7.
Anthrax
v
Questions and Answers About Anthrax
What is Anthrax
Anthrax is an acute infectious disease
caused by the spore-forming bacterium Bacillus anthracis. Anthrax
most commonly occurs in wild and domestic lower vertebrates (cattle, sheep,
goats, camels, antelopes, and other herbivores), but it can also occur in
humans when they are exposed to infected animals or to tissue from infected
animals or when anthrax spores are used as a bioterrorist weapon.
What is the difference between exposure
to anthrax and disease caused by anthrax?
A person can be said to be exposed to anthrax
when that person comes in contact with the anthrax bacteria and a culture
taken from that person is positive for anthrax. A person can be exposed
without having disease. A person who might have come in contact with anthrax,
but without a positive culture would be said to be potentially exposed.
Disease caused by anthrax occurs when there is some sign of illness, such
as the skin lesion that occurs with cutaneous anthrax.
Transmission
How is anthrax transmitted?
Anthrax is not contagious; the illness
cannot be transmitted from person to person. B. anthracis spores
can live in the soil for many years, and humans can become infected with
anthrax by handling products from infected animals or by inhaling anthrax
spores from contaminated animal products. Anthrax can also be spread by
eating undercooked meat from infected animals. It is rare to find infected
animals in the United States. Anthrax spores can be used as a bioterrorist
weapon, as was the case in 2001, when Bacillus anthracis spores had
been intentionally distributed through the postal system, causing 22 cases
of anthrax, including 5 deaths.
What are the types of anthrax infection?
Anthrax infection can occur in three forms:
cutaneous (skin), inhalation, and gastrointestinal.
-
Cutaneous :
Most (about 95%) anthrax infections occur when the bacterium enters a cut
or abrasion on the skin, such as when handling contaminated wool, hides,
leather or hair products (especially goat hair) of infected animals.
Skin infection begins as a raised itchy bump that resembles an insect
bite but within 1-2 days develops into a vesicle and then a painless
ulcer, usually 1-3 cm in diameter, with a characteristic black necrotic
(dying) area in the center. Lymph glands in the adjacent area may swell.
About 20% of untreated cases of cutaneous anthrax will result in death.
Deaths are rare with appropriate antimicrobial therapy.
-
Inhalation : Initial
symptoms may resemble a common cold–sore throat, mild fever, muscle aches
and malaise. After several days, the symptoms may progress to severe breathing
problems and shock. Inhalation anthrax is usually fatal.
-
Gastrointestinal :
The intestinal disease form of anthrax may follow the consumption of
contaminated meat and is characterized by an acute inflammation of the
intestinal tract. Initial signs of nausea, loss of appetite, vomiting, fever
are followed by abdominal pain, vomiting of blood, and severe diarrhea.
Intestinal anthrax results in death in 25% to 60% of cases.
What are the case fatality for the various forms of anthrax?
Early treatment of cutaneous anthrax is
usually curative, and early treatment of all forms is important for recovery.
Patients with cutaneous anthrax have reported case fatality rates of 20%
without antibiotic treatment and less than 1% with it. Although case-fatality
estimates for inhalational anthrax are based on incomplete information, the
rate is extremely high, approximately 75%, even with all possible supportive
care including appropriate antibiotics. Estimates of the impact of the delay
in postexposure prophylaxis or treatment on survival are not known. For gastrointestinal
anthrax, the case-fatality rate is estimated to be 25%-60% and the effect
of early antibiotic treatment on that case-fatality rate is not defined.
Symptoms
What are the symptoms for
anthrax?
These symptoms can occur within 7 days
of infection:
-
Fever (temperature greater than 100 degrees F). The fever may be accompanied
by chills or night sweats.
-
Flu-like symptoms.
-
ough, usually a non-productive cough, chest discomfort, shortness of
breath, fatigue, muscle aches
-
Sore throat, followed by difficulty swallowing, enlarged lymph nodes,
headache, nausea, loss of appetite, abdominal distress, vomiting, or
diarrhea
-
A sore, especially on your face, arms or hands, that starts as a raised
bump and develops into a painless ulcer with a black area in the center.
How can I know my cold or flu is not anthrax?
Many human illnesses begin with what are
commonly referred to as "flu-like" symptoms, such as fever and muscle aches.
However, in most cases anthrax can be distinguished from the flu because
the flu has additional symptoms. In previous reports of anthrax cases, early
symptoms usually did not include a runny nose, which is typical of the flu
and common cold.
Testing
Can I get screened or tested
to find out whether I have been exposed to anthrax?
There is no screening test for anthrax;
there is no test that a doctor can do for you that says you’ve been exposed
to or carry it. The only way exposure can be determined is through a public
health investigation. Nasal swabs and environmental tests, are not tests
to determine whether an individual should be treated. These kinds of tests
are used only to determine the extent of exposure in a given building or
workplace.
Does CDC collect samples to test the bacteria?
CDC is engaging its partners in the Laboratory
Response Network (LRN) in states all
across the United States. The LRN is a collaborative partnership and multilevel
system linking state and local public health laboratories with advanced
capacity laboratories–including clinical, military, veterinary, agricultural,
water, and food-testing laboratories– to rapidly identify threat agents,
including anthrax. Local clinical laboratory testing is confirmed at state
and large metropolitan public health laboratories. CDC conducts the definitive
or highly specialized testing for major threat agents. There are 100 laboratories
in the network; none of them are commercial labs.
When an area is tested for the presence
of Bacillus anthracis , how long does it take to get the results?
Before testing can begin, samples must
be collected in a form suitable for testing. The length of time it takes
to get test results depends on both the kind of test to be performed and
the laboratory’s workload. Some tests may take only a short time to perform,
but confirmation takes longer. It may take many days to get the test results.
Testing is a two-step process. The first
test, a screening test, may be positive within 2 hours if the sample is
large and contains a lot of Bacillus anthracis, the organism that causes
the disease anthrax. However, a positive reading on this first test must
be confirmed with a second, more accurate test. This confirmation test, conducted
by a more sophisticated laboratory, takes much longer. The length of time
needed depends in part on how fast the bacteria grow, but results are usually
available 1 to 3 days after the sample is received in the laboratory.
Does CDC recommend the use of home test kits for anthrax?
Hand-held assays (sometimes referred to
as "Smart Tickets") are sold commercially for the rapid detection of Bacillus
anthracis . These assays are intended only for the screening of environmental
samples. First responder and law enforcement communities are using these
as instant screening devices and should forward any positive samples to authorities
for more sensitive and specialized confirmatory testing. The results of these
assays should not be used to make decisions about patient management or
prophylaxis. The utility and validity of these assays are unknown.
At this time, CDC does not have enough
scientific data to recommend the use of these assays. The analytical sensitivity
of these assays is limited by the technology, and data provided by manufacturers
indicate that a minimum of 10,000 spores is required to generate a positive
signal. This number of spores would suggest a heavy contamination of the
area (sample). Therefore a negative result does not rule out a lower level
of contamination. Data collected from field use also indicate specificity
problems with some of these assays. Some positive results have been obtained
with spores of the non-anthrax Bacillus bacteria that may be found in the
environment.
For these reasons, CDC has been asked to
evaluate the sensitivity and specificity of the commercially available,
rapid, hand-held assays for B. anthracis. When this study is completed,
results will be made available. Conclusions from this study are not expected
in the near future.
Are health department laboratories capable of conducting testing?
All state health departments are capable
of obtaining results of tests on suspected infectious agents. Laboratories
are usually classified as Level A, B, C, or D. Level A laboratories are those
typically found in community hospitals, and these laboratories should be
able to perform initial testing on all clinical specimens (usually blood
or some other body fluid). Public health laboratories are usually Level B;
these laboratories are valuable for confirming or refuting preliminary test
results and can usually perform antimicrobial susceptibility tests on bacteria
and viruses. Level C laboratories, which are reference facilities and can
be public health laboratories, can perform more rapid identification tests.
Level D laboratories are designed to perform the most sophisticated tests
and are located in federal facilities such as CDC. Every state has a Laboratory
Response Network (LRN) contact. The LRN links state and local public health
laboratories with advanced-capacity laboratories, including clinical, military,
veterinary, agricultural, water, and food-testing laboratories. Laboratorians
should contact their state public health laboratory to identify their local
LRN representative. CDC’s public bioterrorism Web site provides access to
CDC’s Centers for Public Health Preparedness, a national network of academic
institutions and local health departments whose goal is to ensure that local
public health workers are fully prepared to respond to current and emerging
health threats, including bioterrorism.
How effective and reliable are anthrax tests?
There are many kinds of tests, and the
reliability of each has not been determined. In general, findings from culturing
environmental samples are specific; that is, a positive result reflects
the true presence of Bacillus anthracis, and a negative result likely means
that no B. anthracis is present.
What is subtyping?
Subtyping is a laboratory process to identify
different subtypes of organisms, which is not possible with standard microbiological
testing. Most Bacillus anthracis subtyping is done by examining the organism’s
molecular structure for certain genetic characteristics that can then be
compared with those of other B. anthracis organisms to determine whether
they are the same or different. Differences between these two organisms
would indicate different strains.
Is subtyping different from
polymerase chain reaction (PCR)?
Polymerase chain reaction (PCR) is a laboratory
method used to detect and amplify genetic material from organisms. It can
be used to diagnose disease by identifying genetic material (DNA) commonly
found in all Bacillus anthracis strains or it can be used to subtype the
organism by amplifying specific genetic material and comparing it with known
strains of B. anthracis to see if it matches or if it is different. When
PCR is used for subtyping, the amplified genetic material is usually further
analyzed by other molecular methods, such as DNA sequencing.
What method does CDC use to subtype
Bacillus anthrax?
CDC uses a method called MLVA, which is
the acronym for multi-locus variable-number of tandem (consecutive) repeat
analysis .
How does MLVA (Multi-locus variable number of tandem [consecutive] repeat
analysis) identify different strains of anthrax?
MLVA examines a number of DNA segments
within the chromosome or plasmids of Bacillus anthracis that have specific
repeat patterns of nucleotides (fundamental DNA units). These repeats may
differ by sequence and length, as well as the number of times that they
are repeated. Different types of these repeats and the number of times that
they are repeated provide a specific pattern that will identify different
strains of the organism. More than 100 different strains of B. anthracis
have been identified using this method.
When is environmental sampling performed?
Environmental sampling is the sampling
of the air, soil, dust, water, and physical surfaces to identify the presence
or absence of bacteria, chemicals, and radiological materials (see "Procedures
for Collecting Surface Environmental Samples for Culturing Bacillus anthracis").
In the case of anthrax, this is used to identify its location and presence
in the environment. Environmental sampling would be conducted if there were
a threat or possibility of Bacillus anthracis contamination. However, the
presence of B. anthracis in an environmental sample does not mean the person
will get the disease.
Why is environmental sampling performed?
-
To identify the site or source of B. anthracis that could lead to exposure
and disease,
-
To trace the route of an exposure (e.g., a letter),
-
To guide clean-up efforts in a facility with known exposure, and
- To
assess biosafety procedures in laboratories processing anthrax specimens.
What is the turnaround time for an anthrax test of an environmental sample?
Before testing can begin, samples must
be collected and arrive in a form suitable for testing. The length of time
necessary to get results of tests depends on transportation to the laboratory
and the specific tests to be done. Testing is a two-step process. Initial
screening tests (such as Gram stain) may be positive within two hours if
the sample is large and the concentration of bacteria is high. These tests
are used to narrow the definition of the sample. The confirmation tests take
much longer, depending in part on how fast the bacteria grow, but are usually
available 24-48 hours after the sample is received by the laboratory. (See
"Basic Laboratory Protocols for the Presumptive Identification of Bacillus
anthracis".)
Is the Mayo Clinic/Roche Rapid Anthrax Test a new test?
This is not a "new test." The Laboratory
Response Network (LRN) has been using a validated real-time polymerase chain
reaction (PCR) assay on the LightCycler for some time. CDC has also developed
and validated real-time PCR assays for Bacillus anthracis for the SmartCycler™,
ABI/PE 7700 and 5700. In addition, Idaho Technology has a real-time PCR assay
for B. anthracis that can be used with the R.A.P.I.D.™, which is similar
to the LightCycler™. SmartCycler is a trade name of Cepheid; R.A.P.I.D. is
a trade name of Idaho Technology; LightCycler is a trade name of Roche; Idaho
Technology is the name of a company.
Is the Mayo Clinic assay the same as the assay available to Laboratory
Response Network (LRN) laboratories?
N
o. The Mayo Clinic assay targets the Lethal Factor (lef) gene on the virulence
plasmid p0X1 and the Protective Antigen gene (pag) on p0X2. This assay has
been tested with DNA from 32 strains of Bacillus anthracis, 26 Bacillus species,
and 21 different bacterial genera commonly encountered in human specimens.
The Mayo Clinic assay has not been validated in multiple laboratories.
The CDC assay uses targets on p0X1, p0X2,
and the chromosome. A total of 100 Bacillus anthracis isolates were used
to evaluate the sensitivity of the assay. Of the 100, 77 were selected to
provide the best possible representation with respect to geographic origin
and date isolated. The strains were obtained from infected animals, humans,
and from industrial sites associated with anthrax outbreaks; they span 58
years (1939-1997) and are from various countries. In addition, five p0X1-cured
strains (including the Sterne strain) and one p0X2-cured strain (Pasteur
strain) were included. For evaluation of the specificity of the assay, 54
Bacillus species were used (B. subtilis , 9; B. cereus, 23; B. thuringiensis
, 12; and B. megaterium, 10) as well as 250 other DNAs of various viruses
and bacteria from human, animal, and insect sources. The assay was validated
in a multicenter study by using state public health laboratories that had
the specific platform.
What are the limitations of the Mayo Clinic test?
Because the Mayo Clinic assay uses only
two plasmid targets, it cannot identify Bacillus anthracis strains such as
Sterne or Pasteur that may be present in environmental specimens. This would
not be a problem if the assay were used to confirm the identity of a gram-positive,
non-motile, non-hemolytic rod.
Is CDC going to validate this assay?
CDC is testing samples from the current
anthrax outbreak. When we have sufficient time, we will study this and other
anthrax assays.
Are you aware of any sensitivity or specificity issues
with the Mayo Clinic test? Should we expect a large number of false positive/negative
results?
On the basis of the data provided by the
Mayo Clinic, the assay appears to be sensitive and specific. However, the
results are only as good as the method used to prepare the sample for analysis.
There have been no data provided to indicate the types of samples that can
be assayed or how they are to be processed. The Food and Drug Administration
(FDA) has not seen the package insert for this test. The CDC assay has been
validated for different types of samples and sample processing methods.
Are you furnishing CDC-tested equipment and reagents to
laboratories?
T
hrough the bioterrorism cooperative agreement, CDC has funded the purchase
of platforms for real-time polymerase chain reaction (PCR) assays for the
Laboratory Response Network (LRN). To date, 61 instruments have been purchased
or ordered. Among these are 17 LightCyclers,™ 23 SmartCyclers™, 13 7700s,
and 5 5700s. Reagents for real-time anthrax assays are made at CDC and placed
in inventory. They are available at no charge to LRN laboratories. Currently,
reagents for the LightCycler™ anthrax assay are available; reagents for
the other platforms will be available soon. All of the assays have undergone
the same rigorous validation procedure.
If a laboratory asks your opinion on whether to use Mayo
Clinic/Roche Rapid test, what is your answer?
The Food and Drug Administration (FDA)
considers this an investigational assay. As such it should be used only in
conjunction with other tests, such as culture tests. Currently, polymerase
chain reaction (PCR) assays are not considered confirmatory assays.
Will CDC accept results
from laboratories that use this assay?
Currently, polymerase chain reaction (PCR)
assays are not considered confirmatory tests for anthrax. PCR-positive specimens
(or cultures) should be forwarded to the nearest LRN laboratory for confirmation.
Diagnosis
How is anthrax diagnosed?
A
nthrax is diagnosed by isolating B. anthracis from the blood, skin lesions,
or respiratory secretions or by measuring specific antibodies in the blood
of persons with suspected cases.
In patients with symptoms compatible with
anthrax, providers should confirm the diagnosis by obtaining the appropriate
laboratory specimens based on the clinical form of anthrax that is suspected
(i.e., cutaneous, inhalational, or gastrointestinal).
Cutaneous - vesicular fluid and blood
Inhalational - blood, cerebrospinal fluid
(if meningeal signs are present) or chest X-ray
Gastrointestinal - blood
For more information, read Update: Investigation
of Bioterrorism-Related Anthrax and Interim Guidelines for Clinical Evaluation
of Persons with Possible Anthrax.
What are the standard diagnostic tests used by the laboratories?
Presumptive identification to identify
to genus level ( Bacillus family of organisms) requires Gram stain and colony
identification.
Presumptive identification to identify
to species level (B. anthracis) requires tests for motility, lysis by gamma
phage, capsule production and visualization, hemolysis, wet mount and malachite
green staining for spores.
Confirmatory identification of B. anthracis
carried out by CDC may include phage lysis, capsular staining, and direct
fluorescent antibody (DFA) testing on capsule antigen and cell wall polysaccharide.
What is a nasal swab test?
A nasal swab involves placing a swab inside
the nostrils and taking a culture. The CDC and the U.S. Department of Health
and Human Services do not recommend the use of nasal swab testing by clinicians
to determine whether a person has been exposed to Bacillus anthracis, the
bacteria responsible for anthrax, or as a means of diagnosing anthrax. At
best, a positive result may be interpreted only to indicate exposure; a negative
result does not exclude the possibility of exposure. Also, the presence of
spores in the nose does not mean that the person has inhalational anthrax.
The nose naturally filters out many things that a person breathes, including
bacterial spores. To have inhalational anthrax, a person must have the bacteria
deep in the lungs, and also have symptoms of the disease. Another reason
not to use nasal swabs is that most hospital laboratories cannot fully identify
anthrax spores from nasal swabs. They are able to tell only that bacteria
that resemble anthrax bacteria are present.
When is a nasal swab indicated?
Nasal swabs and screening may assist in
epidemiologic investigations, but should not be relied upon as a guide for
prophylaxis or treatment. Epidemiologic investigation in response to threats
of exposure to B. anthracis may employ nasal swabs of potentially exposed
persons as an adjunct to environmental sampling to determine the extent of
exposure.
Why were nasal swabs used to screen individuals in the
Florida investigation for anthrax?
The nasal swab test was used as a screening
tool because, following initial recognition of the case of confirmed inhalational
anthrax, there were no known sources of exposure. Determining whether anyone
else associated with the case-patient might have been exposed was important.
In this setting, the nasal swab method was used for a rapid assessment of
exposure among people, and as a tool for rapid environmental assessment.
When the source of exposure is not known, nasal swabs can help investigators
determine that information. They are not used for diagnosing people with
anthrax, and they are not 100 percent effective in determining all who may
have been exposed. See also http://www.bt.cdc.gov/documentsapp/faqanthrax.asp#Q602.
Is there an X-ray for detecting anthrax?
A chest X-ray can be used to help diagnose
inhalation anthrax in people who have symptoms. It is not useful as a test
for determining anthrax exposure or for people with no symptoms.
Preventive/Vaccine
What is the therapy for preventing inhalational anthrax?
Interim recommendations for postexposure
prophylaxis for prevention of inhalational anthrax after intentional exposure
to B. anthracis may be found in the MMWR at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5041a1.htm.
Is there a vaccination for anthrax?
A protective vaccine has been developed
for anthrax; however, it is primarily given to military personnel. Vaccination
is recommended only for those at high risk, such as workers in research laboratories
that handle anthrax bacteria routinely. The antibiotics used in post exposure
prophylaxis are very effective in preventing anthrax disease from occurring
after an exposure.
Is the anthrax vaccine available to the public?
A vaccine has been developed for anthrax
that is protective against invasive disease, but it is currently only recommended
for high-risk populations. CDC and academic partners are continuing to support
the development of the next generation of anthrax vaccines.
Who should be vaccinated against anthrax?
T
he Advisory Committee on Immunization Practices (ACIP) has recommended anthrax
vaccination for the following groups:
-
Persons who work directly with the organism in the laboratory.
-
Persons who work with imported animal hides or furs in areas where standards
are insufficient to prevent exposure to anthrax spores.
-
Persons who handle potentially infected animal products in high-incidence
areas; while incidence is low in the United States, veterinarians who
travel to work in other countries where incidence is higher should consider
being vaccinated.
-
Military personnel deployed to areas with high risk for exposure to
the organism.
Treatment
What is the treatment for patients with inhalational and
cutaneous anthrax?
Treatment protocols for cases of inhalational
and cutaneous anthrax associated with this bioterrorist attack are found
in the MMWR , 10/26/2001; 50(42), 909-919. (http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5042a1.htm)
If an anthrax event occurs,
should people buy and store antibiotics?
There is no need to buy or store antibiotics,
and indeed, it can be detrimental to both the individual and to the community.
First, only people who are exposed to anthrax should take antibiotics, and
health authorities must make that determination. Second, individuals may
not stockpile or store the correct antibiotics. Third, under emergency plans,
the Federal government can ship appropriate antibiotics from its stockpile
to wherever they are needed.
What drugs are FDA-approved for postexposure prophylaxis
(PEP) and treatment?
Ciprofloxacin and doxycycline are FDA-approved
for PEP, and ciprofloxacin, doxycycline, and amoxicillin are FDA-approved
for treatment. In the current situation of intentional anthrax distribution,
doxycycline and ciprofloxacin are the recommended drugs for prophylaxis.
Inhalational anthrax treatment protocol
for cases associated with this bioterrorism attack: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5042a1.htm
- (table 1)
Cutaneous anthrax treatment protocol for
cases associated with this bioterrorism attack: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5042a1.htm
- (table 2)
Who should receive antibiotics for 60 days?
People at risk for inhalational anthrax
should receive 60 days of antibiotics. These people include the following:
-
People who have been exposed to an air space known to have been contaminated
with aerosolized B. anthracis.
-
People who share the air space within a facility where others have acquired
inhalational anthrax.
-
People who have been along the transit pathway of an envelope (or other
vehicle) containing B. anthracis that may have been aerosolized.
-
Unvaccinated laboratory workers who have handled powder that has tested
positive for B. anthracis and who may not have used appropriate biosafety
precautions.
If patients are suspected of being exposed to anthrax,
should they be quarantined or should other family members be tested?
Direct person-to-person spread of anthrax
is extremely unlikely and anthrax is not contagious. Therefore, there is
no need to quarantine individuals suspected of being exposed to anthrax
or to immunize or treat contacts of persons ill with anthrax, such as household
contacts, friends, or coworkers, unless they also were also exposed to the
same source of infection.
What if I develop side effects from the antibiotic?
If you develop side effects from the antibiotic,
call your healthcare provider immediately. Depending on the type of side
effects, you may be able to continue taking the medicine, or may be switched
to an alternative antibiotic. If necessary, your physician may contact your
State Department of Health for consultation on possible alternate antibiotics.
Has CDC tested the anthrax isolates for sensitivity to different
antibiotics?
Yes. Antibiotic sensitivity testing performed
at CDC has determined that the strain of anthrax was sensitive to a wide
range of antibiotics, including penicillin and ciprofloxacin, giving public
health officials important treatment information.
What are the risks of using
tetracyclines and fluoroquinolones in children? Are alternatives available?
Risks of using tetracyclines and fluoroquinolones
in children must be weighed carefully against the risk for developing a life-threatening
disease due to B. anthracis. Both agents can have adverse health reactions
in children. If adverse reactions are suspected, therapy may be changed
to amoxicillin or penicillin.
Are there special instructions for
taking ciprofloxacin or doxycycline?
As with all antibiotics, take the medication
according to the schedule you were instructed, and even if you begin to feel
better, continue taking it for the full number of days. If you need an extension
of the antibiotic at the end of your prescribed number of days, local emergency
healthcare workers or your healthcare provider will inform and tell you
how to get more medicine. They may also tell you to discontinue the antibiotic,
or will change the type of antibiotic, depending on results of laboratory
tests.
After I have started taking
ciprofloxacin to protect me from developing anthrax, what side effects could
I get from taking this antibiotic?
Side effects which sometimes occur include
nausea, mild diarrhea, stomach pain, headache and dizziness. Talk with your
doctor if you have any of these problems while you are taking the antibiotic.
Certain foods and medications should not be taken with ciprofloxacin; this
should be discussed at the time the antibiotic is prescribed, so that side
effects will not occur from the combinations. Ciprofloxacin also can cause
sun sensitivity which increases the chances of sunburn. More serious side
effects include central nervous system side effects such as confusion, tremors,
hallucinations, depression, and increased risk of seizures. High blood pressure
and blurred vision are also possible. Allergic reactions could cause difficulty
breathing; closing of the throat; swelling of the lips, tongue, or face;
hives or severe diarrhea. Pain, inflammation, or rupture of a tendon are
possible and also severe tissue inflammation of the colon could occur. Call
your doctor or seek medical advice right away if you are having any of these
side effects. This list is NOT a complete list of side effects reported with
ciprofloxacin. Your healthcare provider can discuss with you a more complete
list of side effects.
