otherwise meet the requirements specified at 45 CFR 164.508.Minimum necessary. Covered entities are required reasonably to limit the amount of protected health information disclosed under 45 CFR 164.512(l) to the minimum necessary to accomplish the workers’ compensation purpose. Under this requirement, protected health information may be shared for such purposes to the full extent authorized by State or other law.
In addition, covered entities are required reasonably to limit the amount of protected health information disclosed for payment purposes to the minimum necessary. Covered entities are permitted to disclose the amount and types of protected health information that are necessary to obtain payment for health care provided to an injured or ill worker.
Where a covered entity routinely makes disclosures for workers’ compensation purposes under 45 CFR 164.512(l) or for payment purposes, the covered entity may develop standard protocols as part of its minimum necessary policies and procedures that address the type and amount of protected health information to be disclosed for such purposes.
Where protected health information is requested by a State workers’ compensation or other public official, covered entities are permitted to reasonably rely on the official’s representations that the information requested is the minimum necessary for the intended purpose. See 45 CFR 164.514(d)(3)(iii)(A).
Covered entities are not required to make a minimum necessary determination when disclosing protected health information as required by State or other law, or pursuant to the individual’s authorization. See 45 CFR 164.502(b).
The Department will actively monitor the effects of the Privacy Rule, and in particular, the minimum necessary standard, on the workers’ compensation systems and consider proposing modifications, where appropriate, to ensure that the Rule does not have any unintended negative effects that disturb these systems.
v Notice of Privacy Practice For Protected Health Information [45 CFR 164.520]
Background
The HIPAA Privacy Rule gives individuals a fundamental new right to be informed of the privacy practices of their health plans and of most of their health care providers, as well as to be informed of their privacy rights with respect to their personal health information. Health plans and covered health care providers are required to develop and distribute a notice that provides a clear explanation of these rights and practices. The notice is intended to focus individuals on privacy issues and concerns, and to prompt them to have discussions with their health plans and health care providers and exercise their rights.
How the Rule Works
General rule. The Privacy Rule provides that an individual has a right to adequate notice of how a covered entity may use and disclose protected health information about the individual, as well as his or her rights and the covered entity’s obligations with respect to that information. Most covered entities must develop and provide individuals with this notice of their privacy practices.
The Privacy Rule does not require the following covered entities to develop a notice:
|
Health care clearinghouses, if the only protected health information they create or receive is as a business associate of another covered entity. See 45 CFR 164.500(b)(1). | |
|
A correctional institution that is a covered entity (e.g., that has a covered health care provider component). | |
|
A group health plan that provides benefits only through one or more contracts of insurance with health insurance issuers or HMOs, and that does not create or receive protected health information other than summary health information or enrollment or disenrollment information. See 45 CFR 164.520(a). |
Content of the notice
. Covered entities are required to provide a notice in plain language that describes:|
How the covered entity may use and disclose protected health information about an individual. | |
|
The individual’s rights with respect to the information and how the individual may exercise these rights, including how the individual may complain to the covered entity. | |
|
The covered entity’s legal duties with respect to the information, including a statement that the covered entity is required by law to maintain the privacy of protected health information. | |
|
Whom individuals can contact for further information about the covered entity’s privacy policies. |
The notice must include an effective date. See 45 CFR 164.520(b) for the specific requirements for developing the content of the notice.
A covered entity is required to promptly revise and distribute its notice whenever it makes material changes to any of its privacy practices. See 45 CFR 164.520(b)(3), 164.520(c)(1)(i)(C) for health plans, and 164.520(c)(2)(iv) for covered health care providers with direct treatment relationships with individuals.
Providing the notice.
|
A covered entity must make its notice available to any person who asks for it. | |
|
A covered entity must prominently post and make available its notice on any web site it maintains that provides information about its customer services or benefits. |
Health plans must also:
|
Provide the notice to individuals then covered by the plan no later than April 14, 2003 (April 14, 2004, for small health plans) and to new enrollees at the time of enrollment. | |
|
Provide a revised notice to individuals then covered by the plan within 60 days of a material revision. | |
|
Notify individuals then covered by the plan of the availability of and how to obtain the notice at least once every three years. |
Covered direct treatment providers must also:
|
Provide the notice to the individual no later than the date of first service delivery (after the April 14, 2003 compliance date of the Privacy Rule) and, except in an emergency treatment situation, make a good faith effort to obtain the individual’s written acknowledgment of receipt of the notice. If an acknowledgment cannot be obtained, the provider must document his or her efforts to obtain the acknowledgment and the reason why it was not obtained. | |
|
When first service delivery to an individual is provided over the Internet, through e-mail, or otherwise electronically, the provider must send an electronic notice automatically and contemporaneously in response to the individual’s first request for service. The provider must make a good faith effort to obtain a return receipt or other transmission from the individual in response to receiving the notice. | |
|
In an emergency treatment situation, provide the notice as soon as it is reasonably practicable to do so after the emergency situation has ended. In these situations, providers are not required to make a good faith effort to obtain a written acknowledgment from individuals. | |
|
Make the latest notice (i.e., the one that reflects any changes in privacy policies) available at the provider’s office or facility for individuals to request to take with them, and post it in a clear and prominent location at the facility. | |
|
A covered entity may e-mail the notice to an individual if the individual agrees to receive an electronic notice. |
See 45 CFR 164.520(c) for the specific requirements for providing the notice.
Organizational options
.|
Any covered entity, including a hybrid entity or an affiliated covered entity, may choose to develop more than one notice, such as when an entity performs different types of covered functions (i.e., the functions that make it a health plan, a health care provider, or a health care clearinghouse) and there are variations in its privacy practices among these covered functions. Covered entities are encouraged to provide individuals with the most specific notice possible. | |
|
Covered entities that participate in an organized health care arrangement may choose to produce a single, joint notice if certain requirements are met. For example, the joint notice must describe the covered entities and the service delivery sites to which it applies. If any one of the participating covered entities provides the joint notice to an individual, the notice distribution requirement with respect to that individual is met for all of the covered entities. See 45 CFR 164.520(d). |
v Restriction on Government Access to Health Information [45 CFR Part 160, Subpart C; 164.512(f)]
Background
Under the HIPAA Privacy Rule, government-operated health plans and health care providers must meet substantially the same requirements as private ones for protecting the privacy of individual identifiable health information. For instance, government-run health plans, such as Medicare and Medicaid plans, must take virtually the same steps to protect the claims and health information that they receive from beneficiaries as private insurance plans or health maintenance organizations (HMO). In addition, all Federal agencies must also meet the requirements of the Privacy Act of 1974, which restricts what information about individual citizens – including any personal health information – can be shared with other agencies and with the public.
The only new authority for government involves enforcement of the protections in the Privacy Rule itself. To ensure that covered entities protect patients’ privacy as required, the Rule requires that health plans, hospitals, and other covered entities cooperate with efforts by the Department of Health and Human Services (HHS) Office for Civil Rights (OCR) to investigate complaints or otherwise ensure compliance.