After I have started taking
doxycycline to protect me from developing anthrax, what side effects could
I get from taking this antibiotic?
Less serious side effects include diarrhea,
upset stomach, nausea, sore mouth or throat, sensitivity to sunlight, vaginal
yeast infection or itching of the mouth lasting more than 2 days. You should
talk with your doctor if you have any of these problems while taking doxycycline.
Certain foods and medications should not be taken with doxycycline, and
this should be discussed with your healthcare provider at the time the antibiotic
is prescribed, so that side effects will not occur from the combinations.
Doxycycline also causes sun sensitivity which increases the chances of sunburn.
Serious side effects of doxycycline that are possible but uncommon include:
life-threatening allergic reaction (symptoms are trouble breathing; closing
of the throat; swelling of the lips, tongue, or face; hives), blood problems
(symptoms are unusual bleeding or bruising), liver damage (symptoms are
yellowing of the skin or eyes, dark urine, nausea ,vomiting, loss of appetite,
abdominal pain), irritation of the esophagus. Call your doctor or seek medical
attention right away if you are having any of these side effects. This list
is NOT a complete list of side effects reported with doxycycline. Your healthcare
provider can discuss with you a more complete list of side effects.
Why is CDC recommending
doxycycline instead of ciprofloxacin for the treatment and prevention of
anthrax?
Both doxycycline and ciprofloxacin are
effective in treating Bacillus anthracis that we are dealing with in these
investigations. Although CDC first recommended the use of either drug for
postexposure prophylaxis for the prevention of inhalational anthrax, we
are now recommending doxycycline in order to prevent other bacteria from
developing resistance to ciprofloxacin. Ciprofloxacin is part of the fluoroquinolone
family of drugs, a relatively new class of antibiotics used to treat infections
caused by organisms for which doctors do not have information about antimicrobial
susceptibility. This kind of treatment is known as empiric therapy. Ciprofloxacin
and other fluoroquinolones are used for empiric treatment for a variety
of serious and common infections in the United States, including pneumonia,
gastrointestinal infections, and urinary tract infections. The number of
people who have been exposed to B. anthracis and need antibiotics has increased
dramatically since CDC first issued guidelines for treatment. If all those
people take ciprofloxacin, other bacteria they carry in their bodies may
develop resistance to fluoroquinolones, potentially limiting the usefulness
of these drugs as empiric therapy. Doxycycline is less frequently used for
empiric treatment than ciprofloxacin; therefore, we have fewer concerns regarding
this drug and the emergence of new resistant bacteria.
Why are people who have
been exposed to B. anthracis being given antibiotics for different amounts
of time?
The initial number of people placed on
prophylaxis may reflect conservative estimates with wide safety margins
based on limited preliminary information. As the investigation progresses,
and a clearer picture of exposure develops, the number of people advised
to continue prophylaxis may be reduced. As of the last week of October 2001,
when preliminary tests show that people have been exposed to Bacillus anthracis,
those exposed may be provided with a starter packet of antibiotics; the number
of days for which antibiotics are prescribed can vary according to the specific
situation and person. Additional tests are then conducted of the area where
exposure occurred and to determine the extent of exposure. Based on the results
of these additional tests, those exposed may be instructed to return to a
centralized location for additional care or to seek additional care from
their primary care providers; additional antibiotics may be prescribed based
on the particular situation and person. Lastly, it is recommended that people
found to be at risk of inhalation anthrax be prescribed 60 days of antibiotics.
These general procedures may change at any time as new information is gathered.
Are there different strains of B. anthracis
? Do they all respond to antibiotics?
Yes, there are different strains of Bacillus
anthracis . Some strains of B. anthracis may be naturally resistant to certain
antibiotics and not others. In addition, there may be biologically mutant
strains that are engineered to be resistant to various antibiotics. A laboratory
analysis can help to define which strain of B. anthracis is present and
which antibiotic would be the most effective in treating the resulting anthrax.
What is the FDA telling physicians and other
health professionals about prescriptions for ciprofloxacin?
Although FDA does not regulate the practice
of medicine, the agency is strongly recommending that physicians not prescribe
ciprofloxacin for individual patients to have on hand for possible use against
inhaled anthrax. Indiscriminate and widespread use of ciprofloxacin could
hasten the development of drug-resistant organisms and lessen the effects
of these agents against many infections.
Can other
fluoroquinolones be used instead of
ciprofloxacin for postexposure prophylaxis
(PEP)/treatment?
Other fluoroquinolones, such as ofloxacin
and levofloxacin, are not specifically recommended as alternatives to ciprofloxacin
because of a lack of sufficient data on their efficacy. However, if first-line
drugs were not available, these other fluoroquinolones may be effective.
Why do I need 60 days of antibiotics?
Anthrax spores grow like plant seeds. If
you plant seeds and give them sun and water, they will grow into plants.
If you give anthrax spores the right environment, such as the human body,
they can grow into the harmful form of the bacteria that can cause anthrax
disease. It takes anthrax spores an average of 7 days to grow into the harmful
form of the bacteria, but it can take longer. For this reason, you must continue
taking preventive antibiotics for the full 60 days.
What happens if I take ciprofloxacin,
doxycycline, or amoxicillin for a few days, stop, and then restart the antibiotics?
You should complete the 60-day course of
antibiotics that you were given. It is best to take antibiotics as prescribed
and not to skip any doses.
The ciprofloxacin I am taking gives
me headaches. Is there anything I can do to
help this?
If you don’t have a history of headaches,
then your headache may be related to the medicine. Changing the time of day
that you take the ciprofloxacin or eating after you take the medicine may
help. Pain relievers such as acetaminophen may help your headache. If your
headache does not go away, you should consult your doctor.
The ciprofloxacin, doxycycline,
or amoxicillin I am taking makes me feel sick to my stomach. Is there anything
I can do to help this?
Taking your antibiotic with food may help
reduce this sick feeling. Ciprofloxacin and doxycycline should not be taken
within 2 hours of taking antacids. Ciprofloxacin and doxycycline should not
be taken with dairy or calcium-fortified products (such as ice cream or calcium-fortified
orange juice).
The ciprofloxacin, doxycycline, or amoxicillin
I am
taking gives me diarrhea. Is there anything I can do to help this?
Antibiotics may disrupt bacteria in the
gastrointestinal tract, causing diarrhea. Food may help relieve the diarrhea.
If the diarrhea does not go away, your doctor may recommend another antibiotic.
If you develop severe, long-lasting diarrhea, you may have a serious condition
and should consult your doctor.
If taking one of the recommended
antibiotics makes me feel terrible, can I switch to another of these antibiotics?
If you have tried taking the medicine with
food or changing the time of your dose but still feel terrible, you should
ask your doctor about switching antibiotics.
I am having terrible yeast infections while
taking ciprofloxacin, doxycycline, or amoxicillin. Is there anything I can take for
this?
Occasionally, women develop yeast infections
while taking amoxicillin. You may treat the infection with over-the-counter
medicines such as clotrimazole. If the symptoms do not go away, you should
consult your doctor.
I feel much better if I
take only one pill of ciprofloxacin, doxycycline, or amoxicillin each day.
Is that okay?
No. The drug must be taken twice a day
to kill the bacteria. If your body contains anthrax bacteria and you do
not take the full dose, the bacteria may start to grow again and become
harder to kill.
My prescription says to take one pill
every 12 hours. If 15 hours have passed since my last dose, is it still okay to take the
pill?
Yes. It is okay to take the next pill even
if 15 hours have elapsed. However, you should not make a habit of this. The
medicine works best when taken every 12 hours.
What side effects are serious
enough that I should go to a doctor?
Any side effect that forces you not to
take your medicine is serious enough that you should consult or see your
doctor.
Serious side effects of ciprofloxacin include
seizures, mental confusion, rash that does not go away, or excessive diarrhea.
If you have any of these effects, call
your doctor.
Serious side effects of doxycycline include
jaundice (yellow eyes or skin), rash that does not go away, or excessive
diarrhea. If you have any of these effects, call your doctor.
Any reaction that causes a rapid swelling
of the lips and face, shortness of breath, or hives is a medical emergency.
You should call 911. These types of reactions are extremely rare.
Can I drink alcohol if I
am taking ciprofloxacin, doxycycline, or amoxicillin?
Social drinking of alcohol (fewer than
2 drinks a day) should not cause any side effects unless you already have
a liver problem. However, drinking too much alcohol can cause the medicine
to leave your body faster, which will decrease the effectiveness of the
medicine. If you drink more than two drinks a day, you should tell your doctor
so that different medicines can be prescribed.
The ciprofloxacin, doxycycline,
or amoxicillin I am taking makes me feel itchy all over. Is there anything
I can do to help this?
Rashes that appear suddenly or do not go
away after a few days may be signs of an allergic reaction. You should see
your doctor immediately.
The ciprofloxacin, doxycycline, or amoxicillin gave me
an allergic reaction and I stopped taking it. What should I do?
If the allergic reaction was severe or
rapid, you should notify your doctor before taking another antibiotic. Your
doctor will prescribe a different antibiotic that will kill the bacteria
without causing an allergic reaction. Remember: you should complete the
entire 60 days of treatment even if you change antibiotics.
Why can’t
I take a shot, wear a patch, or take one large dose of
the medicine instead of taking it for 60 days?
Spores can stay in your body for some time
before they start growing and causing you to become ill. When the spores
are not growing, antibiotics are not effective. Only after the spores start
to grow can the antibiotics work. Therefore, you need a constant level of
antibiotic in your body for 60 days to make sure that when the spores start
to grow, the antibiotic is there to kill them.
Ciprofloxacin and doxycycline
look different and come in different doses. Is one better than the other?
Ciprofloxacin 500 mg and doxycycline 100
mg both have the same killing power in your bloodstream and are equally
effective against anthrax bacteria. Doxycycline is available in both tablet
and capsule form. Both will give you the same amount of medicine in your
bloodstream to kill the bacteria.
Should all patients who
have flu-like symptoms be treated with antibiotics?
No. CDC does not recommend treating all
patients who have flu-like illness with antibiotics. Antibiotics do not
kill viruses, which cause the flu. If the patient is not at risk for developing
anthrax, antibiotics are not recommended because the person may experience
serious side effects. Also, taking antibiotics can increase the chance that
the medicine will not be as effective against other bacterial infections.
Does a patient have immunity after recovering from anthrax
infection?
We do not have enough data at this time
to make this determination. However, it is theoretically possible to gain
post-infection immunity.
How do doctors treat inhalational
anthrax to reduce the risk of death in patients?
When inhalational anthrax is suspected,
physicians prescribe antibiotics to treat the disease. To be effective,
antibiotic therapy should be initiated as soon as possible after exposure.
Other treatment includes supportive care in hospital. B. anthracis usually
responds effectively to several antibiotics including penicillin, doxycycline,
and fluoroquinolones (such as ciprofloxacin).
I was told that I had been exposed to Bacillus
anthracis and prescribed antibiotics. I took the
medicine for a couple weeks. Wouldn’t that weaken any anthrax that’s in
my body?
You should take the full 60 days of antibiotics
even if you feel better. Inhaled anthrax spores become lodged in the body
and may activate after initial exposure. Antibiotics have little or no effect
when the spores are inactive. To be effective in preventing inhalational
anthrax, the antibiotics must be in your system when the spores activate.
It is necessary to take the medicine for at least 60 days to ensure the best
protection against inhalational anthrax.
Why was ciprofloxacin ever publicized as the best drug
for anthrax? How can we know which antibiotic is best?
At the beginning of the recent anthrax
outbreak, investigators did not know which drugs would kill the strains
of Bacillus anthracis responsible for the outbreak. They used ciprofloxacin
because very few bacteria are resistant to it. Recent laboratory tests using
all of the B. anthracis strains from the recent outbreak have indicated
that all the strains are susceptible to ciprofloxacin, doxycycline, and
other antibiotics.
Besides anthrax, what else is ciprofloxacin
prescribed for? Has there been resistance to ciprofloxacin when used in other
instances (historically)?
Ciprofloxacin is a broad-spectrum, highly
effective antibiotic that has been part of the "international traveler’s"
kit at CDC for at least a year. It can be used against most bacterial infections.
However, ciprofloxacin is frequently overused for many diseases that can
be treated with less powerful, narrower-spectrum drugs. Right now, most bacteria
are susceptible to ciprofloxacin, which is why we want to be cautious about
its use. Overuse of ciprofloxacin could lead to the development of resistance.
Is there a generic form
of ciprofloxacin?
No, there is currently no generic form
of ciprofloxacin in the United States.
Pregnancy
I’m taking
medication to prevent anthrax, and I just found out that I’m pregnant. What should I do?
It is very important that you continue
to take as directed the medication you have been prescribed. You should
also contact your doctor or local public health officials right away to
let them know that you are pregnant. They will want to discuss which medicine
would be the best choice for you—to prevent anthrax and to be safe for both
you and the fetus.
I’m pregnant. What medicine should I take to prevent anthrax?
You should take medication to prevent anthrax
only if a public health official confirms that you have had a potential exposure
to anthrax. You and your doctor will want to discuss the risks and benefits
of the various antibiotics that can be used to prevent anthrax. Which medicine
is most appropriate for you will depend on the specific place and situation
of your exposure and on your general medical history (including other medicines
you may be taking and any medication allergies you may have). Currently,
there are three main antibiotics used to prevent anthrax: ciprofloxacin,
amoxicillin, and doxycycline. Ciprofloxacin is effective against anthrax
and is unlikely to cause major problems for the fetus, but there is not enough
experience or data involving ciprofloxacin during pregnancy to say for certain
that there is no risk to the fetus. Doctors are more confident about the
safety of amoxicillin for the fetus, but amoxicillin may not always be effective
against anthrax. Before prescribing amoxicillin for you, your doctor would
want to make sure that the anthrax you were exposed to is not resistant
to amoxicillin. Doxycycline can sometimes cause tooth and bone problems
in the fetus. Therefore, you should not take doxycycline unless there is
a specific reason why you cannot take either ciprofloxacin or amoxicillin.
I’ve heard
that doctors don’t generally prescribe ciprofloxacin to pregnant women. Why is
that? Why are they recommending it for anthrax prevention?
Ciprofloxacin is not likely to cause major
problems for a fetus, but there is not enough experience and data involving
ciprofloxacin during pregnancy to say for certain that there is no risk
to the fetus. Ciprofloxacin is not commonly used during pregnancy because
most infections that pregnant women get can be treated with other drugs whose
safety for pregnant women and their fetuses is better documented. However,
because anthrax is a life-threatening disease, the benefits of using ciprofloxacin
may outweigh potential risks to the fetus.
I was started on ciprofloxacin to prevent
anthrax. I’ve heard that amoxicillin may
be a safer drug for me to take during my pregnancy. How do I know if I can be switched
to amoxicillin?
Doctors are often more confident about
using amoxicillin than ciprofloxacin in pregnancy because they have more
information on the safety of amoxicillin for the mother and the fetus. But
in some situations, amoxicillin may not be effective against anthrax; this
is because the bacteria that cause anthrax can sometimes develop resistance
to penicillins such as amoxicillin. Before prescribing amoxicillin for you,
your doctor will want to learn more about the specific place and situation
of your exposure to anthrax and also about your general medical history.
(For instance, some women cannot take amoxicillin because they are allergic
to it.)
Doxycycline is being recommended for my coworkers
who aren’t pregnant . Is doxycycline a better medicine against anthrax than ciprofloxacin?
No. There are no data to suggest that doxycycline
is better than ciprofloxacin for preventing anthrax.
I’m having a lot of heartburn during my pregnancy. Can
I take ciprofloxacin at the same time as I take antacids?
No. Antacids should not be taken at the
same time as ciprofloxacin because they may make ciprofloxacin less effective.
(They can interfere with the absorption of ciprofloxacin.) You should not
take antacids in the 6 hours before you take a ciprofloxacin pill or for
2 hours after you take ciprofloxacin.
I’ve been trying to get pregnant and have just started taking
medication to prevent anthrax. Can I continue to try to get pregnant while
taking this medication?
Whether to try to become pregnant while
taking medication to prevent anthrax is your personal decision. When making
this decision, you should discuss the possible risks and benefits with your
family and your doctor. Some women may prefer to wait until after completing
the full course of antibiotics before becoming pregnant. If you decide not
to wait, it may be best not to take doxycycline unless there is a specific
reason why you cannot take either ciprofloxacin or amoxicillin.
I just recently found out I’m pregnant,
and I was exposed to anthrax at work. I want to take the best medication for my
fetus and me, but I don’t yet want my employer to know that I’m pregnant. What
should I do?
It is very important that you tell your
doctor or local public health officials that you are pregnant. They will
not be required to tell your employer.
Risk
What is the risk for an individual
if he or she is treated with antibiotics and is exposed to Bacillus
anthracis again?
Because inhalational anthrax in humans
is so rare, we cannot be certain about the risk of reinfection; therefore,
CDC recommends that another course of antibiotic treatment be given promptly
if a person is reexposed to Bacillus anthracis. In animal studies of inhalational
anthrax, animals given anthrax vaccine and antibiotics after exposure did
not develop anthrax when reexposed 4 months after the original exposures,
while animals treated with antibiotics alone became ill when reexposed.
Can the spores that cause
anthrax multiply outside of a human or animal host?
We do not think so, but we are not certain.
What are the odds of my getting anthrax?
(What is the average risk of contracting anthrax in the United States?)
In an average year, the chance that any
one individual in the United States will contract anthrax is extremely low
- about one case in nearly 300 million. In 2001, even with the intentional
release of Bacillus anthracis spores in some environments, the nationwide
risk was still extremely low - about 23 cases in nearly 300 million people.
Can anthrax
affect pregnancy? Should pregnant women exposed to anthrax take antibiotics?
Anthrax is a serious illness in all humans,
including pregnant women. Inhalational anthrax has a high fatality rate,
and cutaneous (skin) anthrax also is serious, but less frequently fatal.
Because these infections are potentially fatal, it has been recommended that
ciprofloxacin, or similar antibiotic drugs, be prescribed for pregnant women
believed to have been exposed to anthrax. Clinical studies of the use of
the ciprofloxacin in pregnant women have not been conducted, so ciprofloxacin
and related drugs are not generally recommended for pregnant women with less
serious illnesses.
Can anthrax be transmitted by handling money?
The Department of the Treasury sponsored
a study to investigate this risk, and it revealed no evidence that anthrax
can be spread by handling money.
What is the risk for anthrax in
employees of a facility with a
positive environmental sample?
The risk would depend on where the environmental
sample was, the amount (quantity) of material, and if it was collected in
an air sample or on a surface. The risk would also depend on the person’s
contact with the type of sample in terms of breathing or touching the sample.
Finding a positive surface or air sample
does not mean that employees of a facility are at risk for anthrax. Heavily
contaminated surfaces may pose a small risk for cutaneous anthrax, which
can be minimized by clean-up. Laboratory test results of environmental surface
samples should not be the only criterion for starting, continuing, or stopping
preventive antibiotic therapy for inhalational disease.
Anthrax and Influenza
Influenza (flu) and inhalation anthrax can
have
similar symptoms. Does CDC recommend that I get a flu shot to help diagnose anthrax?
You should get a flu shot only to prevent
the flu. CDC does not recommend you get the flu shot so doctors can tell
whether you have the flu or anthrax. Many illnesses (including anthrax) begin
with flu-like symptoms, which include fever, body aches, tiredness, and headaches.
In fact, most illnesses with flu-like symptoms are not either the flu or
anthrax.
The flu vaccine is the best protection you can get to prevent the flu and
its severe complications, especially among those who are at the highest
risk (e.g., people older than 65 years old or younger people with chronic
disease such as diabetes or heart disease). The flu shot can prevent 70%-90%
of flu infections, but it will not prevent illnesses with flu-like symptoms
caused by anything other than influenza.
Is there a way to distinguish between early inhalational
anthrax and flu?
Early inhalational anthrax symptoms can
be similar to those of much more common infections. However, a runny nose
is a rare feature of anthrax. This means that a person who has a runny nose
along with other common influenza-like symptoms is by far more likely to
have the common cold than to have anthrax.
In addition, most people with inhalational
anthrax have high white blood cell counts and no increase in the number
of lymphocytes. On the other hand, people with infections such as flu usually
have low white blood cell counts and an increase in the number of lymphocytes.
Chest X-rays are also critical diagnostic tools. Chest X-rays showed that
all patients with inhalational anthrax have some abnormality, although for
some patients, the abnormality was subtle. CT scans can confirm these abnormalities.
Is there a quick test that
doctors can do to tell whether I have anthrax or an illness like the flu?
Some influenza detection tests give results
fairly quickly. However, these tests are not perfect and are not appropriate
for every patient. Rapid influenza tests can provide results within 24 hours;
viral culture provides results in 3-10 days. However, as many as 30% of samples
that test positive for influenza by viral culture may give a negative rapid
test result. And, some rapid test results may indicate influenza when a person
is not infected with influenza.
Safety Issues/Mail
How can mail get cross-contaminated with
anthrax?
CDC does not have specific studies to address
this, however, cross-contamination of the mail could occur during the processing,
sorting, and delivery of mail when an envelope comes in contact with an envelope,
piece of equipment (e.g., an electronic sorting machine), or other surface
that is contaminated with Bacillus anthracis spores. In addition, airborne
spores in contaminated postal facilities before they were cleaned might play
a role.
Can the presence of Bacillus
anthracis spores be detected by a characteristic appearance, odor, or t
aste?
Bacillus anthracis
spores do not have a characteristic appearance (e.g., color), smell, or
taste. Spores themselves are too small to be seen by the naked eye, but
have been mixed with powder to transport them. The U.S. Postal Service advises
that individuals be suspicious of letters or packages with any powdery substance
on them, regardless of color.
How long do anthrax spores
live?
Anthrax spores can survive for decades
in soil.
What is the importance of
knowing the genetic information about anthrax?
Genetic information about B. anthracis,
particularly to determine genetic similarity among strains, is an important
part of a disease investigation, but it is not immediately required for taking
action to prevent or treat anthrax in those who may have been exposed to
or infected by B. anthracis. Genetic information is often used to determine
the similarity of strains if a common source is suspected.
Does the similarity in strains from Florida, New York,
and Washington, D.C. mean that they came from the same source or are these
just the most common strains?
The strains of anthrax identified in Florida,
New York, and Washington, D.C., are similar and consistent with a naturally
occurring strain that shows no evidence of genetic alteration or bioengineering.
All are sensitive and susceptible to the antibiotics recommended by CDC
for those who have been exposed to or infected with B. anthracis.
When there is a known incident,
how can I prevent anthrax exposure from cross- contaminated mail?
There are no scientifically proven recommendations
for preventing exposure. However, there are some common-sense steps people
can take:
-
Do not open suspicious mail
-
Keep mail away from your face when you open it
-
Do not blow or sniff mail or mail contents
-
Avoid vigorous handling of mail, such as tearing or shredding
-
Wash your hands after handling the mail
-
Discard envelopes after opening mail.
What kind of mail should be considered suspicious?
Identifying Suspicious Packages and Envelopes
Some characteristics of suspicious packages
and envelopes include the following:
- Inappropriate
or unusual labeling
- Excessive postage
- Handwritten or poorly typed addresses
- Misspellings of common words
- Strange return address or no return
address
- Incorrect titles or title without
a name
- Not addressed to a specific person
- Marked with restrictions, such as
"Personal," "Confidential," or "Do not x-ray"
- Marked with any threatening language
- Postmarked from a city or state that
does not match the return address
- Powdery substance felt through
or appearing on the package or envelope
- Oily stains, discolorations, or
odor
- Lopsided or uneven envelope
- Excessive packaging material such
as masking tape, string, etc.
- Excessive weight
- Ticking sound
- Protruding wires or aluminum
foil
If a package or envelope appears
suspicious, Do Not Open It.
What should people do when they get a letter or
package with powder?
Handling of Suspicious Packages
or Envelopes*
-
Do not shake or empty the contents of any suspicious package or envelope.
-
Do not carry the package or envelope, show it to others or allow others
to examine it.
-
Put the package or envelope down on a stable surface; do not sniff,
touch, taste, or look closely at it or at any contents which may have
spilled.
-
Alert others in the area about the suspicious package or envelope.
Leave the area, close any doors, and take actions to prevent others
from entering the area. If possible, shut off the ventilation system.
-
WASH hands with soap and water to prevent spreading potentially infectious
material to face or skin. Seek additional instructions for exposed or
potentially exposed persons.
-
If at work, notify a supervisor, a security officer, or a law enforcement
official. If at home, contact the local law enforcement agency.
-
If possible, create a list of persons who were in the room or area
when this suspicious letter or package was recognized and a list of
persons who also may have handled this package or letter. Give this
list to both the local public health authorities and law enforcement
officials.
*
These recommendations were published on October 26, 2001, in "Update: Investigation
of bioterrorism-related anthrax and interim guidelines for exposure management
and antimicrobial therapy" MMWR 2001; 50(42):909-919. (http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5042a1.htm)
What is the risk
for getting anthrax from handling my own mail?
If there is a risk for inhalational
anthrax associated with exposure to cross-contaminated mail, it is very low.
For example, about 85 million pieces of mail were processed on the few days
in 2001 after envelopes containing Bacillus anthracis (addressed to two U.S.
senators) passed through the New Jersey and District of Columbia sorting
facilities until they were closed. Despite the fact that both of these facilities
had evidence of widespread environmental contamination with B. anthracis
spores and the fact that public health officials had been aggressively looking
for anthrax cases, no new cases of anthrax were identified during that time.
As a postal employee,
am I at risk for getting anthrax from handling mail on the job when there
is an anthrax cross-contaminated mail event?
If there is a risk for inhalational
anthrax associated with exposure to cross-contaminated mail, it is very low,
even for postal employees and persons who work in company mailrooms. CDC
has published interim recommendations that are intended to assist personnel
responsible for occupational health and safety in developing a comprehensive
program to reduce potential cutaneous or inhalational exposures to Bacillus
anthracis spores among workers in work sites where mail is handled
or processed. Detailed guidelines may be found on these Web sites:
When the possibility of cross-contamination
of the mail exists, should I take antibiotics?
Preventive antibiotics are not
recommended for persons who routinely open or handle mail, either at home
or at the workplace. Antimicrobial prophylaxis is recommended only in certain
specific situations such as for persons exposed to an air space known to
be contaminated with aerosolized Bacillus anthracis or for persons in a postal
sorting facility in which an envelope containing B. anthracis spores was
processed. CDC’s complete recommendations on antimicrobial prophylaxis are
contained in the November 9, 2001 MMWR. (http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5044a1.htm)
What should I do
to protect my family and myself if a dangerous chemical agent were released
in my community?
Emergency management teams would
lead efforts in the event of a chemical attack and would let you know if
you need to evacuate the area or seek some type of shelter.
Should I purchase
a gas mask as protection from any chemical agent release such as anthrax?
No, CDC does not recommend purchasing
gas masks. The likelihood that you would be involved in a chemical attack
is low, and your protection is the responsibility of state and federal law
enforcement officials. They are on high alert to ensure that such an event
does not happen. In addition, CDC believes that purchasing a gas mask causes
a false sense of security and can do more harm than good. Masks that aren’t
used properly or that do not fit well will not give you adequate protection.
What kinds of anthrax
worker safety guidelines have been issued?
The recommendations are divided
into four categories. They are engineering controls, administrative controls,
housekeeping controls, and personal protective equipment for workers. The
guidelines describe measures that should be implemented in mail-handling/processing
sites to prevent potential exposures to B. anthracis spores.
Worker Safety
If these recommendations are
followed does it mean workers will stop
getting sick with anthrax?
The interim recommendations that
have been developed are based on the limited information available on ways
to avoid infection and the effectiveness of various prevention strategies.
As new information becomes available the guidelines will be updated. These
recommendations do not address instances where a known or suspected exposure
has occurred. Workers should be trained in how to recognize and handle a
suspicious piece of mail (www.bt.cdc.gov/documentsapp/anthrax/10312001/han51.asp).
In addition, each work site should develop an emergency plan describing
appropriate actions to be taken when a known or suspected exposure to B.
anthracis occurs.
Is CDC telling all
mail handling operations to adopt these anthrax worker safety guidelines
immediately?
Every facility is different and
should be evaluated based on the recommendations in the guidelines, and
the recommendations implemented should be selected on the basis of an initial
evaluation of the work site. This evaluation should focus on determining
which processes, operations, jobs, or tasks would be most likely to result
in an exposure should a contaminated envelope or package enter the work
site. Many of these measures (e.g., administrative controls, use of HEPA
filter-equipped vacuums, wet-cleaning, use of protective gloves) can be
implemented immediately; implementation of others will require additional
time and efforts.
What kinds of engineering
controls should mail-handling/processing operations consider implementing
for detecting anthrax spores?
B. anthracis
spores can be aerosolized during the operation and maintenance of high-speed,
mail-sorting machines, potentially exposing workers and possibly entering
heating, ventilation, or air-conditioning (HVAC) systems. Engineering controls
can provide the best means of preventing worker exposure to potential aerosolized
particles, thereby reducing the risk for inhalational anthrax, the most severe
form of the disease. In settings where such machinery is in use, the following
engineering controls should be considered:
-
An industrial vacuum cleaner equipped with a high-efficiency particulate
air (HEPA) filter for cleaning high-speed, mail-sorting machinery
-
Local exhaust ventilation at pinch roller areas
-
HEPA-filtered exhaust hoods installed in areas where dust is generated
(e.g., areas with high-speed, mail-sorting machinery)
-
Air curtains (using laminar air flow) installed in areas where large
amounts of mail are processed
-
HEPA filters installed in the building’s HVAC systems (if feasible)
to capture aerosolized spores. (Note: Machinery should not be cleaned
using compressed air [i.e., blow-down/blow-off].)
What administrative controls should
mail-handling/processing sites consider implementing to protect workers from
exposure to B. anthracis spores?
Strategies should be developed
to limit the number of people working at or near sites where aerosolized
particles may be generated, such as mail-sorting machinery and places where
mailbags are unloaded or emptied. In addition, restrictions should be in
place to limit the number of people including support staff and nonemployees
entering areas where aerosolized particles may be generated. This recommendation
applies to contractors, business visitors, and support staff.
What housekeeping
controls in mail-handling/processing sites are recommended to protect workers
from exposure to B. anthracis spores?
In the mail-handling worksite,
dry sweeping and dusting should be avoided. Instead, the area should be wet-cleaned
and vacuumed with HEPA-equipped vacuum cleaners.
What personal protective
equipment for workers in mail-handling/processing sites is recommended to
protect workers from exposure to B. anthracis spores?
Personal protective equipment for
workers in mail-handling/processing work sites must be selected on the basis
of the potential for cutaneous or inhalational exposure to B. anthracis spores.
Handling packages or envelopes may result in skin exposure. In addition,
because certain machinery such as electronic mail sorters can generate aerosolized
particles, people who operate, maintain, or work near such machinery may
be exposed through inhalation. People who handle or sort mail or work at
other sites where airborne particles may be generated such as where mailbags
are unloaded or emptied may also be exposed through inhalation.
What are some examples of personal
protective equipment and clothing that could
be used to protect workers who
handle mail from exposure to B. anthracis spores?
-
Protective, impermeable gloves should be worn by all workers who handle
mail. In some cases, workers may need to wear cotton gloves under their
protective gloves for comfort and to prevent dermatitis. Skin rashes
and other dermatological conditions are a potential hazard of wearing
gloves. Latex gloves should be avoided because of the risk of developing
skin sensitivity or allergy.
-
Gloves should be provided in a range of sizes to ensure proper fit.
-
The choice of glove material such as nitrile or vinyl should be based
on safety, fit, durability, and comfort. Sterile gloves such as surgical
gloves are not necessary.
-
Different gloves or layers of gloves may be needed depending on the
task, the dexterity required, and the type of protection needed. Protective
gloves can be worn under heavier gloves such as leather, heavy cotton
for operations where gloves can easily be torn or if more protection
against hand injury is needed.
-
For workers involved in situations where a gloved hand presents a hazard
such as those who work close to moving machine parts, the risk for potential
injury resulting from glove use should be measured against the risk
for potential exposure to B. anthracis.
-
Workers should avoid touching their skin, eyes, or other mucous membranes
since contaminated gloves may transfer B. anthracis spores to other
body sites.
-
Workers should consider wearing long-sleeved clothing and long pants
to protect exposed skin.
-
Gloves and other personal protective clothing and equipment can be
discarded in regular trash once they are removed or if they are visibly
torn, unless a suspicious piece of mail is recognized and handled. If
a suspicious piece of mail is recognized and handled for anthrax, the
worker’s protective gear should be handled as potentially contaminated
material (See " Guideline For Handwashing And Hospital Environmental
Control," 1985, available at www.cdc.gov/ncidod/hip/guide/handwash.htm)
-
Workers should wash their hands thoroughly with soap and water when
gloves are removed, before eating, and when replacing torn or worn gloves.
Soap and water will wash away most spores that may have contacted the
skin; disinfectant solutions are not needed.
Are there some areas in the postal
setting that present a greater risk to some workers than others for anthrax
exposure?
-
People working with or near machinery capable of generating aerosolized
particles, such as electronic mail sorters, or at other work sites where
such particles may be generated should be fitted with NIOSH-approved
respirators that are at least as protective as an N95 respirator.
-
People working in areas where oil mist from machinery is present should
be fitted with respirators equipped with P-type filters.
-
Because facial hair interferes with the fit of protective respirators,
workers with facial hair like beards or large moustaches may require
alternative respirators such as powered air-purifying respirators [PAPRs]
with loose-fitting hoods.
-
Workers who cannot be fitted properly with a half-mask respirator based
on a fit test may require the use of alternative respirators, such as
full facepiece, negative-pressure respirators, PAPRs equipped with HEPA
filters, or supplied-air respirators.
-
If a worker is medically unable to wear a respirator, the employer
should consider reassigning that worker to a job that does not require
respiratory protection.
-
In addition, the use of disposable aprons or goggles by persons working
with or near machinery capable of generating aerosolized particles may
provide an extra margin of protection.
How can I recognize suspicious packages
that have anthrax?
Only specially trained personnel
can distinguish between a real bioterrorism attack and a false one. If you
suspect that a package, letter, or anything else contains a harmful biological
agent, call 911 to activate the local emergency response system; in communities
without 911 systems, notify local law enforcement authorities. Guidance
on identifying suspicious packages and letters and what to do until the
authorities arrive are available on CDC’s Web site at www.bt.cdc.gov/documentsapp/anthrax/10312001/han50.asp.
What can the consumer
buy to protect against germ or chemical warfare such as anthrax?
Currently, the CDC does not recommend
consumers buy any particular product to protect against biological or chemical
attacks.
What should be done with clothing
contaminated with anthrax? Is washing in a regular home washer and dryer
ok?
Does CDC recommend adding bleach to the wash?
Contact your state or local public
health department for advice. Clothing can be decontaminated using soap
and water, and 0.5% hypochlorite solution (one part household bleach to
9 parts water).
Are other solutions used at hospitals
for cleaning blood spills also effective against anthrax?
(Source: Interim Recommendation
for Firefighters and other First Responders) The recommendation for decontaminating
equipment is a 0.5% hypochlorite solution (1 part household bleach to ten
parts water). www.bt.cdc.gov/documentsapp/anthrax/protective/10242001protect.asp.
What actions need
to be taken if a facility is found to have an environmental sample positive
for anthrax?
The number and location of positive
environmental samples will be used to guide clean-up efforts. Positive environmental
samples alone do not indicate the need for antibiotics or the need to close
a facility. Employees, visitors, and family members of employees at these
facilities are advised to monitor their own health carefully and report
any unusual symptoms to a physician.
Reporting
If tests confirm that I was potentially
exposed to Bacillus anthracis or have anthrax, how will it be reported to the
proper authorities?
Your doctor should immediately
report any suspected isolate of Bacillus anthracis or any suspected case
of anthrax to your local or state public health department. The state public
health department is available to your doctor for consultation 24 hours a
day. If local or state health department officials suspect that cases of
illness may be due to a bioterrorist incident, they will notify CDC and an
investigation will be conducted. If the investigation confirms that a bioterrorist
incident has occurred or is thought probable, the FBI will be notified. Public
health officials will also involve other response partners using a preestablished
notification list. The CDC bioterrorism Web site displays the protocol that
health officials will use for further reporting:
http://www.bt.cdc.gov/EMContact/Protocols.asp.
How should healthcare workers
respond to suspected exposure to a bioterrorist agent? Who should healthcare
workers call first, second, third? CDC,
FBI, local police, local health department?
Healthcare providers, clinical
laboratory personnel, and infection control professionals who notice illness
patterns and diagnostic clues that might indicate an unusual infectious
disease outbreak associated with intentional release of a biologic agent
should report any clusters or findings to their local or state health department.
(Guidelines for recognizing a number of biologic agents, including anthrax,
plague, botulism, smallpox, inhalational tularemia, and hemorrhagic fever,
are described in CDC’s Morbidity and Mortality Weekly Report, Vol. 50, No.
41, dated October 19, 2001.)
Source: Centers for Disease Control
v
Anthrax as a Biological Weapon
Introduction
Of the numerous biological agents
that may be used as weapons, the Working Group on Civilian Biodefense has
identified a limited number of organisms that could cause disease and deaths
in sufficient numbers to cripple a city or region. Anthrax is one of the
most serious of these diseases.
High hopes were once vested in
the Biological Weapons and Toxins Convention, which prohibited offensive
biological weapons research or production and was signed by most countries.
However, Iraq and the former Soviet Union, both signatories of the convention,
have subsequently acknowledged having offensive biowarfare programs; a number
of other countries are believed to have such programs, as have some autonomous
terrorist groups. The possibility of a terrorist attack using bioweapons
would be especially difficult to predict, detect, or prevent, and thus, it
is among the most feared terrorist scenarios.
1
Biological agents have seldom been
dispersed in aerosol form, the exposure mode most likely to inflict widespread
disease. Therefore, historical experience provides little information about
the potential impact of a biological attack or the possible efficacy of
postattack measures such as vaccination, antibiotic therapy, or quarantine.
Policies and strategies must therefore rely on interpretation and extrapolation
from an incomplete knowledge base. The Working Group on Civilian Biodefense
reviewed the available literature and expertise and developed consensus
recommendations for medical and public health measures to be taken following
such an attack.
History of Current Threat
For centuries, anthrax has caused
disease in animals and, uncommonly, serious illness in humans throughout
the world. 2
Research on anthrax as a biological weapon began more than 80 years ago.
3 Today, at least 17 nations
are believed to have offensive biological weapons programs
4; it is uncertain how many are
working with anthrax. Iraq has acknowledged producing and weaponizing anthrax.
5
Most experts concur that the manufacture
of a lethal anthrax aerosol is beyond the capacity of individuals or groups
without access to advanced biotechnology. However, autonomous groups with
substantial funding and contacts may be able to acquire the required materials
for a successful attack. One terrorist group, Aum Shinrikyo, responsible
for the release of sarin in a Tokyo, Japan, subway station in 1995,
6 dispersed aerosols of anthrax
and botulism throughout Tokyo on at least 8 occasions. For unclear reasons,
the attacks failed to produce illness.
7
The accidental aerosolized release
of anthrax spores from a military microbiology facility in Sverdlovsk in
the former Soviet Union in 1979 resulted in at least 79 cases of anthrax
infection and 68 deaths and demonstrated the lethal potential of anthrax
aerosols. 8
An anthrax aerosol would be odorless and invisible following release and
would have the potential to travel many kilometers before disseminating.
9-10 Evidence suggests that
following an outdoor aerosol release, persons indoors could be exposed to
a similar threat as those outdoors.
11
In 1970, a World Health Organization
(WHO) expert committee estimated that casualties following the theoretical
aircraft release of 50 kg of anthrax over a developed urban population of
5 million would be 250,000, 100,000 of whom would be expected to die without
treatment. 9
A 1993 report by the US Congressional Office of Technology Assessment estimated
that between 130,000 and 3 million deaths could follow the aerosolized release
of 100 kg of anthrax spores upwind of the Washington, DC, area—lethality
matching or exceeding that of a hydrogen bomb.
12 An economic model developed
by the Centers for Disease Control and Prevention (CDC) suggested a cost
of $26.2 billion per 100,000 persons exposed.
13
Epidemilogy
Naturally occurring anthrax is
a disease acquired following contact with anthrax-infected animals or anthrax-contaminated
animal products. The disease most commonly occurs in herbivores, which are
infected by ingesting spores from the soil. Large anthrax epizootics in
herbivores have been reported; during a 1945 outbreak in Iran, 1 million
sheep died. 14
Animal vaccination programs have reduced drastically the animal mortality
from the disease. 15
However, anthrax spores continue to be documented in soil samples from
throughout the world.16-18
In humans, 3 types of anthrax infection
occur: inhalational, cutaneous, and gastrointestinal. Naturally occurring
inhalational anthrax is now a rare cause of human disease. Historically,
wool sorters at industrial mills were at highest risk. Only 18 cases were
reported in the United States from 1900 to 1978, with the majority occurring
in special-risk groups, including goat hair mill or goatskin workers and
wool or tannery workers. Two of the 18 cases were laboratory associated.
19
Cutaneous anthrax is the most common
naturally occurring form, with an estimated 2000 cases reported annually.
18 Disease typically follows
exposure to anthrax-infected animals. In the United States,
22 4 cases of cutaneous anthrax
were reported between 1944 and 1994.
20 The largest reported epidemic
occurred in Zimbabwe between 1979 and 1985, when more than 10,000 human
cases of anthrax were reported, nearly all of them cutaneous.
21
Gastrointestinal anthrax is uncommonly
reported .18, 22-23
However, gastrointestinal outbreaks have been reported in Africa and Asia.
24 Gastrointestinal anthrax
follows ingestion of insufficiently cooked contaminated meat and includes
2 distinct syndromes, oral-pharyngeal and abdominal.
22, 24-27 In 1982, there were
24 cases of oral-pharyngeal anthrax in a rural northern Thailand outbreak
following the consumption of contaminated buffalo meat.
24 In 1987, there were 14 cases
of gastrointestinal anthrax reported in northern Thailand with both oral-pharyngeal
and abdominal disease occurring.
25
No case of inhalational anthrax
has been reported in the United States since 1978,
19-20 making even a single
case a cause for alarm today. As was demonstrated at Sverdlovsk in 1979,
inhalational anthrax is expected to account for most morbidity and essentially
all mortality following the use of anthrax as an aerosolized biological weapon.
8, 28 In the setting of an
anthrax outbreak resulting from an aerosolized release, cutaneous anthrax
would be less common than inhalational anthrax, easier to recognize, simpler
to treat, and associated with a much lower mortality. In the Sverdlovsk
experience, there were no deaths in patients developing cutaneous anthrax.
8 There is little information
available about the risks of direct contamination of food or water with
anthrax spores. Although human infections have been reported, experimental
efforts to infect primates by direct gastrointestinal instillation of anthrax
spores have not been successful.
29
Microbiology
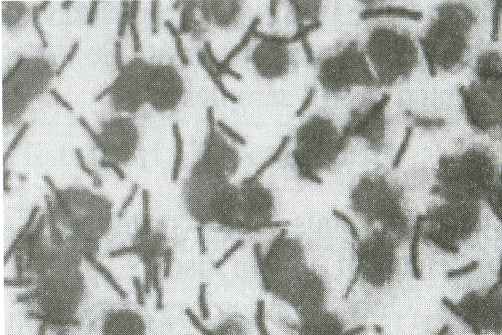
Figure 1.
Gram Stain of Bacillus anthracis. Gram-positive anthrax bacilli
in a peripheral blood smear from a rhesus monkey that died of inhalational
anthrax. Reprinted with permission from Zajtchuk and Bellamy.
23
|
Bacillus anthracis
derives from the Greek word for coal, anthrakis, because the disease
causes black, coal-like skin lesions. Bacillus anthracis is
an aerobic, gram-positive, spore-forming, nonmotile Bacillus species.
The nonflagellated vegetative cell is large (1-8 µm in length, 1-1.5
µm in breadth). Spore size is approximately 1 µm. Spores grow readily
on all ordinary laboratory media at 37°C, with a "jointed
bamboo-rod" cellular appearance and a unique "curled-hair" colonial
appearance, and display no hemolysis on sheep agar (Figure 1). This
cellular and colonial morphology theoretically should make its identification
by an experienced microbiologist straightforward, although few practicing
microbiologists outside the veterinary community have seen anthrax
colonies other than in textbooks.
30 |
Anthrax spores germinate when they
enter an environment rich in amino acids, nucleosides, and glucose, such
as that found in the blood or tissues of an animal or human host. The rapidly
multiplying vegetative anthrax bacilli, on the contrary, will only form spores
after local nutrients are exhausted, such as when anthrax-infected body fluids
are exposed to ambient air.16-17
Full virulence requires the presence of both an antiphagocytic capsule
and 3 toxin components (i.e., protective antigen, lethal factor, and edema
factor). 30
Vegetative bacteria have poor survival outside of an animal or human host;
colony counts decline to undetectable within 24 hours following inoculation
into water.17
This contrasts with the environmentally hardy properties of the B. anthracis
spore, which can survive for decades.
30
Pathogenesis and Clinical
Manifestations
Inhalational Anthrax
Inhalational anthrax follows deposition
of spore-bearing particles of 1 to 5 µm into alveolar spaces.
31-32 Macrophages ingest the
spores, some of which undergo lysis and destruction. Surviving spores are
transported via lymphatics to mediastinal lymph nodes, where germination
may occur up to 60 days later.
28-29,33 The process responsible
for the delayed transformation of spores to vegetative cells is poorly understood
but well documented. In Sverdlovsk, cases occurred from 2 to 43 days after
exposure. 8
In experimental monkeys, fatal disease occurred up to 58 days
28 and 98 days
34 after exposure. Viable spores
have been demonstrated in the mediastinal lymph nodes of monkeys 100 days
after exposure.35
Once germination occurs, disease
follows rapidly. Replicating bacteria release toxins leading to hemorrhage,
edema, and necrosis. 23, 36
In experimental animals, once toxin production has reached critical threshold,
death occurs even if sterility of the bloodstream is achieved with antibiotics.
19 Based on primate data, it
has been estimated that for humans the LD 50 (lethal dose sufficient to
kill 50% of persons exposed to it) is 2500 to 55,000 inhaled anthrax spores.
37
The term inhalational anthrax reflects
the nature of acquisition of the disease. The term anthrax pneumonia is misleading.
Typical bronchopneumonia does not occur. Postmortem pathological study of
patients who died because of inhalational anthrax in Sverdlovsk showed hemorrhagic
thoracic lymphadenitis and hemorrhagic mediastinitis in all patients. In
up to half of the patients, hemorrhagic meningitis also was seen. No patients
who underwent autopsy had evidence of a bronchoalveolar pneumonic process,
although 11 of 42 patients undergoing autopsy had evidence of a focal, hemorrhagic,
necrotizing pneumonic lesion analogous to the Ghon complex associated with
tuberculosis.38
These findings are consistent with other human case series and experimentally
induced inhalational anthrax in animals.
33, 39-40
Early diagnosis of inhalational
anthrax would be difficult and would require a high index of suspicion. Clinical
information is available from only some of the 18 cases reported in the
United States in this century and from the limited available information
from Sverdlovsk. The clinical presentation has been described as a 2-stage
illness. Patients first developed a spectrum of nonspecific symptoms, including
fever, dyspnea, cough, headache, vomiting, chills, weakness, abdominal pain,
and chest pain. 8, 19
Signs of illness and laboratory studies were nonspecific. This stage of
illness lasted from hours to a few days. In some patients, a brief period
of apparent recovery followed. Other patients progressed directly to the
second, fulminant stage of illness.
2, 19, 41
This second stage
developed abruptly, with sudden fever, dyspnea, diaphoresis, and shock. Massive
lymphadenopathy and expansion of the mediastinum led to stridor in some
cases. 42-43
A chest radiograph most often showed a widened mediastinum consistent with
lymphadenopathy (Figure 2). 42
Up to half of patients
developed hemorrhagic meningitis with concomitant meningismus, delirium,
and obtundation. In this second stage of illness, cyanosis and hypotension
progress rapidly; death sometimes occurs within hours.
2, 19, 41
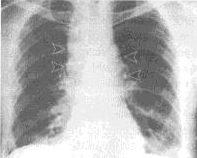
Figure 2.
Chest radiograph of a patient with inhalational anthrax. Chest radiograph
of a 51-year-old laborer with occupational exposure to airborne
anthrax spores taken on day 2 of illness. Lobulated mediastinal
widening (arrowheads) is present, consistent with lymphadenopathy,
with a small parenchymal infiltrate at the left lung base. Reprinted
with permission from Zajtchuk and Bellamy.
23
|
The
mortality rate of occupationally acquired cases in the United States
is 89%, but the majority of cases occurred before the development
of critical care units and, in some cases, before the advent of
antibiotics. 19
At Sverdlovsk, it is reported that 68 of the 79 patients with
inhalational anthrax died, although the reliability of the diagnosis
in the survivors is questionable.
8 Patients who had
onset of disease 30 or more days after release of organisms had a
higher reported survival rate compared with those with earlier disease
onset. Antibiotics, antianthrax globulin, and vaccine were used
to treat some residents in the affected area some time after exposure,
but which patients received these interventions and when is not
known. In fatal cases, the interval between onset of symptoms and
death averaged 3 days. This is similar to the disease course and case
fatality rate in untreated experimental monkeys, which have developed
rapidly fatal disease even after a latency as long as 58 days.
28 |
Modern mortality rates in the setting
of contemporary medical and supportive therapy might be lower than those
reported historically. However, the 1979 Sverdlovsk experience is not instructive.
Although antibiotics, antianthrax globulin, corticosteroids, and mechanical
ventilation were used, individual clinical records have not been made public.8
It is also uncertain if the B. anthracis strain to which patients were exposed
was susceptible to the predominant antibiotics that were used during the
outbreak.
Physiological sequelae of severe
anthrax infection in animal models have been described as hypocalcemia,
profound hypoglycemia, hyperkalemia, depression and paralysis of respiratory
center, hypotension, anoxia, respiratory alkalosis, and terminal acidosis.
44-45 Those animal studies
suggest that in addition to the rapid administration of antibiotics, survival
might improve with vigilant correction of electrolyte disturbances and acid-base
imbalance, glucose infusion, and early mechanical ventilation and vasopressor
administration.
Cutaneous Anthrax
Cutaneous anthrax occurs following
the deposition of the organism into skin with previous cuts or abrasions
especially susceptible to infection.
21, 46 Areas of exposed skin,
such as arms, hands, face, and neck, are the most frequently affected. There
are no data to suggest the possibility of a prolonged latency period in
cutaneous anthrax. In Sverdlovsk, cutaneous cases occurred only as late
as 12 days after the original aerosol release.
8 After the spore germinates
in skin tissues, toxin production results in local edema (Figure 3). An
initially pruritic macule or papule enlarges into a round ulcer by the second
day. Subsequently, 1- to 3-mm vesicles may appear, which discharge clear
or serosanguinous fluid containing numerous organisms on Gram stain. As
shown in Figure 3, development of a painless, depressed, black eschar follows,
often associated with extensive local edema. The eschar dries, loosens,
and falls off in the next 1 to 2 weeks, most often leaving no permanent scar.
Lymphangitis and painful lymphadenopathy can occur with associated systemic
symptoms. Although antibiotic therapy does not appear to change the course
of eschar formation and healing, it does decrease the likelihood of systemic
disease. Without antibiotic therapy, the mortality rate has been reported
to be as high as 20%; with antibiotics, death due to cutaneous anthrax is
rare.2
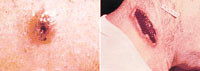
|
Figure 3.
Cutaneous Anthrax
Left, Forearm lesion on day 7 of illness
shows vesiculation and ulceration of the initial macular or papular
anthrax skin lesion. Right, Eschar of the neck on day 15 of illness
is typical of the last stage of the lesion before it resolves over
1 to 2 weeks. Reprinted with permission from Binford CH, Connor DH,
eds. Pathology of Tropical and Extraordinary Diseases. Vol 1. Washington,
DC: Armed Forces Institute of Pathology; 1976:119. AFIP negative.
71-1290-2.
|
Gastrointestinal Anthrax
Gastrointestinal anthrax occurs
following deposition and subsequent germination of spores in the upper or
lower gastrointestinal tract. The former results in the oral-pharyngeal
form of disease. 24-26
An oral or esophageal ulcer leads to development of regional lymphadenopathy,
edema, and sepsis. 24-26
The latter results in primary intestinal lesions occurring predominantly
in the terminal ileum or cecum,
38 presenting initially with
nausea, vomiting, and malaise and progressing rapidly to bloody diarrhea,
acute abdomen, or sepsis.22
Massive ascites has occurred in some cases of gastrointestinal anthrax.
27 Advanced infection may appear
similar to the sepsis syndrome occurring in either inhalational or cutaneous
anthrax. 2
Some authors suggest that aggressive medical intervention such as would
be recommended for inhalational anthrax may reduce mortality, although,
given the difficulty of early diagnosis, mortality almost inevitably would
be high.2, 22
Diagnosis
Given the rarity of anthrax infection
and the possibility that early cases are a harbinger of a larger epidemic,
the first suspicion of an anthrax illness must lead to immediate notification
of the local or state health department, local hospital epidemiologist, and
local or state health laboratory. By this mechanism, definitive tests can
be arranged rapidly through a reference laboratory and, as necessary, the
US Army Medical Research Institute of Infectious Diseases (USAMRIID), Fort
Detrick, Md.
The first evidence of a clandestine
release of anthrax as a biological weapon most likely will be patients seeking
medical treatment for symptoms of inhalational anthrax. The sudden appearance
of a large number of patients in a city or region with an acute-onset flulike
illness and case fatality rates of 80% or more, with nearly half of all deaths
occurring within 24 to 48 hours, is highly likely to be anthrax or pneumonic
plague (Table 1). Currently, there are no effective atmospheric warning systems
to detect an aerosol cloud of anthrax spores.
47
Rapid diagnostic tests for diagnosing
anthrax, such as enzyme-linked immunosorbent assay for protective antigen
and polymerase chain reaction, are available only at national reference
laboratories. Given the limited availability of these tests and the time required
to dispatch specimens and perform assays, rapid diagnostic testing would
be primarily for confirmation of diagnosis and determining in vitro susceptibility
to antibiotics. In addition, these tests will be used in the investigation
and management of anthrax hoaxes, such as the series occurring in late 1998.
48 They would also be of value
should suspicious materials in the possession of a terrorist be identified
as possibly containing anthrax.
|
Table 1. Diagnosis of Inhalational Infection |
| Epidemiology |
Sudden
appearance of multiple cases of severe flu like illness with fulminant
course and high mortality |
|
Diagnostic studies
Microbiology
|
Chest
radiograph: widened mediastinum
Peripheral blood smear: gram-positive
bacilli on unspun smear
|
| Blood
culture growth of large gram-positive bacilli with preliminary identification
of Bacillus species |
| Pathology |
Hemorrhagic
mediastinitis, hemorrhagic thoracic lymphadenitis, hemorrhagic meningitis |
If only small numbers of cases
present contemporaneously, the clinical similarity of early inhalational
anthrax to other acute respiratory tract infections may delay initial diagnosis
for some days. However, diagnosis of anthrax could soon become apparent
through the astute recognition of an unusual radiological finding, identification
in the microbiology laboratory, or recognition of specific pathologic findings.
A widened mediastinum on chest radiograph (Figure 2) in a previously healthy
patient with evidence of overwhelming flulike illness is essentially pathognomonic
of advanced inhalational anthrax and should prompt immediate action.
23, 42 Although treatment at
this stage would be unlikely to alter the outcome of illness in the patient
concerned, it might lead to earlier diagnosis in others.
Microbiologic studies can also
demonstrate B. anthracis and may be the means for initial detection of an
outbreak. The bacterial burden may be so great in advanced infection that
bacilli are visible on Gram stain of unspun peripheral blood, as has been
demonstrated in primate studies (Figure 1). While this is a remarkable finding
that would permit an astute clinician or microbiologist to make the diagnosis,
the widespread use of automated cell-counter technology in diagnostic laboratories
makes this unlikely.41
The most useful microbiologic test
is the standard blood culture, which should show growth in 6 to 24 hours.
If the laboratory has been alerted to the possibility of anthrax, biochemical
testing and review of colonial morphology should provide a preliminary diagnosis
12 to 24 hours later. Definitive diagnosis would require an additional 1
to 2 days of testing in all but a few national reference laboratories. It
should be noted, however, that if the laboratory has not been alerted to
the possibility of anthrax, B. anthracis may not be correctly identified.
Routine laboratory procedures customarily identify a Bacillus species from
a blood culture approximately 24 hours after growth, but most laboratories
do not further identify Bacillus species unless specifically requested
to do so. In the United States, the isolation of
species most often represents
growth of Bacillus cereus. The laboratory and clinician must determine whether
its isolation represents specimen contamination.
49 There have been no B. anthracis
bloodstream infections reported for more than 20 years. However, given the
possibility of anthrax being used as a weapon and the importance of early
diagnosis, it would be prudent for laboratory procedures to be modified so
that B. anthracis is excluded after identification of a Bacillus species
bacteremia.
Sputum culture and Gram stain are
unlikely to be diagnostic, given the lack of a pneumonic process.
30 If cutaneous anthrax is
suspected, a Gram stain and culture of vesicular fluid will confirm the
diagnosis.
A diagnosis of inhalational anthrax
also might occur at postmortem examination following a rapid, unexplained
terminal illness. Thoracic hemorrhagic necrotizing lymphadenitis and hemorrhagic
necrotizing mediastinitis in a previously healthy adult are essentially
pathognomonic of inhalational anthrax.
38, 43 Hemorrhagic meningitis
should also raise strong suspicion of anthrax infection.
23, 38, 43, 50 Despite pathognomonic
features of anthrax on gross postmortem examination, the rarity of anthrax
makes it unlikely that a pathologist would immediately recognize these findings.
If the case were not diagnosed at gross examination, additional days would
likely pass before microscopic slides would be available to suggest the
disease etiology.
Vaccination
The US anthrax vaccine, an inactivated
cell-free product, was licensed in 1970 and is produced by Bioport Corp,
Lansing, Mich (formerly called the Michigan Biologic Products Institute).
The vaccine is licensed to be given in a 6-dose series and has recently
been mandated for all US military active- and reserve-duty personnel.
51 The vaccine is made from
the cell-free filtrate of a nonencapsulated attenuated strain of B. anthracis.
52 The principal antigen responsible
for inducing immunity is the protective antigen.
18, 23 A similar vaccine has
been shown in one small placebo-controlled human trial to be efficacious
against cutaneous anthrax.53
As of March 1, 1999, approximately 590,000 doses of anthrax vaccine have
been administered to US Armed Forces (Gary Strawder, Department of Defense,
Falls Church, Va, oral communication, April 1999); no serious adverse events
have been causally related (Miles Braun, Food and Drug Administration, Rockville,
Md, written communication, April 1999). In a study of experimental monkeys,
inoculation with this vaccine at 0 and 2 weeks was completely protective
against an aerosol challenge at 8 and 38 weeks and 88% effective at 100
weeks. 54
A human live attenuated vaccine
is produced and used in countries of the former Soviet Union.
55 In the Western world, live
attenuated vaccines have been considered unsuitable for use in humans.
55
Current vaccine supplies are limited
and the US production capacity is modest. It will be years before increased
production efforts can make available sufficient quantities of vaccine for
civilian use. However, even if vaccine were available, populationwide vaccination
would not be recommended at this time, given the costs and logistics of a
large-scale vaccination program and the unlikely occurrence of a bioterrorist
attack in any given community. Vaccination of some essential service personnel
should be considered if vaccine becomes available. Postexposure vaccination
following a biological attack with anthrax would be recommended with antibiotic
administration to protect against residual retained spores, if vaccine were
available.
Therapy
Recommendations regarding antibiotic
and vaccine use in the setting of a biological anthrax attack are conditioned
by a limited number of studies in experimental animals, current understanding
of antibiotic resistance patterns, and the possible requirement to treat
large numbers of casualties. A number of possible therapeutic strategies
have yet to be fully explored experimentally or submitted for approval to
the FDA. For these reasons, the working group offers consensus recommendations
based on the best available evidence. The recommendations do not represent
uses currently approved by the FDA or an official position on the part of
any of the federal agencies whose scientists participated in these discussions
and will need to be revised as further relevant information becomes available.
Given the rapid course of symptomatic
inhalational anthrax, early antibiotic administration is essential. A delay
of antibiotic treatment for patients with anthrax infection even by hours
may substantially lessen chances for survival.
56-57 Given the difficulty
in achieving rapid microbiologic diagnosis of anthrax, all persons with
fever or evidence of systemic disease in an area where anthrax cases are
occurring should be treated for anthrax until the disease is excluded.
There are no clinical studies of
the treatment of inhalational anthrax in humans. Thus, antibiotic regimens
commonly recommended for empirical treatment of sepsis have not been studied
in this setting. In fact, natural strains of B. anthracis are resistant to
many of the antibiotics used in these empirical regimens, such as those of
the extended-spectrum cephalosporins.
58-59 Most naturally occurring
anthrax strains are sensitive to penicillin, and penicillin historically
has been the preferred therapy for the treatment of anthrax. Penicillin is
approved by the FDA for this indication,
41, 56-57 as is doxycycline.
60 In studies of small numbers
of monkeys infected with susceptible strains of B. anthracis, oral doxycycline
has proved efficacious. 41
|
Table 2. Working Group Recommendations for Medical Therapy for
Patients With
Clinically Evident Inhalational Anthrax
Infection in the Contained Casualty
Setting28,41,60,62,63†
|
|
Optimal Therapy if
Initial Therapy
Strain Is Proven Susceptible Duration, d
‡
|
|
Adults
Ciprofloxacin,
400 mg intravenously Penicillin G, 4 million U intravenously
60
every 12 h every
12 h
Doxycycline, 100 mg intravenously
every 12 h§
|
|
Children Ciprofloxacin, 20-30 mg/kg per day Age<12 y: penicillin
G, 50 0000 U/kg 60
divided into 2 daily doses,
intravenously every 6 h
not to exceed 1 g/d Age
ł12 y: penicillin G, 4 million U
intravenously every 4 h
|
|
Pregnant women¶
Same for nonpregnant adults
|
|
Immunosuppressed persons Same
as for nonimmunosuppressed adults and children
|
|
* Most recommendations are based on animal
studies or in-vitro studies and are not approved by the US Food and Drug
Administration (FDA). These recommendations are not FDA approved but were
reached by consensus the working group. See text for explanations and
alternatives.
† In vitro studies suggest ofloxacin, 400 mg intravenously
every 12 hours, or levofloxacin, 500 mg intravenously every 24 hours,
could be substituted for ciprofloxacin.
‡ Oral antibiotics should be substituted for intravenous
antibiotics as soon as clinical condition improves.
§ In vitro studies suggest tetracycline could be substituted
for doxycycline.
| Doxycycline could also be used. For children heavier than
45 kg. use adult dosage. For children 45 kg or lighter, use 2.5 mg/kg
doxycycline intravenously every 12 hours. Refer to management of pediatric
population in text for details.
¶ Refer to section on management of pregnant women in text
for details.
|
|
Table
3. Working Group Recommendations for Medical Therapy for Patients
With
Clinically Evident Anthrax Infection in the Mass Casualty Setting
or for
Postexposure Prophylaxis 41
*
|
|
Optimal Therapy if
Initial
Therapy†
Strain Is Proven Susceptible
Duration, d‡
|
|
Adults Ciprofloxacin, 500 mg
Amoxicillin, 500 mg every 8 hr 60
intravenously every 12 h Doxycycline,
100 mg by mouth
every 12 h‡
|
|
Children§ Ciprofloxacin, 20-30 mg/kg Weight
ł 20 kg: amoxicillin, 500 mg
60
per day by mouth divided into 2 by mouth every
8 hr
daily doses, not to exceed 1 g/d Weight <20
kg: amoxicillin, 40 mg/kg
divided into 3 doses to be taken every 8 h
|
|
Pregnant Ciprofloxacin, 500 mg by mouth Amoxicillin, 500 mg
by mouth every 8 h 60
women|| every 12 h
|
| Immunosuppressed
persons Same as for nonimmunosuppressed adults and
children |
|
* Most recommendations are
based on animal studies or in-vitro studies and are not approved
by the US Food and Drug Administration (FDA). These recommendations
are not FDA approved but were reached by consensus of the working
group. See text for explanations and alternatives.
† In vitro studies suggest ofloxacin, 400
mg intravenously every 12 hours, or levofloxacin, 500 mg by mouth
every 24 hours, could be substituted for ciprofloxacin.
‡ In vitro studies suggest tetracycline,
500 mg by mouth every 6 hours, could be substituted for doxycycline.
§ Doxycycline could also be used. For children
heavier than 45 kg. use adult dosage. For children 45 kg or lighter,
use 2.5 mg/kg doxycycline by mouth every 12 hours. Refer to management
of pediatric population in text for details.
| Refer to section on management of pregnant
women in text for details.
|
Doxycycline is the
preferred option from the tetracycline class of antibiotics because of its
proven efficacy in monkey studies and its ease of administration. Other
members of this class of antibiotics are suitable alternatives. Although
treatment of anthrax infection with ciprofloxacin has not been studied in
humans, animal models suggest excellent efficacy.
28, 41, 61 In vitro data suggest that other
fluoroquinolone antibiotics would have equivalent efficacy in treating anthrax
infection, although no animal data exist for fluoroquinolones other than
ciprofloxacin. 59
Reports have been
published of a B. anthracis vaccine strain that has been engineered by Russian
scientists to resist the tetracycline and penicillin classes of antibiotics.
62 Although the engineering of quinolone-resistant
B. anthracis may also be possible, to date there have been no published
accounts of this.
Balancing considerations
of efficacy with concerns regarding resistance, the working group recommends
that ciprofloxacin or other fluoroquinolone therapy be initiated in adults
with presumed inhalational anthrax infection. Antibiotic resistance to penicillin-
and tetracycline-class antibiotics should be assumed following a terrorist
attack until laboratory testing demonstrates otherwise. Once the antibiotic
susceptibility of the B. anthracis strain of the index case has
been determined, the most widely available, efficacious, and least toxic
antibiotic should be administered to patients and persons requiring postexposure
prophylaxis.
In a contained casualty
setting (a situation in which a modest number of patients require therapy),
the working group recommends intravenous antibiotic therapy, as shown in
Table 2. If the number of persons requiring therapy is sufficiently high
(i.e., a mass casualty setting), the working group recognizes that intravenous
therapy will no longer be possible for reasons of logistics and/or exhaustion
of equipment and antibiotic supplies, and oral therapy will need to be used
(Table 3). The threshold number of cases at which parenteral therapy becomes
impossible depends on a variety of factors, including local and regional
health care resources.
In experimental
animals, antibiotic therapy during anthrax infection has prevented development
of an immune response. 28, 62
This suggests that even if the antibiotic-treated patient survives anthrax
infection, risk for recurrence remains for at least 60 days because of the
possibility of delayed germination of spores. Therefore, the working group
recommends that antibiotic therapy be continued for 60 days, with oral therapy
replacing intravenous therapy as soon as a patient’s clinical condition improves.
If vaccine were to become widely available, postexposure vaccination in patients
being treated for anthrax infection might permit the duration of antibiotic
administration to be shortened to 30 to 45 days, with concomitant administration
of 3 doses of anthrax vaccine at 0, 2, and 4 weeks.
The treatment of
cutaneous anthrax historically has been with oral penicillin. The working
group recommends that oral fluoroquinolone or tetracycline antibiotics as
well as amoxicillin in the adult dosage schedules described in Table 2 and
Table 3 would be suitable alternatives if antibiotic susceptibility is proved.
Although previous guidelines have suggested treating cutaneous anthrax for
7 to 10 days, 23, 49
the working group recommends treatment for 60 days in the setting of bioterrorism,
given the presumed exposure to the primary aerosol. Treatment of cutaneous
anthrax generally prevents progression to systemic disease, although it
does not prevent the formation and evolution of the eschar. Topical therapy
is not useful.2
Other antibiotics
effective against B. anthracis in vitro include chloramphenicol,
erythromycin, clindamycin, extended-spectrum penicillins, macrolides, aminoglycosides,
vancomycin hydrochloride, cefazolin, and other first-generation cephalosporins.
58-59,64 The efficacy of these antibiotics
has not been tested in humans or animal studies. The working group recommends
the use of these antibiotics only if the previously cited antibiotics are
unavailable or if the strain is otherwise antibiotic resistant. Natural
resistance of B. anthracis strains exists against sulfamethoxazole, trimethoprim,
cefuroxime, cefotaxime sodium, aztreonam, and ceftazidime.
58-59,64 Therefore, these antibiotics should
not be used in the treatment or prophylaxis of anthrax infection.
Postexposure
Prophylaxis
Guidelines regarding
which populations would require postexposure prophylaxis following the release
of anthrax as a biological weapon would need to be developed quickly by
state and local health departments in consultation with national experts.
These decisions require estimates of the timing and location of the exposure
and the relevant weather conditions in an outdoor release.65 Ongoing monitoring
of cases would be needed to define the high-risk areas, direct follow-up,
and guide the addition or deletion of groups to receive postexposure prophylaxis.
There are no FDA-approved
postexposure antibiotic regimens following exposure to an anthrax aerosol.
For postexposure prophylaxis, the working group recommends the same antibiotic
regimen as that recommended for treatment of mass casualties; prophylaxis
should be continued for 60 days (Table 3).
Management
of Special Groups
Consensus recommendations
for special groups as set forth herein reflect the clinical and evidence-based
judgments of the working group and at this time do not necessarily correspond
with FDA-approved use, indications, or labeling.
Children
It has been recommended
that ciprofloxacin and other fluoroquinolones should not be used in children
younger than 16 to 18 years because of a link to permanent arthropathy in
adolescent animals and transient arthropathy in a small number of children.
60 However, balancing these risks against the
risks of anthrax caused by an engineered antibiotic-resistant strain, the
working group recommends that ciprofloxacin be used in the pediatric population
for initial therapy or postexposure prophylaxis following an anthrax attack
(Table 2). If antibiotic susceptibility testing allows, penicillin should
be substituted for the fluoroquinolone.
As a third alternative,
doxycycline could be used. The American Academy of Pediatrics has recommended
that doxycycline not be used in children younger than 9 years because the
drug has resulted in retarded skeletal growth in infants and discolored
teeth in infants and children.60
However, the serious risk of infection following an anthrax attack supports
the consensus recommendation that doxycycline be used in children if antibiotic
susceptibility testing, exhaustion of drug supplies, or allergic reaction
preclude use of penicillin and ciprofloxacin.
In a contained casualty
setting, the working group recommends that children receive intravenous
antibiotics (Table 2). In a mass casualty setting and as postexposure prophylaxis,
the working group recommends that children receive oral antibiotics (Table
3).
The US vaccine is
licensed for use only in persons aged 18 to 65 years because studies to date
have been conducted exclusively in this group.
52 No data exist for children, but based on
experience with other inactivated vaccines, it is likely that the vaccine
would be safe and effective.
Pregnant
Women
Fluoroquinolones
are not generally recommended during pregnancy because of their known association
with arthropathy in adolescent animals and small numbers of children. Animal
studies have discovered no evidence of teratogenicity related to ciprofloxacin,
but no controlled studies of ciprofloxacin in pregnant women have been conducted.
Balancing these possible risks against the concerns of anthrax due to engineered
antibiotic-resistant strains, the working group recommends that ciprofloxacin
be used in pregnant women for therapy and postexposure prophylaxis following
an anthrax attack (Table 2 and Table 3). No adequate controlled trials of
penicillin or amoxicillin administration during pregnancy exist. However,
the CDC recommends penicillin for the treatment of syphilis during pregnancy
and amoxicillin as a treatment alternative for chlamydial infections during
pregnancy. 60
The working group
recommends that pregnant women receive fluoroquinolones in the usual adult
dosages. If susceptibility testing allows, intravenous penicillin in the
usual adult dosages should be substituted for fluoroquinolones. As a third
alternative, intravenous doxycycline could be used. The tetracycline class
of antibiotics has been associated with both toxic effects in the liver in
pregnant women and fetal toxic effects, including retarded skeletal growth.
60 Balancing the risks of anthrax infection
with those associated with doxycycline use in pregnancy, the working group
recommends that doxycycline be used in pregnant women for therapy and postexposure
prophylaxis if antibiotic susceptibility testing, exhaustion of drug supplies,
or allergic sensitivity preclude the use of penicillin and ciprofloxacin.
If doxycycline is used in pregnant women, periodic liver function testing
should be performed if possible.
Ciprofloxacin (and
other fluoroquinolones), penicillin, and doxycycline (and other tetracyclines)
are each excreted in breast milk. Therefore, a breast-feeding woman should
be treated or given prophylaxis with the same antibiotic as her infant based
on what is most safe and effective for the infant (see pediatric guidelines
herein) to minimize risk to the infant.
Immunosuppressed
Persons
The antibiotic treatment
or postexposure prophylaxis for anthrax among those who are immunosuppressed
has not been studied in human or animal models of anthrax infection. Therefore,
the working group consensus recommendation is to administer antibiotics
as for immunocompetent adults and children (Table 2 and Table 3).
Infection Control
There are no data
to suggest patient-to-patient transmission of anthrax occurs.
8, 46 Thus, standard barrier isolation precautions
are recommended for hospitalized patients with all forms of anthrax infection,
but the use of high-efficiency particulate air filter masks or other measures
for airborne protection are not indicated.66
There is no need to immunize or provide prophylaxis to patient contacts
(e.g., household contacts, friends, coworkers) unless a determination is
made that they, like the patient, were exposed to the aerosol at the time
of the attack.
In addition to immediate
notification of the hospital epidemiologist and state health department,
the local hospital microbiology laboratories should be notified at the first
indication of anthrax so that safe specimen processing under biosafety level
2 conditions can be undertaken.41, 67
A number of disinfectants used for standard hospital infection control,
such as hypochlorite, are effective in cleaning environmental surfaces contaminated
with infected bodily fluids.17, 66
Proper burial or
cremation of humans and animals who have died because of anthrax infection
is important in preventing further transmission of the disease. Serious consideration
should be given to cremation. Embalming of bodies could be associated with
special risks. 66
If autopsies are performed, all related instruments and materials should
be autoclaved or incinerated. 66
Animal transmission might occur if infected animal remains are not cremated
or buried. 16, 21
Decontamination
Recommendations
regarding decontamination in the event of an intentional aerosolization
of anthrax spores are based on evidence concerning aerosolization, anthrax
spore survival, and environmental exposures at Sverdlovsk and among goat
hair mill workers. The greatest risk to human health following an intentional
aerosolization of anthrax spores occurs during the period in which anthrax
spores remain airborne, called primary aerosolization. The duration
for which spores remain airborne and the distance spores travel before they
become noninfectious or fall to the ground is dependent on meteorological
conditions and aerobiological properties of the dispersed aerosol.
8, 65 Under circumstances of maximum survival
and persistence, the aerosol would be fully dispersed within hours to 1
day at most, well before the first symptomatic cases would be seen. Following
the discovery that a bioweapon has been used, anthrax spores may be detected
on environmental surfaces using rapid assay kits or culture, but they provide
no indication as to the risk of reaerosolization.
The risk that anthrax
spores might pose to public health after the period of primary aerosolization
can be inferred from the Sverdlovsk experience, investigations in animal
hair processing plants, and modeling analyses by the US Army. At Sverdlovsk,
new cases of inhalational anthrax developed as late as 43 days after the
presumed date of release, but none occurred during the months and years afterward.
68 Some have questioned whether any of those
cases with onset of disease beyond 7 days might have represented illness
following resuspension of spores from the ground or other surfaces, a process
that has been called secondary aerosolization. While it is impossible to
state with certainty that secondary aerosolizations did not occur, it appears
unlikely. It should be noted that few efforts were made to decontaminate
the environment after the accident and only 47,000 of the city’s 1 million
inhabitants were vaccinated.8
The epidemic curve (Figure 4) is typical for a common-source epidemic,
and it is possible to account for virtually all patients having been within
the area of the plume on the day of the accident. Moreover, if secondary
aerosolization had been important, new cases almost certainly would have
continued for a period well beyond the observed 43 days.
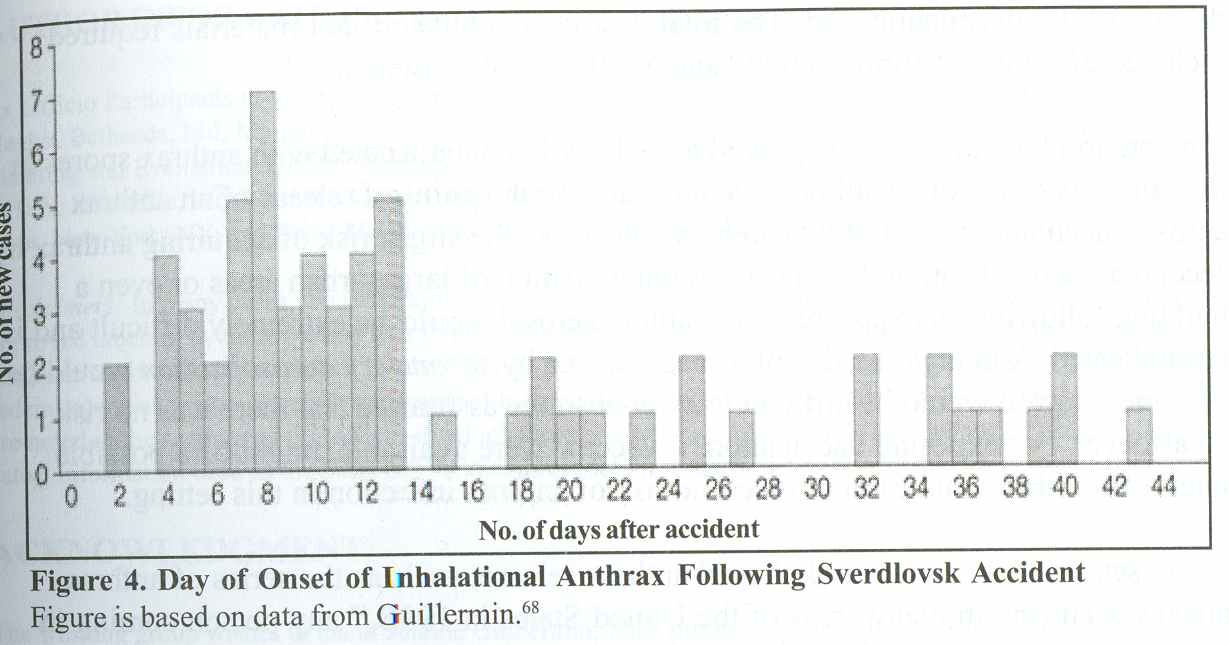
Although persons working with animal
hair or hides are known to be at increased risk of developing inhalational
or cutaneous anthrax, surprisingly few of those exposed in the United States
have developed disease. During the first half of this century, a significant
number of goat hair mill workers were likely exposed to aerosolized spores.
Mandatory vaccination became a requirement for working in goat hair mills
only in the 1960s. Meanwhile, many unvaccinated person-years of high-risk
exposure had occurred, but only 13 cases of inhalational anthrax were reported.
19, 44 One study of environmental exposure
was conducted at a Pennsylvania goat hair mill at which workers were shown
to inhale up to 510 B. anthracis particles of at least 5 µm in diameter
per person per 8-hour shift. These concentrations of spores were constantly
present in the environment during the time of this study,
44 but no cases of inhalational anthrax occurred.
Modeling analyses
have been carried out by US Army scientists seeking to determine the risk
of secondary aerosolization. One study concluded that there was no significant
threat to personnel in areas contaminated by 1 million spores per square
meter either from traffic on asphalt-paved roads or from a runway used by
helicopters or jet aircraft. 69
A separate study showed that in areas of ground contaminated with 20 million
Bacillus subtilis spores per square meter, a soldier exercising actively
for a 3-hour period would inhale between 1000 and 15,000 spores.
70
Much has been written
about the technical difficulty of decontaminating an environment contaminated
with anthrax spores. A classic case is the experience at Gruinard Island
in the United Kingdom. During World War II, British military undertook explosives
testing with anthrax spores on this island off the Scottish coast. Spores
persisted and remained viable for 36 years following the conclusion of testing.
Decontamination of the island occurred in stages, beginning in 1979 and ending
in 1987, when the island was finally declared fully decontaminated. The total
cost is unpublished, but materials required included 280 tons of formaldehyde
and 2000 tons of seawater.17, 71
If an environmental
surface is proved to be heavily contaminated with anthrax spores in the immediate
area of a spill or close proximity to the point of release of an anthrax
aerosol, decontamination of that area may decrease the slight risk of acquiring
anthrax by secondary aerosolization. However, decontamination of large urban
areas or even a building following an exposure to an anthrax aerosol would
be extremely difficult and is not indicated. Although the risk of disease
caused by secondary aerosolization would be extremely low, it would be
difficult to offer absolute assurance that there was no risk whatsoever.
Postexposure vaccination, if vaccine were available, might be a possible
intervention that could further lower the risk of anthrax infection in this
setting.
In the setting of
an announced alleged anthrax release, such as the series of anthrax hoaxes
occurring in many areas of the United States in 1998,
48 any person coming in direct physical contact
with a substance alleged to be anthrax should perform thorough washing of
the exposed skin and articles of clothing with soap and water.72 Further
decontamination of directly exposed individuals or of others is not indicated.
In addition, any person in direct physical contact with the alleged substance
should receive postexposure antibiotic prophylaxis until the substance is
proved not to be anthrax. If the alleged substance is proved to be anthrax,
immediate consultation with experts at the CDC and USAMRIID should be obtained.
ADDITIONAL RESEARCH
To develop a maximally
effective response to a bioterrorist incident involving anthrax, the medical
community will require new knowledge of the organism, its genetics and pathogenesis,
improved rapid diagnostic techniques, improved prophylactic and therapeutic
regimens, and an improved second-generation vaccine.
47 A recently published Russian study indicates
that genes transferred from the related B cereus can act to enable
B. anthracis to evade the protective effect of the live attenuated Russian
vaccine in a rodent model.73
Research is needed to determine the role of these genes with respect to
virulence and ability to evade vaccine-induced immunity. Furthermore, the
relevance of this finding for the US vaccine needs to be established. An accelerated
vaccine development effort is needed to allow the manufacture of an improved
second-generation product that requires fewer doses. Finally, an expanded
knowledge base is needed regarding possible maximum incubation times after
inhalation of spore-containing aerosols and optimal postexposure antibiotic
regimens.
AUTHOR INFORMATION
Ex Officio Participants
in the Working Group on Civilian Biodefense: George Curlin, MD, National
Institutes of Health, Bethesda, Md; Margaret Hamburg, MD, and William Raub,
PhD, Office of Assistant Secretary for Planning and Evaluation, DHHS, Washington,
DC; Robert Knouss, MD, Office of Emergency Preparedness, DHHS, Rockville,
Md; Marcelle Layton, MD, Office of Communicable Disease, New York City Health
Department, New York, NY; and Brian Malkin and Stuart Nightingale, MD, FDA,
Rockville.
Disclaimers: In
many cases, the indication and dosages and other information are not consistent
with current approved labeling by the US Food and Drug Administration (FDA).
The recommendations on the use of drugs and vaccine for uses not approved
by the FDA do not represent the official views of the FDA or of any of the
federal agencies whose scientists participated in these discussions. Unlabeled
uses of the products recommended are noted in the sections of this article
in which these products are discussed. Where unlabeled uses are indicated,
information used as the basis for the recommendation is discussed.
ACKNOWLEDGEMENT
The working group
wishes to thank Jeanne Guillermin, PhD, professor of sociology, Boston College,
Boston, Mass, for her comments on the manuscript. Starting in 1992, Dr Guillermin
directed the interview project to verify onset, hospital, and death data
for the 1979 Sverdlovsk victims, which will be detailed in Anthrax, A Book
of Names, from California Press. We also thank Matthew Meselson, Timothy
Townsend, MD, Martin Hugh-Jones, MA, VetMB, MPH, PhD, and Philip Brachman,
MD, for their review and commentary of the manuscript.
Corresponding Author
and Reprints: Thomas V. Inglesby, MD, Johns Hopkins Center for Civilian
Biodefense Studies, Johns Hopkins University, Candler Bldg, Suite 850
, 11 1 Market Pl, Baltimore, MD 21202 (e-mail: tvijhmi.edu).
Author Affiliations:
The Center for Civilian Biodefense Studies (Drs Inglesby, Henderson, Bartlett,
O’Toole, Perl, and Russell), and the Schools of Medicine (Drs Inglesby, Bartlett,
and Perl) and Public Health (Drs Henderson, O’Toole, and Russell), Johns
Hopkins University, Baltimore, Md; Viral and Rickettsial Diseases, California
Department of Health, Berkeley (Dr Ascher); US Army Medical Research Institute
of Infectious Diseases, Frederick, Md (Drs Eitzen, Friedlander, and Parker);
Office of Emergency Management, New York, NY (Mr Hauer); Centers for Disease
Control and Prevention, Atlanta, Ga (Dr McDade); Acute Disease Epidemiology,
Minnesota Department of Health, Minneapolis (Dr Osterholm); and the Office
of Emergency Preparedness, Department of Health and Human Services, Rockville,
Md (Dr Tonat).
REFERENCES
-
Carter A, Deutsch J, Zelicow P. Catastrophic terrorism. Foreign Aff
. 1998;77:80-95.
-
Lew D. Bacillus anthracis (anthrax). In: Mandell GL, Bennett JE, Dolin
R, eds. Principles and Practices of Infectious Disease.New York, NY: Churchill
Livingstone Inc; 1995:1885-1889.
-
Christopher GW, Cieslak TJ, Pavlin JA, Eitzen EM. Biological warfare:
a historical perspective. JAMA. 1997;278:412-417.
-
Cole LA. The specter of biological weapons. Sci Am. December 1996:60-65.
-
Zilinskas RA. Iraq’s biological weapons: the past as future? JAMA .
1997;278:418-424.
-
Public Health Service Office of Emergency Preparedness. Proceedings
of the Seminar on Responding to the Consequences of Chemical and Biological
Terrorism. Washington, DC: US Dept of Health and Human Services; 1995.
-
WuDunn S, Miller J, Broad W. How Japan germ terror alerted world. New
York Times. May 26, 1998:1-6.
-
Meselson M, Guillemin J, Hugh-Jones M, et al. The Sverdlovsk anthrax
outbreak of 1979. Science. 1994;266:1202-1208.
-
World Health Organization. Health Aspects of Chemical and Biological
Weapons. Geneva, Switzerland: World Health Organization; 1970:98-99.
-
Simon JD. Biological terrorism: preparing to meet the threat. JAMA
. 1997;278:428-430.
-
Cristy GA, Chester CV. Emergency Protection Against Aerosols. Oak Ridge,
Tenn: Oak Ridge National Laboratory; 1981. Publication ORNL-5519.
-
Office of Technology
Assessment, US Congress. Proliferation of Weapons of Mass Destruction.
Washington, DC: US Government Printing Office; 1993:53-55. Publication
OTA-ISC-559.
-
Kaufmann AF, Meltzer MI, Schmid GP. The economic impact of a bioterrorist
attack. Emerg Infect Dis. 1997;3:83-94.
-
Kohout E, Sehat A, Ashraf M. Anthrax: a continuous problem in south
west Iran. Am J Med Sci. 1964;247:565.
-
Pienaar UV. Epidemiology of anthrax in wild animals and the control
on anthrax epizootics in the Kruger National Park, South Africa. Fed
Proc. 1967;26:1496-1591.
-
Dragon DC, Rennie RP. The ecology of anthrax spores. Can Vet J. 1995;36:295-301.
-
Titball RW,
Turnbull PC, Hutson RA. The monitoring and detection of Bacillus anthracis
in the environment. J Appl Bacteriol. 1991;70(suppl):9S-18S.
-
Brachman PS, Friedlander A. Anthrax. In: Plotkin SA, Orenstein WA, eds.
Vaccines 3rd ed. Philadelphia, Pa: WB Saunders Co; 1999:629-637.
-
Brachman PS. Inhalation anthrax. Ann N Y Acad Sci. 1980;353:83-93.
-
Centers for Disease Control and Prevention. Summary of notifiable diseases,
1945-1994. MMWR Morb Mortal Wkly Rep. 1994;43:70-78.
-
Myenye KS, Siziya S, Peterson D. Factors associated with human anthrax
outbreak in the Chikupo and Ngandu villages of Murewa district in Mashonaland
East Province, Zimbabwe. Cent Afr J Med. 1996;42:312-315.
-
Tekin A, Bulut N, Unal T. Acute abdomen due to anthrax. Br J Surg. 1997;84:813.
-
Friedlander A. Anthrax. In: Zajtchuk R, Bellamy RF, eds. Textbook of
Military Medicine: Medical Aspects of Chemical and Biological WarfareWashington,
DC: Office of the Surgeon General, US Dept of the Army; 1997:467-478.
-
Sirisanthana T, Nelson KE, Ezzell JW, Abshire TG. Serological studies
of patients with cutaneous and oral-pharyngeal anthrax from northern Thailand.
Am J Trop Med Hyg. 1988;39:575-581.
-
Kunanusont C, Limpakarnjanarat K, Foy HM. Outbreak of anthrax in Thailand.
Ann Trop Med Parasitol. 1989;84:507-512.
-
Sirisanthana T, Navachareon N, Tharavichitkul P, Sirisanthana V, Brown
AE. Outbreak of oral-pharyngeal anthrax. Am J Trop Med Hyg. 1984;33:144-150.
-
Dutz W, Saidi F, Kouhout E. Gastric anthrax with massive ascites. Gut.
1970;11:352-354.
-
Friedlander A, Welkos SL, Pitt ML, et al. Postexposure prophylaxis against
experimental inhalation anthrax. J Infect Dis. 1993;167:1239-1242.
-
Lincoln RE, Hodges DR, Klein F, et al. Role of the lymphatics in the
pathogenesis of anthrax. J Infect Dis. 1965;115:481-494.
-
Williams RP. Bacillus anthracis and other spore forming bacilli. In:
Braude AI, Davis LE, Fierer J, eds. Infectious Disease and Medical Microbiology.Philadelphia,
Pa: WB Saunders Co; 1986:270-278.
-
Druett HA, Henderson DW, Packman L, Peacock S. Studies on respiratory
infection. J Hyg. 1953;51:359-371.
-
Hatch TF. Distribution and deposition of inhaled particles in respiratory
tract. Bacteriol Rev. 1961;25:237-240.
-
Ross JM. The pathogenesis of anthrax following the administration of
spores by the respiratory route. J Pathol Bacteriol. 1957;73:485-495.
-
Glassman HN. Industrial inhalation anthrax. Bacteriol Rev. 1966;30:657-659.
-
Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of
experimental pulmonary anthrax in the monkey. J Hyg. 1956;54:28-36.
-
Smith H, Keppie J. Observations on experimental anthrax. Nature. 1954;173:869-870.
-
Defense Intelligence Agency. Soviet Biological Warfare Threat. Washington,
DC: US Dept of Defense; 1986. Publication DST-161OF-057-86.
-
Amramova FA, Grinberg LM, Yampolskaya O, Walker DH. Pathology of inhalational
anthrax in 42 cases from the Sverdlovsk outbreak in 1979. Proc Natl Acad
Sci U S A. 1993;90:2291-2294.
-
Dalldorf F, Kaufmann AF, Brachman PS. Woolsorters’ disease. Arch Pathol.
1971;92:418-426.
-
Gleiser CA, Berdjis CC, Harman HA, Gochenour WS. Pathology of experimental
respiratory anthrax in Macaca Mulatta. Br J Exp Pathol. 1963;44:416-426.
-
Franz DR, Jahrling PB, Friedlander A, et al. Clinical recognition and
management of patients exposed to biological warfare agents. JAMA. 1997;278:399-411.
-
Vessal K, Yeganehdoust J, Dutz W, Kohout E. Radiologic changes in inhalation
anthrax. Clin Radiol. 1975;26:471-474.
-
Albrink WS, Brooks SM, Biron RE, Kopel M. Human inhalation anthrax.
Am J Pathol. 1960;36:457-471.
-
Dahlgren CM, Buchanan LM, Decker HM, et al. Bacillus anthracis aerosols
in goat hair processing mills. Am J Hyg. 1960;72:24-31.
-
Walker JS, Lincoln RE, Klein F. Pathophysiological and biochemical changes
in anthrax. Fed Proc. 1967;26:1539-1544.
-
Pile JC, Malone JD, Eitzen EM, Friedlander A. Anthrax as a potential
biological warfare agent. Arch Intern Med. 1998;158:429-434.
-
Institute of Medicine National Research Council. Improving Civilian Medical
Response to Chemical and Biological Terrorist Incidents. Washington, DC:
National Academy Press; 1998:1-70.
-
Centers for Disease Control and Prevention. Bioterrorism alleging use
of anthrax and interim guidelines for management—United States, 1998. MMWR
Morb Mortal Wkly Rep. 1999;48:69-74.
-
Penn C, Klotz SA. Anthrax. In: Gorbach SL, Bartlett JG, Blacklow NR,
eds.Infectious DiseasesPhiladelphia, Pa: WB Saunders Co; 1998:1575-1578.
-
Brachman PS. Anthrax. In: Hoeprich PD, Jordan MC, Ronald AR, eds. Infectious
Diseases Philadelphia, Pa: JB Lippincott; 1994:1003-1008.
-
Anthrax vaccine, military use in Persian Gulf region [press release].
Washington, DC: US Dept of Defense; September 8, 1998.
-
Michigan Department of Public Health. Anthrax Vaccine Absorbed. Lansing:
Michigan Dept of Public Health; 1978.
-
Brachman PS, Gold H, Plotkin SA, Fekety FR, Werrin M, Ingraham NR. Field
evaluation of human anthrax vaccine. Am J Public Health. 1962;52:632-645.
-
Ivins BE, Fellows P, Pitt ML, et al. Efficacy of standard human anthrax
vaccine against Bacillus anthracis aerosol spore challenge in rhesus monkeys.
Salisbury Med Bull. 1996;87:125-126.
-
Turnbull PC. Anthrax vaccines: past, present and future. Vaccine. 1991;9:533-539.
-
Barnes JM. Penicillin and B anthracis. J Pathol Bacteriol . 1947;194:113-125.
-
Lincoln RE, Klein F, Walker JS, et al. Successful treatment of monkeys
for septicemic anthrax. In: Antimicrobial Agents and Chemotherapy—1964Washington,
DC: American Society for Microbiology; 1965:759-763.
-
Odendaal MW, Peterson PM, de Vos V, Botha AD. The antibiotic sensitivity
patterns of Bacillus anthracis isolated from the Kruger National Park.
Onderstepoort J Vet Res. 1991;58:17-19.
-
Doganay M, Aydin N. Antimicrobial susceptibility of Bacillus anthracis.
Scand J Infect Dis. 1991;23:333-335.
-
American Hospital Formulary Service. AHFS Drug Information. Bethesda,
Md: American Society of Health System Pharmacists; 1996.
-
Kelly D, Chulay JD, Mikesell P, Friedlander A. Serum concentrations of
penicillin, doxycycline, and ciprofloxacin during prolonged therapy in
rhesus monkeys. J Infect Dis. 1992;166:1184-1187.
-
Stepanov AV, Marinin LI, Pomerantsev AP, Staritsin NA. Development of
novel vaccines against anthrax in man. J Biotechnol. 1996;44:155-160.
-
Schaad UB, Abdus Salam M, Aujard Y, et al. Use of fluoroquinolones in
pediatrics. Pediatr Infect Dis J. 1995;14:1-9.
-
Lightfoot NF, Scott RJ, Turnbull PC. Antimicrobial susceptibility ofBacillus
anthracis: proceedings of the international workshop on anthrax. Salisbury
Med Bull. 1990;68:95-98.
-
Perkins WA. Public health implications of airborne infection. Bacteriol
Rev. 1961;25:347-355.
-
American Public Health Association. Anthrax. In: Benenson AS, ed. Control
of Communicable Diseases ManualWashington, DC: American Public Health
Association; 1995:18-22.
-
Morse S, McDade J. Recommendations for working with pathogenic bacteria.
Methods Enzymol. 1994;235:1-26.
-
Guillermin J. Anthrax: The Investigation of a Lethal Outbreak. Berkeley:
University of California Press. In press.
-
Chinn KS. Reaerosolization Hazard Assessment for Biological Agent-Contaminated
Hardstand Areas. Life Sciences Divon, Dugway Proving Ground, Utah: US
Dept of the Army; 1996:1-40. Publication DPG/JCP-96/012.
-
Resnick IG, Martin DD, Larsen LD. Evaluation of Need for Detection of
Surface Biological Agent Contamination. Dugway Proving Ground, Life Sciences
Divon, US Dept of the Army; 1990:1-35. Publication DPG-FR-90-711.
-
Manchee RJ, Stewart WD. The decontamination of Gruinard Island. Chem
Br. July 1988;690-691.
-
US Army Medical Research Institute of Infectious Diseases, Centers for
Disease Control and Prevention, and US Food and Drug Administration. Medical
Response to Biological Warfare and Terrorism. Gaithersburg, Md: US Army
Medical Research Institute of Infectious Diseases, Centers for Disease
Control and Prevention, and US Food and Drug Administration; 1998.
-
Pomerantsev AP, Staritsin NA, Mockov YV, Marinin LI. Expression of cereolysine
AB genes in Bacillus anthracis vaccine strain ensures protection against
experimental hemolytic anthrax infection. Vaccine. 1997;15:1846-1850.
Reprinted with permission.
The Journal of American Medical Association. Vol. 281, No. 18,
May 12, 1999.
v
Update: Investigation of Bioterrorism-Related Anthrax and Interim Guidelines
for Exposure Management and Antimicrobial Therapy, October 2001
Since October 3, 2001, CDC and state and local public health authorities
have been investigating cases of bioterrorism-related anthrax. This report
updates previous findings, provides new information on case investigations
in two additional areas, presents the susceptibility patterns of Bacillus
anthracis isolates, and provides interim recommendations for managing potential
threats and exposures and for treating anthrax.
As
of October 24, investigations in the District of Columbia (DC), Florida,
New Jersey, New York City (NYC), Maryland, Pennsylvania, and Virginia have
identified 15 (11 confirmed and four suspected) cases of anthrax according
to the CDC surveillance case definition(1). Seven of the 15 cases were inhalational
anthrax and eight were cutaneous. Of the seven inhalational cases, five occurred
in postal workers in New Jersey and DC, and one in a person who sorted and
distributed mail at a media company in Florida. Two letters mailed to two
different recipients in NYC and one letter mailed to a recipient in DC are
known to have contained B. anthracis spores. Six cases were identified in
employees of media companies; one was a 7-month-old infant who visited a
media company; and eight cases are consistent with exposures along the postal
route of letters known to be contaminated with B. anthracis spores in New
Jersey and DC. Using molecular typing, analysis of B. anthracis isolates
from cases in Florida, NYC, and DC indicated that the isolates are indistinguishable(2).
Epidemiologic investigations and surveillance in other locations are continuing;
no additional cases have been identified.
Florida
As
of October 24, investigations in Florida have identified two confirmed cases
of inhalational anthrax in persons who worked at the same media company;
no additional cases of disease have been identified since the last report
(1). A pleural
biopsy for the second confirmed patient was positive for B. anthracis by
immunohistochemical (IHC) staining. In addition, a >4-fold increase in
levels of serum antibody (IgG) to the protective antigen (PA) component of
the anthrax toxin using enzyme-linked immunosorbant assay (ELISA) was demonstrated.
Environmental
sampling of the work site revealed B. anthracis contamination and implicated
one or more mailed letters or packages as the likely source of exposure.
Several environmental specimens from regional and local postal centers that
provided mail services to the work site were culture-positive for B. anthracis.
Thirty postal workers had no evidence of B. anthracis exposure by nasal
swab testing. No cases of disease have been identified among postal workers.
On the basis of the positive environmental swabs, focused clean-up procedures
continue at regional and local postal centers. The Environmental Protection
Agency (EPA), in consultation with health officials, is conducting decontamination
of the work site.
Approximately
1,100 persons were started on antimicrobial prophylaxis for suspected B.
anthracis exposure; 555 worked either full- or part-time in the affected
building. The majority of other persons reported spending at least 1 hour
in the affected building since August 1. Additional follow-up for compliance
with prophylaxis recommendations and monitoring adverse events associated
with long-term antimicrobial prophylaxis is ongoing.
New York
Investigations
in NYC have identified five (three confirmed and two suspected) cutaneous
anthrax cases; three cases (one confirmed and two suspected) have been identified
since the last report (1)
. These five cases were associated with four media companies (A-D). The
two previously reported cases were related to work sites A and B, and the
three additional cases were related to work sites C, D, and A, respectively.
No cases among postal workers have been identified.
On
October 1, a 27-year-old woman who regularly handled mail at work site C
sought medical care at a local hospital for two lesions on the left cheek,
which developed surrounding erythema and edema and local adenopathy. A biopsy
obtained on October 16 was positive by IHC staining for the cell wall antigen
of B. anthracis and serologic testing was weakly reactive. No suspicious
letter was identified from her work site.
Two
suspected cases of cutaneous anthrax also have been detected. The first suspected
case, a 29-year-old woman with onset of illness on September 22, frequently
handled mail at work site D. At her work site, an unopened letter postmarked
September 18, which contained powder contaminated with B. anthracis was
found on October 19. The second suspected case, a 23-year-old woman with
onset of illness on September 28, handled a suspicious letter postmarked
September 18 from work site A. All three patients were treated with ciprofloxacin
and have shown clinical improvement. A total of three persons were confirmed
by nasal swabs to have been exposed to B. anthracis, presumably acquired
during handling and processing of specimens during the investigation of the
first confirmed case (1)
.
In
work site A, potentially exposed persons were identified and prescribed antimicrobial
prophylaxis. An environmental investigation of work site A was conducted
subsequently; environmental samples taken from work site A were culture-positive
for B. anthracis. Of 1,360 persons who were tested by nasal swabs from work
site A, all were confirmed negative. Nasal swabs were obtained from 1,202
persons from work sites B, C, and D; 1,183 tested negative and 19 are pending
final results. Environmental samples taken from work site A were positive.
Testing of environmental specimens from work sites B, C, and D is ongoing.
Prophylaxis
was recommended for potentially exposed persons at work site A. Antimicrobial
prophylaxis was initiated for nine persons who had recent contact with the
sealed letter containing B. anthracis in work site D.
New Jersey
T
o date, investigations in New Jersey and Pennsylvania have identified four
(two confirmed and two suspected) anthrax cases. Cutaneous disease has been
diagnosed in three patients and one has illness suspected to be inhalational
anthrax, but laboratory tests to confirm the diagnosis are pending. All four
of these patients worked at one of two postal facilities in New Jersey. Although
no specific contaminated letter was identified, contaminated letters destined
for both NYC and DC passed through at least one of these postal facilities
in New Jersey.
On
October 1, a 45-year-old female mail carrier sought medical care at a local
hospital for a 4-day history of worsening skin lesions on her right forearm.
A biopsy was obtained and arrived at CDC on October 17 and later that night
was found positive by IHC. In addition, tissue was positive for B. anthracis
by polymerase chain reaction (PCR), and serologic testing was reactive.
The patient’s condition improved on antimicrobial therapy.
On
October 16, a 35-year-old male mail processor, with a history of a chronic,
bullous-like skin condition, was taken to a local hospital complaining of
a 2-day history of a large pustular lesion on his neck. He returned 1 day
later with increasing ulceration of the skin lesion associated with fatigue,
chills, and a swollen throat; he was afebile but had vesicles and bullae
around the pustular lesion. Biopsy was positive by IHC, and serologic testing
was reactive to B. anthracis . The patient’s lesions responded to antimicrobial
therapy.
Two
suspected cases also have been detected. The first case occurred in a 39-year-old
male machine mechanic who was taken to a local hospital on September 26 for
two bullous, vesicular lesions with surrounding erythema, edema, and induration
on the right forearm, which progressed to black eschars. The patient was
treated for cellulitis with ceftriaxone followed by amoxicillin/clavulanate.
The patient was reported to CDC on October 17 and serologic testing at CDC
was reactive to B. anthracis. No biopsy was obtained. The patient’s condition
improved.
O
n October 14, the second suspected case occurred in a 56-year-old female
postal worker who sought medical care for fever, diarrhea, and vomiting at
a local hospital. On October 14, the second suspected case occurred in a 56-year-old
female postal worker who sought medical care for fever,
diarrhea, and vomiting at a local hospital. On October 19, the patient was
admitted to the hospital with chills, dry cough, and pleuritic chest pain.
A chest radiograph showed a small right infiltrate and bilateral effusions,
but no evidence of a widened mediastinum. The next day, her respiratory status
and pleural effusions worsened. A chest computerized tomography (CT) showed
an enlarged mediastinal and cervical lymph nodes without parenchymal disease.
The pleural fluid was positive for B. anthracis by PCR. Bilateral pleural
effusions have complicated the patient’s hospital course and she continues
to require supplemental oxygen.
On
October 20, the postal facility was closed; the New Jersey Department of
Health and Senior Services recommended that postal workers at both postal
facilities initiate antimicrobial prophylaxis pending further epidemiologic
and environmental investigation. Both facilities have been closed pending
results of further environmental evaluation. Environmental sampling is being
conducted at both postal facilities. In one facility, 13 of 23 samples from
high-risk areas were preliminarily culture-positive for B. anthracis. Clean-up
efforts are ongoing. Results of cultures from samples taken in the second
facility and results from approximately 600 nasal swab cultures obtained
from postal employees are pending.
District of Columbia
To
date, investigations in DC, Maryland, and Virginia have identified four
confirmed anthrax cases. All patients had inhalational illness and all worked
at a single postal facility in DC.
On
October 15, a staff member in the office of a U.S. Senator noted a small
burst of dust released while opening a tightly sealed letter. The U.S. Capitol
Police and Federal Bureau of Investigation (FBI) were notified and the area
was vacated and secured immediately; ventilation systems for the Senator’s
offices were deactivated within 45 minutes of recognizing the threat. The
letter and surrounding carpet were removed and sent for testing. On October
16, the letter tested positive for B. anthracis by PCR, and an epidemiologic
investigation was initiated by the health officials from the Office of Attending
Physician, U.S. Capitol; DC Department of Health (DCDOH); Infectious Disease
Service, National Naval Medical Center; and CDC.
Based
on the initial investigation, the area of exposure was determined to consist
of two floors in the southeast quadrant of the building where the U.S. Senator’s
office is located. Approximately 340 staff and visitors potentially were
exposed. Beginning October 15, nasal swab testing was performed on these
persons and approximately 5,000 additional persons who referred themselves
for testing. Twenty-eight persons had evidence of exposure by nasal swab
testing; 13 were in the immediate office space where the letter was opened,
nine were in adjacent areas, and six were first responders. Antimicrobial
prophy laxis was administered to persons from the
area of exposure and first-responders to the incident. Environmental specimens
were collected at the affected building and other buildings in the U.S.
Capitol complex. To date, environmental specimens are positive from the
area of exposure as well as two mail rooms in the U.S. Capitol complex;
one of the mail rooms did not process the contaminated letter. None of the
mail room personnel and none of the postal workers at the post office serving
the mail rooms had positive nasal swabs. These mail handlers were all offered
prophylactic antibiotics. Initially, a single positive environmental sample
for the post office serving these mail rooms was positive. Subsequent samples
from this post office and the mail distribution center serving this post
office were positive.
On
October 19, enhanced regional surveillance activities (a collaborative effort
between DCDOH, Maryland Department of Health and Mental Hygiene, and the
Virginia Department of Health) identified a case of pulmonary illness in
a postal worker. The postal worker, a 56-year-old man, sought medical care
at a Virginia hospital for fever, chills, chest heaviness, malaise, and minimally
productive cough of 3 days’ duration. Initial evaluation in the emergency
department (ED) revealed a widened mediastinum on a chest radiograph; a subsequent
CT scan revealed mediastinal lymphadenopathy and small, bilateral pleural
effusions. The patient was hospitalized for suspected inhalational anthrax
and was treated with broad spectrum antimicrobial agents, including ciprofloxacin.
Blood cultures grew gram-positive rods within 15 hours of collection, later
confirmed to be B. anthracis at the Virginia State Health Laboratory and
CDC on October 21. The patient is clinically stable and remains hospitalized.
On
October 20, a second postal worker, also a 56-year-old man, who worked at
the same distribution center, was admitted to the hospital with a 3-day
history of progressively worsening headache and night sweats. He had no
fever, stiff neck, or other symptoms or signs consistent with meningitis.
He had a mild sore throat and occasional dry cough. Because the patient
was linked epidemiologically to the index case of inhalational anthrax,
a chest radiograph and chest CT scan were performed that revealed mediastinal
lymphadenopathy and a right middle lobe infiltrate. Antimicrobial therapy
was initiated. Blood cultures grew B. anthracis within 18 hours.
The patient is clinically stable and remains hospitalized.
On
October 21, a third postal worker, a 55-year-old man, who worked at the same
distribution center was admitted to the hospital with suspected inhalational
anthrax. The patient had initially sought medical care at a physician’s office
on October 18 for 2 days of progressive fatigue, myalgias, and fever. The
patient had a temperature of 102 F (38.9 C) and normal white blood cell count
and was sent home. The patient returned to the ED on October 21 with persistent
symptoms, including chills, vague chest tightness, and temperature of 102
F (38.9 C). Chest radiograph revealed right middle and lower lobe alveolar
infiltrates and right hilar and peritracheal soft tissue fullness. Evaluation
revealed hypoxia, leukocytosis, and hemoconcentration. Antimicrobial therapy
was initiated, and the patient was mechanically ventilated. The patient’s
condition deteriorated, and he died on October 21. Blood cultures obtained
on admission to the hospital grew gram-positive bacilli, which were confirmed
later as B. anthracis at CDC.
On
October 22, a fourth postal worker, a 47-year-old man, who worked at the
same distribution center was admitted to the hospital with suspected inhalational
anthrax. The patient had initially presented to the ED on October 21 with
complaints of 5 days of progressive fatigue, nausea, vomiting, and diarrhea,
and syncope. The patient was afebrile and had orthostatic hypotension. A
chest radiograph was obtained and reported to be normal. The patient received
intravenous fluids and was discharged. He returned to the ED 26 hours later
following another syncopal episode and persistent gastrointestinal complaints.
The patient was afebrile, hypotensive, diaphoretic, and in respiratory distress.
A second chest radiograph and a chest CT revealed mediastinal lymphadenopathy
and bilateral pleural effusions. Subsequent review of the first chest radiograph
revealed an ill-defined area of increased density in the right subhilar
region. Laboratory evaluation revealed leukocytosis and hemoconcentration.
Antimicrobial therapy was initiated, and the patient was mechanically ventilated.
Peripheral blood smear demonstrated gram-positive bacilli; blood cultures
grew gram-positive bacilli within 18 hours and were confirmed as B. anthracis
at CDC. The patient died on October 22.
On
October 20, CDC and DCDOH initiated an investigation of the postal facility
where the four patients were employed. Although no specific exposure event
was identified, the contaminated tightly sealed letter that was mailed to
the Senator’s office was processed at this facility on October 12 before
entering the Capitol mail distribution system. The postal facility was closed
on October 21, and antimicrobial prophylaxis was recommended to employees
working in proximity to the same mail sorting area of the first patient.
In addition, visitors to nonpublic operations areas of this facility also
were offered antimicrobial prophylaxis.
On
October 22, because of concern about the potential for unrecognized aerosol
exposures among postal workers, antimicrobial therapy was recommended for
all workers and visitors to nonpublic areas in this postal facility. Subsequently,
this recommendation has been extended to all postal workers in the DC area
directly served by this postal facility pending results of ongoing epidemiologic
and environmental investigation.
The
first patient also worked at a second postal facility. On October 21, this
facility also was closed. Antimicrobial prophylaxis also was recommended
for workers at this facility pending further epidemiologic and environmental
testing.
Susceptibility Testing
of B. anthracis Isolates
Antimicrobial
susceptibility patterns were determined for 11 B. anthracis isolates associated
with intentional exposures in Florida, NYC, and DC. Susceptibility breakpoints
for interpreting minimum inhibitory concentration (MIC) results for B. anthracis
have not been determined by the National Committee for Clinical Laboratory
Standards (NCCLS); thus, breakpoints for staphylococci were used (3). All
B. anthracis isolates were susceptible to ciprofloxacin (MIC<0.06
µg/mL), doxycycline (MIC<0.03 µg/mL), chloramphenicol (MIC=4 µg/mL), clindamycin
(MIC< 0.5 µg/mL), tetracycline (MIC=0.06 µg/mL), rifampin (MIC<0.5
µg/mL), and vancomycin (MIC=1-2 µg/mL). Limited testing of imipenem suggests
that these organisms are also susceptible to this agent (MIC<0.12
µg/mL) and are likely susceptible to meropenem. Susceptibility of the isolates
was considered intermediate to erythromycin (MIC=1 µg/mL) and borderline
susceptible to azithromycin (MIC=2 µg/mL); clarithromycin was considered
susceptible (MIC=0.25 µg/mL).
B.
anthracis isolates were susceptible to penicillin
(MIC range: <0.06 ug/mL-0.12 µg/mL) and amoxicillin (MIC<
0.06 µg/mL); ceftriaxone (MIC=16) was considered intermediate. NCCLS has
not defined either a B. anthracis or staphylococcal interpretive breakpoint
for ceftriaxone results; thus, breakpoints for gram-negative organisms were
used to interpret ceftriaxone results. These ceftriaxone MICs and additional
laboratory data at CDC indicate the presence in B. anthracis isolates of
a cephalosporinase, an enzyme that inhibits the antibacterial activity of
cephalosporins such as ceftriaxone. Additional studies were performed with
some of the B. anthracis isolates to identify other beta-lactamases, the
general class of enzymes that inactivate penicillins, cephalosporins, and
related drugs. These preliminary studies indicate the presence of a class
B cephalosporinase and suggest that a penicillinase also may be present.
These enzymes often are present in naturally occurring B. anthracis
isolates.
This
information is current as of October 24, 2001, 9 p.m. eastern daylight time.
Intensive surveillance activities and environmental and case investigations
are in progress to identify and treat all U.S. Postal Service workers and
others at potential risk for anthrax. Surveillance also is being conducted
to monitor adverse events associated with antimicrobial prophylaxis for anthrax.
CDC and FBI are collaborating to accelerate all aspects of the investigation
surrounding these events.
Reported
by: J Malecki, MD, Palm Beach County Health Dept, Palm Beach; S Wiersma,
MD, State Epidemiologist, Florida Dept of Health. K Cahill, MD; M Grossman,
MD, Columbia Presbyterian Medical Center, New York City; H Hochman, MD, Lenox
Hill Hospital, New York City; A Gurtman, MD, Mount Sinai School of Medicine,
New York City; Communicable Disease Program, New York City Dept of Health.
E Bresnitz, MD, State Epidemiologist, G DiFerdinando, MD, New Jersey Dept
of Health and Senior Svcs. P Lurie, MD, K Nalluswami, MD, Pennsylvania Dept
of Health. L Siegel, MD, S Adams, I Walks, MD, J Davies-Coles,
PhD, District of Columbia Dept of Health. R Brechner, State Epidemiologist,
Maryland Dept of Health and Mental Hygiene. E Peterson, MD, Virginia Dept
of Health. D Frank, MD, Greater SE Hospital, District of Columbia. S Bresoff-Matcha,
MD, Mid-Atlantic Permanente Medical Group and Inova Fairfax Hospital, Falls
Church, Virginia. C Chiriboga, MD, Southern MD Hosp, Clinton, Maryland.
J Eisold, MD, G Martin, MD, Office of the Attending Physician, US Capitol.
EIS officers, CDC.
Editorial
Note: Bioterrorism attacks using B. anthracis
spores sent through the mail have resulted in 15 anthrax cases and three
deaths. The initial anthrax cases occurred among persons with known or suspected
contact with opened letters contaminated with B. anthracis spores. Later,
investigations identified four confirmed cases and one suspected case among
postal workers who had no known contact with contaminated opened letters.
This suggests that sealed envelopes contaminated with B. anthracis passing
through the postal system may be the source of exposure. The number of contaminated
envelopes passing through the postal system is not known. In addition, automated
sorting could damage envelopes and release spores into postal environments;
other circumstances that could contribute to the contamination of postal
facility environments may be identified.
Because
these cases are the result of intentional exposures, FBI and other law enforcement
authorities are investigating these events as criminal acts and are working
to identify and eliminate the source of these exposures. Until that occurs,
the possibility of further exposure to B. anthracis and subsequent clinical
illness exists. Clinicians and laboratorians should be vigilant for symptoms
or laboratory findings that indicate B. anthracis infection, particularly
among mail handlers. Information to guide health-care providers and laboratorians
is available at <http://www.bt.cdc.gov>.
Managing Threats
Letters
containing B. anthracis spores have been sent to persons in NYC
and DC. Prompt identification of a threat and institution of appropriate
measures may prevent inhalational anthrax. To prevent exposure to B. anthracis
and subsequent infection, suspicious letters or packages should be recognized
and appropriate protective steps taken.
Characteristics
of suspicious packages and letters include inappropriate or unusual labeling,
strange return address or no return address, postmarks from a city or state
different from the return address, excessive packaging material, and others.
If a package appears suspicious, it should not be opened. The package should
be handled as little as possible. The room should be vacated and secured
promptly and appropriate security or law enforcement agencies promptly notified
(Box 1).
Box 1. Handling of
Suspicious Packages or Envelopes
-
Do not shake or empty the contents of a suspicious package or envelope.
-
Do not carry the package or envelope, show it to others, or allow
others to examine it.
-
Put the package or envelope on a stable surface; do not sniff,
touch, taste, or look closely at it or any contents that may have
spilled.
-
Alert others in the area about the suspicious package or envelope.
Leave the area, close any doors, and take actions to prevent others
from entering the area. If possible, shut off the ventilation system.
-
Wash hands with soap and water to prevent spreading potentially
infectious material to face or skin. Seek additional instructions
for exposed or potentially exposed persons.
-
If at work, notify a supervisor, a security officer, or a law enforcement
official. If at home, contact the local law enforcement agency.
-
If possible, create a list of persons who were in the room or area
when this suspicious letter or package was recognized and a list
of persons who also may have handled this package or letter. Give
the list to both the local public health authorities and law enforcement
officials.
|
Managing Exposures
Identification of a patient with anthrax or a confirmed exposure to B. anthracis
should prompt an epidemiologic investigation. The highest priority is to
identify at-risk persons and initiate appropriate interventions to protect
them. The exposure circumstances are the most important factors that direct
decisions about prophylaxis. Persons with an exposure or contact with an
item or environment known, or suspected to be contaminated with B. anthracis--regardless
of laboratory tests results–should be offered antimicrobial prophylaxis.
Exposure or contact, not laboratory test results, is the basis for initiating
such treatment. Culture of nasal swabs is used to detect anthrax spores.
Nasal swabs can occasionally document exposure, but cannot rule out exposure
to B. anthracis . As an adjunct to epidemiologic evaluations, nasal swabs
may provide clues to help assess the exposure circumstances. In addition,
rapid evaluation of contaminated powder, including particle size and characteristics,
may prove useful in assessing the risk for inhalational anthrax.
CDC is working with U.S. Postal Service employees and managers on several
strategies to address the risk for anthrax among workers involved in mail
handling. These strategies include personal protective equipment for workers
handling mail and engineering controls in mail facilities. Clinicians and
laboratorians should be vigilant for symptoms or laboratory findings that
indicate possible anthrax infection, particularly among workers involved
in mail sorting and distribution. Information to guide health-care providers
and laboratories is available at <http://www.bt.cdc.gov>
(1).
Antimicrobial Treatment
A high index of clinical suspicion and rapid administration of effective
antimicrobial therapy is essential for prompt diagnosis and effective treatment
of anthrax. Limited clinical experience is available and no controlled trials
in humans have been performed to validate current treatment recommendations
for inhalational anthrax. Based on studies in nonhuman primates and other
animal and in vitro data, ciprofloxacin or doxycycline should be used for
initial intravenous therapy until antimicrobial susceptibility results are
known (Table 1). Because of the mortality associated with inhalational anthrax,
two or more antimicrobial agents predicted to be effective are recommended;
however, controlled studies to support a multiple drug approach are not
available. Other agents with in vitro activity suggested for use in conjunction
with ciprofloxacin or doxycycline include rifampin, vancomycin, imipenem,
chloramphenicol, penicillin and ampicillin, clindamycin, and clarithromycin;
but other than for penicillin, limited or no data exist regarding the use
of these agents in the treatment of inhalational B. anthracis infection.
Cephalosorins and trimethoprim-sulfamethoxazole should not be used for therapy.
Regimens being used to treat patients described in this report include ciprofloxacin,
rifampin, and vancomycin; and ciprofloxacin, rifampin, and clindamycin.
Penicillin is labelled for use to treat inhalational anthrax. However, preliminary
data indicate the presence of constitutive and inducible beta-lactamases
in the B. anthracis isolates from Florida, NYC, and DC. Thus, treatment
of systemic B. anthracis infection using a penicillin alone (i.e., penicillin
G and ampicillin) is not recommended. The B. anthracis genome sequence shows
that this organism encodes two beta-lactamases: a penicillinase and a cephalosporinase.
Data in the literature also show that some beta-lactamase negative B. anthracis
strains for which the penicillin MICs are 0.06 µg/mL increase to 64 µg/mL
and become beta-lactamase positive when exposed to semisynthetic penicillins
(4). The
frequency of this induction event is unknown. Although amoxicillin/clavulanic
acid is more active than amoxicillin alone against beta-lactamase, producing
strains in vitro, the combination may not be clinically effective for inhalational
anthrax where large numbers of organisms are likely to be present.
Toxin-mediated morbidity is a major complication of systemic anthrax. Corticosteroids
have been suggested as adjunct therapy for inhalational anthrax associated
with extensive edema, respiratory compromise, and meningitis
(5).
For cutaneous anthrax, ciprofloxacin and doxycycline also are first-line
therapy (Table 2). As for inhalational disease, intravenous therapy with
a multidrug regimen is recommended for cutaneous anthrax with signs of systemic
involvement, for extensive edema, or for lesions on the head and neck (Table
2). In cutaneous anthrax, antimicrobial treatment may render lesions culture
negative in 24 hours, although progression to eschar formation still occurs
(5). Some experts recommend that corticosteroids
be considered for extensive edema or swelling of the head and neck region
associated with cutaneous anthrax. Cutaneous anthrax is typically treated
for 7-10 days; however, in this bioterrorism attack, the risk for simultaneous
aerosol exposure appears to be high. Although infection may produce an effective
immune response, a potential for reactivation of latent infection may exist.
Therefore, persons with cutaneous anthrax associated with this attack should
be treated for 60 days.
Prophylaxis for inhalational anthrax exposure has been addressed in a previous
report (1)
and indicates the use of either ciprofloxacin or doxycycline as first line
agents. High-dose penicillin (e.g., amoxicillin or penicillin VK) may be
an option for antimicrobial prophylaxis when ciprofloxacin or doxycycline
are contraindicated. The likelihood of beta-lactamase induction events that
would increase the penicillin MIC is lower when only small numbers of vegetative
cells are present, such as during antimicrobial prophylaxis.
All medications may have undesirable side effects and allergic reactions
may result from any medication. Clinicians prescribing these medications
should be aware of their side effects and consult an infectious disease specialist
as needed. Patients should be urged to inform their health-care provider
of any adverse event.
This is the first bioterrorism-related anthrax attack in the United States,
and the public health ramifications of this attack continue to evolve. Additional
updates and recommendations will be published in MMWR.
|
Table 1.
Inhalational anthrax treatment protocol for cases associated with
this bioterrorism attack
|
| Category
Adults
|
Initial
therapy (intravenous)
Duration
Ciprofloxacin 400 mg every 12 hrs* IV treatment
initially **. Switch
to oral
or
antimicrobial therapy when clinically
appropriate:
Doxycycline 100 mg every 12 hrs††
Ciprofloxacin 500 mg po
BID
and
or
One or two additional antimicrobials¶
Doxycycline 100 mg pg BID
Continue
for 60 days (IV and po
combined)
|
| Children |
Ciprofloxacin 10-15 mg/kg every 12h¶¶***
IV tratment initially**. Switch
to oral
or
antimicrobial therapy when clinically
Doxycycline:†††,¶¶
appropriate:
>8 yrs and >45 kg: 100 mg every 12 hrs Ciprofloxacin
10-15 mg/kg po
>8 yrs and Ł
2.2 mg/kg every 12 hrs every 12 hours***
Ł 8 yrs: 2.2 mg/kg every 12 hrs
or
and
Doxycycline:†††
One or two additional antimicrobials¶
>8 yrs and >45 kg: 100 mg BID
>8 yrs and Ł 2.2 mg/kg po BID
Ł 8 yrs: 2.2 mg/kg po BID
Continue for 60 days {IV and po
combined}
|
|
Pregnant
women
Immunocompro-
mised person
|
Same for nonpregnant adults (the high IV treatment
initially. Switch to
deatgh rate from the infection outweights or antimicrobial
therapy when
the risk posed by the antimicrobial agent) clinically
appropriate.† Oral therapy
regimens same for nonpregnant adults.
Same for nonimmunocompromised Same for nonimmunocompromised
persons and children
persons and children
|
*For gastrointestinal and oropharyngeal anthrax’
use regimens recommended for inhalational anthrax.
† Ciprofloxacin or doxycycline should be considered
an essential part of first-line therapy for Inhalational anthrax.
§Steroids may be considered as an adjunct therapy
for patients with severe edema and for meningitis based on experience with
bacterial meningitis of other etiologies.
¶Other agents with in vitro activity include rifampin,
vancomycin, penicillin, ampicillin, chloramphenicol, imipenem, clindamycin,
and clarithromycin. Because of concerns of constitutive and inducible beta-lactamases
in Bacillus anthracis, penicillin and ampicillin should not be used alone.
Consultation with an infectious disease specialist is advised.
** Initial therapy may be altered based on clinical
course of the patient; one or two antimicrobial agents (e.g., ciprofloxacin
or doxycycline) may be adequate as the patient improves.
††If meningitis is suited doxycycline may be less
optimal because of poor central nervous system penetration.
§§Because of the potential persistence of spores
after an aerosol exposure, antimicrobial therapy should be continued
for 60 days.
¶¶ If intravenous ciprofloxacin is not available,
oral ciprofloxacin may be acceptable because it is rapidly and well
absorbed from the gastrointestinal tract with no substantial loss
by first-pass metabolism. Maximum serum concentrations are affairs
1-2 hours after oral dosing but may achieved if vomiting or ileus
are present.
*** In children ciprofloxacin dosage should not exceed 1 g/day.
††† The American Academy of Pediatrics recommends treatment of
young children with tetracycline for serious infections (e.g., Rocky
Mountain spotted fever).
¶¶ If intravenous ciprofloxacin is not available, oral
ciprofloxacin may be acceptable because it is rapidly and well absorbed
from the gastrointestinal tract with no substantial loss by first-pass
metabolism.Maximum serum concentrations are affairs 1-2 hours after
oral dosing but may achieved if vomiting or ileus are present.
*** In children ciprofloxacin dosage should not exceed 1 g/day.
††† The American Academy of Pediatrics recommends treatment of
young children with tetracycline for serious infections (e.g., Rocky
Mountain spotted fever).
§§§Although tetracyclines are not recommended
during pregnancy, their use may be indicated for life-threatening
illness. Adverse effects on developing teeth and bones are dose related
therefore doxycycline might be used for a short time 47-14 days)
before 6 months of gestation.
REFERENCES
-
CDC. Update: investigation of anthrax associated with intentional
exposure and interim public health guidelines, October 2001. MMWR
2001;50:889-97.
-
Keim P, Price LB, Klevytska AM, et al. Multiple-locus variable-number
tandem repeat analysis reveals genetic relationships with Bacillus
anthracis. J Baceriol 2000;182:2928-36.
-
National Committee for Clinical Laboratory Standards. Performance
standards for antimicrobial susceptibility testing. Wayne, Pennsylvania:
National Committee for Clinical Laboratory Standards, 2001; 11th informational
supplement M100-S11.
-
Lightfoot NF, Scott RJ, Turnbull PC. Antimicrobial susceptibility
of Bacillus anthracis. Salisbury Med Bull
1990;68:95S-98S.
-
Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J
Med 1999;341:815-26.
-
Inglesby TV, Henderson DA, Bartlett JG, et al. Anthrax as a biological
weapon: medical and public health management. JAMA 1999;281:1735-45.
v
Children and Anthrax: A Fact Sheet for Clinicians
Anthrax is an acute infectious
disease caused by the bacterium Bacillus anthracis. Children, like
adults, may be affected by three clinical forms: cutaneous, inhalational,
or gastrointestinal.
The symptoms and signs of anthrax
infection in children older than 2 months of age are similar to those in
adults. The clinical presentation of anthrax in young infants is not well
defined. When children become ill and present for treatment, making a diagnosis
may be more difficult than in adults because young children have difficulty
reporting what has happened to them or telling a doctor exactly how they
feel. Because respiratory illnesses are much more common in children than
adults, the examining clinician should have an understanding of disease
manifestations in children.
The following are clinical descriptions
(based on experience with adults) of the three forms of anthrax (MMWR
2001;50(41):889-893).
-
Inhalational.
Inhalational anthrax begins with a brief prodrome resembling an
influenza-like viral respiratory illness followed by development of
dyspnea, systemic symptoms, and shock, with radiographic evidence of
mediastinal widening and pleural effusion. Inhalational anthrax is the
most lethal form of anthrax. The incubation period of inhalational
anthrax among humans typically ranges from 1 to 7 days but may be up
to 60 days. Host factors, dose of exposure, and chemoprophylaxis may
affect the duration of the incubation period. Patients frequently develop
meningitis. Case-fatality estimates for inhalational anthrax are extremely
high; the risk for death is high even if patients are provided with
supportive care, including appropriate antimicrobial treatment.
-
Cutaneous. Cutaneous
anthrax is characterized by a skin lesion evolving from a papule, through
a vesicular stage, to a depressed black eschar. The incubation period
ranges from 1 to 12 days. The lesion is usually painless, but patients
also may have fever, malaise, headache, and regional lymphadenopathy.
The case fatality rate for cutaneous anthrax is 20% without, and <1%
with, antimicrobial treatment.
-
Gastrointestinal.
Gastrointestinal anthrax is characterized by severe abdominal pain followed
by fever and signs of septicemia. This form of anthrax usually results
from eating raw or undercooked meat containing B. anthracis, and the
incubation period is usually 1 to 7 days. An oropharyngeal and an abdominal
form of the disease have been described. Involvement of the pharynx is
usually characterized by lesions at the base of the tongue, dysphagia,
fever, and regional lymphadenopathy. Lower bowel inflammation typically
causes nausea, loss of appetite, and fever followed by abdominal pain,
hematemesis, and bloody diarrhea. The case-fatality rate is estimated
to be between 25% and 60%. The effect of early antibiotic treatment on
the case-fatality rate has not been established.
Neither CDC nor the American Academy
of Pediatrics (AAP) recommend dispensing antibiotics for parents to have
on hand in case of a possible exposure to Bacillus anthracis. CDC and its
partner organizations will dispense antibiotics through the National Pharmaceutical
Stockpile (NPS) program if exposure occurs. The NPS was designed to ensure
the availability of lifesaving pharmaceuticals; antimicrobials; chemical
interventions; and medical, surgical, and patient-support supplies, as
well as equipment for prompt delivery to disaster sites. Disasters include
a possible biological or chemical terrorist event anywhere in the United
States. For more detailed information about the NPS, see CDC’s Web site
at www.bt.cdc.gov.
Vaccination
At this time, anthrax vaccine is
not recommended for people younger than 18 years of age. Military personnel
and civilians at high risk for repeated exposure (e.g., laboratory workers
handling powders containing Bacillus anthracis may benefit from the vaccine.
Prophylaxis
Post-exposure prophylaxis is indicated
to prevent inhalational anthrax after a confirmed or suspected aerosol
exposure to Bacillus anthracis. Consultation with public health authorities
is strongly encouraged to identify people who should receive prophylaxis.
When no information is available about the antimicrobial susceptibility
of the implicated strain of Bacillus anthracis, CDC recommends initial
therapy with either ciprofloxacin or doxycycline for children, as follows:
� 10-15 mg/kg/dose po Q12 hours (not to exceed 1 gram per day)
for 60 days.
�
8 years or older and weighing more than 45 kg: 100 mg po BID for 60
days.
�
8 years or older and weighing
45 kg or less: 2.2 mg/kg/dose po BID for 60 days.
� 8 years or younger: 2.2
mg/kg/dose po BID for 60 days
Reference: CDC. Update: Investigation of anthrax associated
with intentional exposure and interim public health guidelines, October,
2001. MMWR 2001;50 (41):889-893.
The National Pharmaceutical
Stockpile (NSP) contains oral and liquid types of both drugs for use by
children who are too small to tolerate pills. Both tetracyclines and fluoroquinolones
can cause adverse health effects in children. These risks must be weighed
carefully against the risk of developing a life-threatening disease due
to Bacillus anthracis. As soon as the penicillin susceptibility of the
organism has been confirmed, prophylactic therapy for children should be
changed to oral amoxicillin 80 mg/kg of body mass per day divided every
8 hours (not to exceed 500 mg three times daily). The NSP also includes
amoxicillin suspension for children. Bacillus anthracis is not susceptible
to cephalosporins or to trimethoprim/sulfamethoxazole, and these agents
should not be used for prophylaxis.
Drug Recommendations
For Pediatric Anthrax Cases
Some antibiotics and other
treatments that have proven effective against anthrax in adults have not
been studied as extensively in children. Therefore, CDC provides the following
recommendations for treating anthrax in children:
For inhalational anthrax:
|
Initial Therapy (intravenous) |
Duration |
|
Ciprofloxacin* 10-15 mg/kg/dose
every 12 hrs.
or
Doxycycline:¶
> 8 years and 45 kg: 100 mg every 12 hrs.
> 8 years and 45 kg or less: 2.2 mg/kg/dose
every 12 hours
8 years or younger: 2.2 mg/kg/dose
every 12 hrs
and
One or two additional antimicrobial§
|
IV treatment initially. Switch to oral antimicro-
bial therapy when clinically appropriate:
Ciprofloxacin 10-15 mg/kg/dose po every 12 hrs
every 12 hours
or
Doxycycline:¶
8 years and > 45 kg: 100 mg po BID
> 8 years and 45 kg or less:
2.2 mg/kg/dose po BID
8 years or younger: 2.2 mg/kg/dose po BID
and
Continue for 60 days (IV and po combined)
|
Antimicrobial therapy should
be continued for 60 days because of the potential persistence of spores
after an aerosol exposure. Initial therapy may be altered on the basis
of the clinical course of the patient; one or two antimicrobial agents
(e.g., ciprofloxacin or doxycycline) may be adequate as the patient improves.
*If intravenous ciprofloxacin
is not available, oral ciprofloxacin may be acceptable because it is rapidly
and well absorbed from the gastrointestinal tract with no substantial loss
by first-pass metabolism. Maximum serum concentrations are attained 1 to
2 hours after oral dosing but may not be achieved if vomiting or ileus
is present. In children, ciprofloxacin dosage should not exceed 1 g/day.
¶ The AAP recommends treatment
of young children with tetracyclines for serious infections (e.g., Rocky
Mountain spotted fever). If meningitis is suspected, doxycycline may be
less optimal because of poor central nervous system penetration.
§Other agents with in vitro
activity include rifampin, vancomycin, penicillin, ampicillin, chloramphenicol,
imipenem, clindamycin, and clarithromycin. Because of concerns of constitutive
and inducible beta-lactamases in Bacillus anthracis isolates involved in
the current bioterrorist attack, penicillin and ampicillin should not be
used alone. Consultation with an infectious disease specialist is advised.
For cutaneous anthrax
, CDC recommends the following treatment:
|
Initial Therapy (oral) |
Duration |
| Ciprofloxacin:
10-15 mg/kg/dose every 12 hrs.
(not to exceed 1 g/day)
or
Doxycycline:
> 8 years and > 45 kg:
100 mg every 12 hrs.
> 8 years and 45 kg or less:
2.2 mg/kg/dose every 12 hrs.
8 years or younger: 2.2 mg/kg/dose
every 12 hrs.
|
60 days
60 days
|
Cutaneous anthrax with signs
of systemic involvement, extensive edema, or lesions on the head or neck
requires intravenous therapy, and a multidrug approach is recommended.
Ciprofloxacin or doxycycline should be considered first-line therapy. Amoxicillin
80 mg/kg/day divided every 8 hours is an option for completion of therapy
after clinical improvement, if the organism is susceptible.
Previous guidelines have
suggested treating cutaneous anthrax for 7 to 10 days, but 60 days is recommended
in the setting of this attack, given the likelihood of exposure to aerosolized
Bacillus anthracis.
For gastrointestinal and
oropharyngeal anthrax, use regimens recommended for inhalational anthrax.
Children are more likely
than adults to suffer side affects from some antibiotics used to prevent
or treat the disease. If a child does develop side effects, testing should
be done to determine whether the bacteria to which the child was exposed
are susceptible to other drugs with fewer side effects, such as amoxicillin.
For Additional Information
The American Academy of Pediatrics
offers more extensive information about children and anthrax at its Web
site, http://www.aap.org/advocacy/releases/smlpoxanthrax.htm. For information
related to preparedness and bioterrorism, see CDC’s Web site at http://www.bt.cdc.gov.
Sources: MMWR
50(41); October 19, 2001; MMWR 50(42); October 26, 2001;
American Academy of Pediatrics Fact Sheet at:
http://www.aap.org/advocacy/releases/smlpoxanthrax.htm.
v Anthrax Technical Information
Clinical features Human anthrax has three major clinical forms depending on the route of
infection: cutaneous, inhalation, and gastrointestinal. Cutaneous anthrax
begins as a pruritic papule or vesicle that enlarges and erodes (1-2 days)
leaving a necrotic ulcer with subsequent formation of a central black eschar;
inhalation anthrax may begin as a prodrome of fever, chills, nonproductive
cough, chest pain, headache, myalgias, and malaise, with more distinctive
clinical hallmarks of hemorrhagic mediastinal lymphadenitis, hemorrhagic
pleural effusions, bacteremia and toxemia resulting in severe dyspnea, hypoxia
and septic shock; gastrointestinal anthrax may result in pharyngeal lesions
with sore throat, dypshagia marked neck swelling and regional lymphadenopathy,
or intestinal infection characterized by fever, severe abdominal pain, massive
ascites, hematemesis, and bloody diarrhea. As with any form of anthrax,
hemorrhagic meningitis can result from hematogenous spread of the organism
from the primary site.
Etiologic agent Bacillus anthracis
is an encapsulated gram-positive, nonmotile, aerobic, spore-forming bacterial
rod with a spore size of approximately 1 m x 2 m. The three virulence factors
of B. anthracis are edema toxin, lethal toxin, and an antiphagocytic capsular
antigen. The toxins are responsible for the primary clinical manifestations
of hemorrhage, edema, and necrosis.
Incidence In the United States, incidence of naturally acquired anthrax is extremely
rare (~ 1-2 cases of cutaneous disease per year). Gastrointestinal anthrax
is rare, but may occur as explosive outbreaks associated with ingestion
of infected animals. Worldwide, the incidence is unknown, though B. anthracis
is present in most of the world. Unreliable reporting makes it difficult
to estimate the true incidence of human anthrax worldwide. However, in
fall 2001, 22 cases of anthrax (11 inhalation, 11 cutaneous) were identified
in the United States following intentional contamination of the mail.
Sequelae If untreated, anthrax in all forms can lead to septicemia, hemorrhagic
meningitis, and death. The case fatality ratio for patients with appropriately
treated cutaneous anthrax is usually <1%, but for inhalation or gastrointestinal
disease it can exceed 50%. Case-fatality rates for inhalation anthrax are
high, even with appropriate antibiotics and supportive care. Among the eighteen
cases of inhalation anthrax in the United States during the twentieth century,
the overall case fatality was >75%. Following the bioterrorist attack
in fall 2001, the case-fatality rate among patients with inhalation disease
was 45% (5/11). The case-fatality rate of gastrointestinal anthrax is unknown
but is estimated to be 25%-60%.
Transmission For humans, the source of infection in naturally acquired disease is through
contact with infected livestock, wild animals, or contaminated animal products
(including carcasses, hides, hair, wool, meat, and bone meal). Person-to-person
transmission is extremely unlikely and only reported with cutaneous anthrax
where discharges from cutaneous lesions are potentially infectious.
Risk groups Cutaneous anthrax is the most common manifestation of naturally acquired
infection with B. anthracis. Inhalation (pulmonary) anthrax occurs in
persons working in certain occupations where spores may be aerosolized from
contaminated animal products, such as animal hair processing or through
intentional release. Occupational risk groups include those coming into
contact with livestock or products from livestock, e.g., veterinarians,
animal handlers, abattoir workers, and laboratorians.
Surveillance For both livestock and humans, anthrax is a notifiable disease in the United
States.
Trends In the United States, the annual incidence of naturally occurring human
anthrax declined from estimated 130 cases annually in the early 1900’s
to 2 cases each in 2000, 2001, and 2002. The recent cases of anthrax that
occurred after B. anthracis spores were distributed through the U.S. mail
have further underscored the potential dangers of this organism as a bioterrorism
threat. In addition to aerosolization, there is a theoretical health risk
associated with B. anthracis spores being introduced into food products
or water supplies.
v Fact Sheet: Anthrax Information for Health Care Providers
|
Cause |
Bacillus anthracis |
|
-
Encapsulated, aerobic, gram-positive, spore-forming, rod-shaped
(bacillus) bacterium
|
|
Systems affected |
-
Skin or cutaneous (most common)
-
Respiratory tract or inhalational (rare)
-
Gastrointestinal (GI) tract (rare)
-
Oropharyngeal form (least common)
|
|
Transmission |
-
Skin: direct skin contact with spores; in nature, contact with
infected animals or animal products (usually related to occupational
exposure)
-
Respiratory tract: inhalation of aerosolized spores
-
GI: consumption of undercooked or raw meat products or dairy
products from infected animals
-
No person-to-person transmission of inhalational or GI anthrax
|
|
Reporting |
-
Report suspected or confirmed anthrax cases immediately to
your local or state department of health.
|
| Cutaneous
Anthrax |
|
|
Incubation period |
Usually an immediate response up to 1 day |
|
Typical signs/symptoms |
-
Local skin involvement after direct contact with spores or
bacilli
-
Localized itching followed by 1) papular lesion that turns
vesicular and 2) subsequent development of black eschar within
7–10 days of initial lesion
|
|
Treatment
(See Cutaneous
Anthrax Treatment Protocol
for specific therapy) |
|
|
Precautions |
-
Standard contact precautions. Avoid direct contact with wound
or wound drainage.
|
|
Inhalational Anthrax
|
|
Incubation period |
-
Usually <1 week; may be prolonged for weeks (up to 2 months)
|
|
Typical signs/ symptoms often biphasic, but
symptoms may progress |
Initial phase
Subsequent phase
• Non-specific symptoms such
• 1–5 days after onset
of
as low-grade fever, nonproductive initial symptoms
cough, malaise, rapidly fatigue, • May be preceded by
1-3 days
myalgias, profound sweat, chest of improvement
discomfort (upper respiratory • Abrupt
onset of high fever and
tract symptoms are rare) severe respiratory
distress
• Maybe rhonchi on exam, (dyspnea, stridor,
cyanosis)
otherwise normal • Shock,
death within 24-36
• Chest X-ray: hours
� mediastinal
widening
� pleural
effusion (often)
� infiltrates
(rare)
|
|
Laboratory |
• Coordinate all aspects of testing,
Clues to diagnosis
packaging, and transporting with • Gram-positive bacilli on unspun
public health laboratory/Labora- peripheral blood smear or
CSF
tory Response Network (LRN). • Aerobic blood culture
• Obtain specimens appropriate growth of large, gram-positive
to system affected: bacilli provides
preliminary
� blood (essential) identification
of Bacillus
� pleural fluid species.
� cerebral spinal fluid
(CSF) skin lesion
|
|
Treatment (See
(Inhalational Anthrax
Treat- ment Protocol
for specific therapy) |
• Obtain specimens for culture Before initiating antimicrobial therapy.
• Initiate antimicrobial
therapy immediately upon suspicion.
• Do Not use extended-spectrum-cephalosporins or trimethoprim/
sulfamethoxazole
because anthrax may be resistant to these drugs.
• Supportive care including controlling pleural effusions
|
|
Precautions |
Standard contact precautions |
|
Gastrointestinal Anthrax |
| Incubation
period |
Usually 1–7 days |
|
Typical signs/symptoms |
Initial phase
Subsequent phase
• Nausea, anorexia,
vomiting, • 2–4 days after onset of
and fever progressing to severe symptoms, ascites develops
abdominal hematemesis, as abdominal
pain decreases
and diarrhea that is almost • Shock death within
2–5
always bloody days of
onset
• Acute abdomen picture with
rebound tenderness may develop.
• Mesenteric adenopathy on
computed tomography (CT)
scan likely.
Mediastinal widening on chest
X-ray has been reported
|
|
Laboratory |
• Coordinate all aspects of testing,
Clues to
diagnosis
packaging, and transporting with • Gram-positive bacilli on
public health laboratory/LRN. unspun peripheral blood
• Obtain specimens appropriate to smear or ascitic fluid
system affected: • Pharyngeal swab
for
�
blood (essential) pharyngeal form
�
ascitic fluid • Aerobic
blood culture
growth
of large, gram-
positive
bacilli provides
preliminary
identification
of Bacillus
species.
|
|
Treatment
(See Inhalational
Anthrax Treatment Protocol
for specific therapy) |
• Obtain specimens
for culture before initiating antimicrobial
therapy.
• Early (during initial phase) antimicrobial therapy is critical.
• Do NOT use extended-spectrum cephalosporins or
trimethoprim/sulfamethoxazole because anthrax may be resistant to
these drugs.
|
|
Precautions |
• Standard
precautions |
|
Oropharyngeal Anthrax |
|
Incubation period |
• Usually
1–7 days |
|
Typical signs/ symptoms |
Initial phase Subsequent
phase
• Fever and marked
unilateral and hyperemic
or bilateral neck swelling • Ulcers
may progress to
caused by regional lymphade- necrosis
nopathy • Swelling
can be severe
• Severe throat pain and enough to
compromise
dysphagia the airway
• Ulcers at the base of the
tongue, initially edematous
|
|
Laboratory |
•
Coordinate all aspects of • Aerobic blood culture
testing, packing, and trans- growth of large, gram-
porting with public health positive bacilli provides
laboratory/LRN. preliminary identification
• Obtain specimens appropriate of Bacillus species
to system affected:
� blood (essential)
� throat
|
|
Treatment (See
Anthrax Treatment
Protocol for
specific therapy)
|
• Obtain specimens for culture before initiating antimicrobial
therapy.
• Do not use extended-spectrum cephalosporins or trimethoprim/sulfame-thoxazole
because anthrax may be resistant to these drugs.
• Supportive care including controlling ascites
|
|
Precautions |
Standard contact
precautions |
v
Drug Therapy
Patient Information: Ciprofloxacin 500 MG Oral Tablet
This drug
belongs to a class of drugs called quinolone antibiotics. You have been given
this drug for protection against possible exposure to an infection-causing
bacteria. This drug prevents:
• Anthrax
You have
been provided a limited supply of medicine. Local emergency health workers
or your healthcare provider will inform you if you need more medicine after
you finish this supply. If so, upon your follow-up visit, you will be told
how to get more medicine. You will be told if no more medicine is needed.
You may also be switched from this medicine to a different medicine based
on laboratory tests.
Take this
medicine as prescribed: one tablet by mouth, two times a day.
You will
be provided special dosing instructions for children.
Keep taking
your medicine, even if you feel okay, unless your doctor tells you to stop.
If you stop taking this medicine too soon, you may become ill.
You should
take this medicine with a full glass of water. Drink several glasses of water
each day while you are taking this medicine. It is best to take this medicine
2 hours after a meal. If it upsets your stomach, you may take it with food,
but do not take it with milk, yogurt, or cheese.
If you
miss a dose, take the missed dose as soon as possible. If it is almost time
for your next regular dose, wait until then to take your medicine, and skip
the missed dose. Do not take two doses at the same time.
Drugs and foods to avoid
: Do not take the following drugs within 2 hours of taking Ciprofloxocin:
antacids such as Maalox or Mylanta, vitamins, iron supplements, zinc supplements,
or sucralfate (Carafate). You may take them 2 hours after or 6 hours before
Ciprofloxocin. Also, make sure your doctor knows if you are taking asthma
medicine like theophylline, gout medicine like probenecid (Benemid), or
a blood thinner such as Coumadin.
Avoid drinking
more than one or two caffeinated beverages (coffee, tea, soft drinks) per
day. Avoid taking this medicine with foods containing large amounts of calcium,
like milk, yogurt, or cheese.
Warnings: If you have epilepsy
or kidney disease, or if you are pregnant, become pregnant, or are breastfeeding,
notify emergency healthcare workers before you start taking this medicine.
Do not
take this medicine if you have had an allergic reaction to ciprofloxacin
or other quinolone medicines such as norfloxacin (Noroxin), ofloxacin (Floxin)
or nalidixic acid (NegGram).
This medicine
may make you dizzy or lightheaded. Avoid driving or using machinery until
you know how it will affect you.
This medicine
increases the chance of sunburn; make sure to use sunscreen to protect your
skin.
Side effects : Call your
doctor or seek medical advice right away if you are having any of these side
effects: rash or hives; swelling of face, throat, or lips; shortness of breath
or trouble breathing; seizures; or severe diarrhea. Less serious side effects
include nausea, mild diarrhea, stomach pain, dizziness, and headache. Talk
with your doctor if you have problems with these side effects.
Patient Information: Doxycycline 100 MG Oral Tablet
This drug
belongs to a class of drugs called tetracycline antibiotics. You have been
given this drug for protection against possible exposure to an infection-causing
bacteria. This drug prevents:
• Anthrax
You have
been provided a limited supply of medicine. Local emergency health workers
or your healthcare provider will inform you if you need more medicine after
you finish this supply. If so, upon your follow-up visit, you will be told
how to get more medicine. You will be told if no more medicine is needed.
You may also be switched from this medicine to a different medicine based
on laboratory tests.
Take
this medicine as prescribed: one tablet by mouth, two times a day.
You will
be provided special dosing instructions for treatment of children under 8
years of age.
Keep
taking your medicine, even if you feel okay, unless your healthcare provider
tells you to stop. If you stop taking this medicine too soon, you may become
ill.
You may
take your medicine with or without food or milk, but food or milk may help
you avoid stomach upset.
If you
miss a dose, take the missed dose as soon as possible. If it is almost time
for your next regular dose, wait until then to take your medicine, and skip
the missed dose. Do not take two doses at the same time.
Drugs and foods to avoid
: Do not take the following medicines within 2 hours of taking
Doxycycline : antacids such
as Maalox or Mylanta, calcium or iron supplements, cholestyramine (Questran)
or colestipol (Colestid).
While
you are taking this medicine, birth control pills may not work as well; make
sure to use another form of birth control.
Warnings : If you have liver
disease, or if you are or might be pregnant, or if you are breastfeeding,
tell emergency healthcare workers before you start taking this medicine.
This
medicine increases the chance of sunburn; make sure to use sunscreen to protect
your skin.
Do not
take this medicine if you have had an allergic reaction to any tetracycline
antibiotics.
Women
may have vaginal yeast infections from taking this medicine.
Side effects : Call your
doctor or seek medical attention right away if you are having any of these
side effects: skin rash, hives, or itching; wheezing or trouble breathing;
swelling of the face, lips, or throat. Less serious side effects include
diarrhea, upset stomach, nausea, sore mouth or throat, sensitivity to sunlight,
or itching of the mouth or vagina lasting more than 2 days. Talk with your
doctor if you have problems with these side effects.
Patient Information: Penicillin VK 500 MG Oral Tablet
This
drug treats infections. It belongs to a class of drugs called penicillin
antibiotics. You have been given this drug for protection against possible
exposure to infection-causing bacteria. This drug treats:
• Anthrax
You
have been provided a limited supply of medicine. Local emergency health workers
or your healthcare provider will inform you if you need more medicine after
you finish this supply. If so, upon your follow-up visit, you will be told
how to get more medicine. You will be told if no more medicine is needed.
You may also be switched from this medicine to a different medicine based
on laboratory tests.
Take
this medicine as prescribed.
Keep
taking your medicine, even if you feel okay, unless your healthcare provider
tells you to stop. If you stop taking this medicine too soon, you may become
ill.
You
may take your medicine with or without food or milk, but food or milk may
help you avoid stomach upset.
If
you miss a dose, take the missed dose as soon as possible. If it is almost
time for your next regular dose, wait until then to take your medicine, and
skip the missed dose. Do not take two doses at the same time.
Drugs and foods to avoid:
Make sure your healthcare provider knows if you are taking the medication
Probenecid. Probenecid causes penicillin to build up in the blood, which
may increase your chance of having side effects.
While
you are taking this medicine, birth control pills may not work as well; make
sure to use another form of birth control.
Warnings : Make sure your
healthcare provider knows if you are pregnant or breast-feeding. Penicillins
as a class are generally considered safe for use in pregnancy. Research suggests
that it is unlikely that penicillin causes birth defects when taken by pregnant
women. Penicillin passes into breast milk, though only in small amounts,
and may cause allergic reactions, diarrhea, fungus infections, and skin rash
in nursing babies.
Do
not take this medicine if you have had an allergic reaction to penicillin
or cephalosporin antibiotics such as Keflex or Ceclor.
Side effects: Call your
doctor or seek medical attention right away if you are having any of these
side effects: wheezing or trouble breathing; skin rash, hives or itching;
swelling of the face, lips, or throat; or severe diarrhea. Less serious side
effects include mild diarrhea, nausea, upset stomach, sore throat or mouth,
itching of the mouth or vagina lasting more than 2 days. Talk with your doctor
if you have problems with these side effects.
Patient Information: Amoxicillin 250 MG – Oral Capsules Or Amoxicillin 250
MG/5 ML– Oral Suspension
This
drug treats infections. It belongs to a class of drugs called penicillin
antibiotics. You have been given this drug for protection against possible
exposure to infection-causing bacteria. This drug treats:
•
Anthrax
You
have been provided a limited supply of medicine. Local emergency health workers
or your healthcare provider will inform you if you need more medicine after
you finish this supply. If so, upon your follow-up visit, you will be told
how to get more medicine. You will be told if no more medicine is needed.
You may also be switched from this medicine to a different medicine based
on laboratory tests.
Take
this medicine as prescribed:
-
You will be provided special dosing instructions if you have a child.
For oral suspension: shake well before using. Measure with marked measuring
device. Keep this medicine refrigerated.
-
Keep taking your medicine, even if you feel okay, unless your healthcare
provider or public health officer tells you to stop. If you stop taking
this medicine too soon, you may become ill.
-
You may take your medicine with or without food or milk, but food or
milk may help you avoid stomach upset.
-
If you miss a dose, take the missed dose as soon as possible. If it
is almost time for your next regular dose, wait until then to take your
medicine, and skip the missed dose. Do not take two doses at the same
time.
Drugs and foods to avoid
: Make sure your healthcare provider knows if you are taking the medication
Probenecid. Probenecid causes amoxicillin to build up in the blood, which
may increase your chance of having side effects.
While
you are taking this medicine, birth control pills may not work as well; make
sure to use another form of birth control.
Warnings : Make sure your
healthcare provider knows if you are pregnant or breast-feeding. An expert
review of published data on experiences with amoxicillin use during pregnancy
concluded that therapeutic doses during pregnancy are unlikely to pose a
substantial risk for birth defects. However, there are no data available
to assess the effects of long-term therapy in pregnant women, such as that
proposed for treatment of anthrax exposure. Amoxicillin passes into breast
milk but is considered "usually compatible with breastfeeding" by the American
Academy of Pediatrics.
Do
not take this medicine if you have had an allergic reaction to amoxicillin
or penicillin or cephalosporin antibiotics such as Keflex or Ceclor.
Side effects : Call your
doctor or seek medical attention right away if you are having any of these
side effects: wheezing or trouble breathing; skin rash, hives or itching;
swelling of the face, lips, or throat; or severe diarrhea. Less serious side
effects include mild diarrhea, nausea, upset stomach, sore throat or mouth,
itching of the mouth or vagina lasting more than 2 days. Talk with your doctor
if you have problems with these side effects.
v Clinical Issues in the Prophylaxis, Diagnosis, and Treatment
of Anthrax
On
November 18, 2001, a meeting was held at the Centers for Disease Control
and Prevention (CDC), Atlanta, Georgia, to discuss the prophylaxis, diagnosis,
and treatment of anthrax. Participants included clinicians and health department
personnel from areas where anthrax cases were identified, infectious disease
experts, representatives of professional societies, and experts from federal
agencies. A patient recovering from inhalational anthrax also described her
illness. The following is a summary of the presentations and discussion.
Prophylaxis
(1)
Ciprofloxacin,
doxycycline, and penicillin G procaine have been approved by the Food and
Drug Administration (FDA) for prophylaxis of inhalational Bacillus anthracis
infection, on the basis of efficacy
data in monkeys and pharmacokinetic, pharmacodynamic, and safety considerations
(1-3) . During the recent bioterrorist
attacks, interim CDC recommendations for anthrax prophylaxis include ciprofloxacin
or doxycycline; amoxicillin (in three daily doses) is an option for children
and pregnant or lactating women exposed to strains susceptible to penicillin
(4-6) , to avoid potential toxicity
of quinolones and tetracyclines. Amoxicillin is not widely recommended as
a first-line prophylactic agent, however, because of lack of FDA approval,
lack of data regarding efficacy, and uncertainty about the drug’s ability
to achieve adequate therapeutic levels at standard doses.
The
optimal duration of prophylaxis is uncertain; however, 60 days was recommended,
primarily on the basis of animal studies of anthrax deaths and spore clearance
after exposure. The possible need for longer prophylaxis and vaccine was
discussed. In monkeys after aerosol challenge, an estimated 0.5%-1% of spores
remained at 75 days and traces were present at 100 days; delaying prophylaxis
up to 20 days after exposure prolonged the incubation period without reducing
disease risk (7)
. In one human case during the Sverdlovsk outbreak (former Soviet Union,
1979), anthrax developed 43 days after spores were released into the atmosphere
(time of exposure unknown) (2,8)
. When prophylaxis is delayed or intermittent, several experts recommended
a total of 60 days of therapy. (On December 18, the Department of Health and
Human Services announced additional options for prophylaxis of inhalational
anthrax for persons who wish to take extra precautions, especially those whose
exposure may have been high. Three options are now offered: 1) 60 days of
antibiotic prophylaxis; 2) 100 days of antibiotic prophylaxis, and 3) 100
days of antibiotic prophylaxis, plus anthrax vaccine as investigational postexposure
treatment [3 doses over a 4-week period]
[9].)
The
need for prophylaxis is determined by public health officials on the basis
of an epidemiologic investigation. Prophylaxis is indicated for persons exposed
to an airspace contaminated with aerosolized B. anthracis. Prophylaxis
is not indicated for health-care and mortuary workers who care for patients
or attend to corpses using standard precautions, for persons who handle or
open mail in the absence of a credible threat, or for prevention of cutaneous
anthrax (10)
.
Successful
implementation of mass prophylaxis requires clarity of public health intent
and communication, as well as coordination and collaboration. A well-communicated
policy on who receives prophylaxis and with which drugs is essential. Agency
spokespersons, local health-care providers, employers, and employee organizations
(e.g., labor unions) should be familiar with the policy. Local or regional
task forces may be helpful in planning and communicating public health policy,
and resolving jurisdictional issues. Prophylaxis teams should be predesignated
to function around the clock. Team members should have contingency plans for
personal needs (e.g., child care). Issues for the point of prophylaxis distribution
include layout and managing of traffic flow; security; availability of medical
and office supplies, antibiotic and disease fact sheets, multilingual staff,
and mental health counselors; legal needs (e.g., for a physician to write
orders); and plans for follow-up, including assessment of adherence, illness,
and possible drug adverse effects. Collaboration among health departments,
health-care delivery organizations, and clinicians is important. In the 2001
outbreak, some patients with possible drug side effects were refused appointments
by their private physicians and were referred back to the health department.
Anthrax
prophylaxis issues needing further consideration or research include efficacy
of additional drugs, optimal duration of prophylaxis, usefulness of a loading
dose, safety of prolonged drug use (especially in children and pregnant women),
concomitant use of vaccine or antitoxin, level of infectious dose, and definition
of high-risk exposure (e.g., according to particle size or degree of environmental
contamination).
Clinical Recognition and Diagnosis
(2)
Twenty-two
confirmed or suspected cases (11 confirmed inhalational; 7 confirmed and
4 suspected cutaneous) were identified in the 2001 outbreak of bioterrorism-related
anthrax. Cases were reported from Florida, New York, New Jersey, the District
of Columbia, and Connecticut.
Inhalational Anthrax
Of
the 11 patients with inhalational anthrax, 9 (and possibly all 11) are believed
to have been exposed to mail containing or contaminated with B. anthracis
spores. Median age was 56 years (range 43-94 years). Average incubation
from known exposure to symptoms was 4 days (range 4-6 days). Fever, chills,
drenching sweats, profound fatigue, minimally productive cough, nausea or
vomiting, and chest discomfort were symptoms reported by most patients. Rhinorrhea
and productive cough were uncommon. Chest X-ray at initial examination showed
mediastinal widening, paratracheal fullness, hilar fullness, and pleural effusions
or infiltrates or both, but in some patients these initial findings were
subtle. Pleural effusions were a complication in all 11 patients; among all
8 patients who had not received antibiotics, B. anthracis grew in
blood cultures drawn at initial examination. Six (55%) of 11 patients have
survived with aggressive supportive care and multidrug antibiotic regimens
including a fluoroquinolone(11)
.
The
differential diagnosis of inhalational anthrax versus influenza-like illness
is challenging. Respiratory viruses, including influenza, are common causes
of influenza-like illness and tend to circulate in winter. These viruses
are readily communicable, in contrast to anthrax, which is not spread from
person to person. A history of influenza vaccination is not helpful in evaluating
the likelihood of anthrax. Influenza-like illnesses have many causes besides
influenza viruses, and influenza vaccine is not 100% effective. Unlike patients
with inhalational anthrax, adults with influenza or other viral respiratory
illnesses do not usually have shortness of breath and vomiting but often
have sore throat or rhinorrhea. Rapid identification tests for influenza
are available but vary widely in sensitivity.
In
the current climate, emergency department and primary-care physicians should
maintain a high index of suspicion for inhalational anthrax. Complicating
diagnosis is the fact that patients initially may not appear very ill
(11). A careful history with
assessment of epidemiologic risk factors for anthrax (e.g., working for the
postal service) should be obtained. Communication between clinicians and
health authorities is critical for obtaining up-to-date assistance with diagnosis
and management.
The
classic chest X-ray findings—widened mediastinum or pleural effusions—may
be subtle or absent on initial medical evaluation. In addition, these radiographic
findings are not unique to anthrax: histoplasmosis, sarcoidosis, tuberculosis,
and lymphoma, for example, are included in the differential diagnosis. A
chest computed tomography scan is helpful in detecting hemorrhagic mediastinal
lymph nodes and edema, peribronchial thickening, and pleural effusions, findings
seen in patients with inhalational anthrax.
Hyperdense
mediastinal and hilar adenopathy plus mediastinal edema suggest anthrax.
The hemorrhagic pleural effusions of inhalational anthrax typically increase
during hospitalization.
Blood
cultures and B. anthracis-specific
polymerase chain reaction (PCR) of sterile fluids (e.g., blood and pleural
fluid) are important in the diagnosis of inhalational anthrax. Serologic
testing has also been valuable. An enzyme-linked immunosorbent assay (ELISA)
to detect immunoglobulin (Ig) G response to B. anthracis protective
antigen (PA) is highly sensitive (detects 98.6% of true positives) but is
only approximately 80% specific. To improve specificity, a PA-competitive
inhibition ELISA is used as a second, confirmatory step. Preliminary studies
indicate that specific IgG anti-PA antibody can be detected as early as 10
days, but peak IgG may not be seen until 40 days after onset of symptoms.
Immunohistochemical
examination of pleural fluid or transbronchial biopsy specimens, using antibodies
to B. anthracis cell wall and capsule, also has an important role in
the diagnosis of inhalational anthrax, especially in patients who have received
prior antibiotics. Immunohistochemical examination can detect intact bacilli
or B. anthracis antigens. PCR, serologic tests, and immunohistochemical
tests are currently available at CDC or at certain laboratories in the Laboratory
Response Network (LRN).
Cutaneous Anthrax
Seven
confirmed and four suspected cases of cutaneous anthrax were identified during
the 2001 outbreak. Skin trauma was not associated with these cases of cutaneous
anthrax. Exposure to contaminated mail was the apparent source of infection
in all patients. The incubation periods after exposure ranged from 1 to 10
days. The initial symptom was often a pruritic papule resembling an insect
bite. The papules vesiculated, with some becoming hemorrhagic. The vesicles
ruptured to form depressed ulcers, often with local edema, ultimately forming
dry eschars. These stages occur regardless of antibiotic therapy. The differential
diagnosis of cutaneous anthrax includes brown recluse spider bite, ecthyma,
ulceroglandular tularemia, accidental vaccinia, and necrotic herpes simplex.
Cutaneous anthrax is painless, does not include rash, and results in a black
eschar. Patients with cutaneous anthrax may have fever, extensive edema, and
other systemic signs.
Gram
stain and culture of the lesion are recommended; however, prior antibiotic
treatment rapidly renders the infected site culture-negative for B. anthracis
. Serologic testing and punch biopsy at the edge of the lesion, examined
by silver staining and immunohistochemical testing, are useful in diagnosing
cutaneous anthrax in patients who have received antibiotic therapy.
Clinical
recognition and diagnosis issues needing further consideration and research
include rapid, reliable, and readily available detection methods (e.g., PCR
and antigen detection); education and ready access to information for clinicians
regarding anthrax clinical features and risk stratification; recognition
of anthrax in children; and the role of serologic testing in the diagnosis
and management of both inhalational and cutaneous anthrax.
Treatment
(3)
Treatment
recommendations for anthrax infections have been based on historical information
and limited data from animals (nonhuman primates), as well as in vitro findings.
Susceptibility testing of 65 historical isolates was performed at CDC. In
the absence of published guidelines for testing for B. anthracis,
the standard National Committee for Clinical Laboratory Standards broth microdilution
method was used with staphylococcal breakpoints. These 65 isolates and all
those associated with the 2001 outbreak were sensitive to the quinolones,
rifampin, tetracycline, vancomycin, imipenem, meropenem, chloramphenicol,
clindamycin, and the aminoglycosides. The isolates have intermediate-range
susceptibility to the macrolides but are resistant to extended-spectrum cephalosporins,
including third-generation agents (e.g., ceftriaxone), and to trimethoprim-sulfamethoxazole
(12).
The
decision regarding the use of penicillins, the drugs historically used for
treatment and prophylaxis of anthrax, is complicated. An inhibition assay
shows beta-lactamase activity at low levels in the isolates. Genomic sequence
data show two beta-lactamases: a potential penicillinase (class A) and a
cephalosporinase (class B), which is expressed. Concern about the use of
penicillin arises because an inducible penicillinase could be activated in
the face of treatment with beta-lactams, particularly if the number of organisms
present is high, as appears typical with inhalational disease. Concerns have
also been raised about the poor penetration of beta-lactams into macrophages,
the site where B. anthracis spores germinate.
Ciprofloxacin
has been recommended on the basis of in vivo (animal) findings; other quinolones
have not been studied in the primate model. Doxycycline, another first-line
agent, should not be used if meningitis is suspected because of its lack of
adequate central nervous system penetration. Bacteremic patients are often
initially treated with a multidrug regimen to which an offending organism
is presumed sensitive; this treatment also allows empiric coverage for other
pathogens. Thus, the recommendation for initial treatment of inhalational
anthrax is a multidrug regimen of either ciprofloxacin or doxycycline along
with one or more agents to which the organism is typically sensitive. After
susceptibility testing and clinical improvement, the regimen may be altered.
The drugs of choice for treatment of cutaneous disease are also ciprofloxacin
or doxycycline. A penicillin such as amoxicillin or amoxacillin/clavulanic
acid may be used to complete the course if susceptibility testing is supportive.
On
the basis of risk for the inhalational form of the disease, cases of both
inhalational and cutaneous anthrax associated with the 2001 outbreak are
being treated with 60 days of antibiotics. Although zoonotic cutaneous anthrax
is treated with a 7- to 10-day regimen, inhaled spores can remain latent
for extended periods.
Two
months after the 2001 outbreak, 6 of 11 patients with inhalational anthrax
had survived. Keys to successful management appear to be early institution
of antibiotics and aggressive supportive care. Chest tube drainage of the
recurrent pleural effusions, which are typically hemorrhagic, often leads
to dramatic clinical improvement. Because these effusions tend to reaccumulate
rapidly, insertion of a chest tube or tubes has been beneficial.
Anthrax
treatment issues meriting further consideration relate to adjunctive therapies.
Clindamycin has been suggested to have antitoxin properties (as in the treatment
of toxic shock associated with group A streptococci, Staphylococcus aureus,
and Clostridium infections). Steroids have been used to control
the edema of cutaneous disease and have been suggested for the treatment
of meningitis or substantial mediastinal edema
(13) . Other antitoxin agents
investigated in vitro include angiotensin-converting enzyme inhibitors,
calcium channel blockers, and tumor necrosis factor inhibitors. Specific
anthrax IgG antisera, collected from military or other vaccinees, may be
an adjunct, as well as administration of the vaccine itself.
Presenters
-
David Ashford, David Bell, Susan Blank, Eddy Bresnitz, M. Dianne Murphy,
Bradley Perkins, Larry Siegel, and Steven Wiersma.
-
Sharon Balter, Carolyn Bridges, James Earls, John Jernigan, Michael
Martin, Thom Mayer, Thomas McGovern, Carlos Omenaca, David Stephens,
Martin Topiel, and Sherif Zaki.
-
John Jernigan, Phyllis Kozarsky, Carlos Omenaca, David Stephens, and
Fred Tenover.
Source: Centers for Disease Control and Prevention, Atlanta, Georgia, USA
David M. Bell, Phyllis E. Kozarsky, and David S. Stephens
REFERNECES
-
Food and Drug Administration. Prescription drug products; doxycycline
and penicillin G procaine administration for inhalational anthrax
(post-exposure). Fed Reg 2001;66-55679-82.
-
Friedlander AM, Welkos SL, Pitt MLM, et al. Postexposure prophylaxis
against inhalation anthrax. J Infect Dis 1993;167:1239-42.
-
Centers for Disease Control and Prevention. Use of anthrax vaccine in
the United States: recommendations of the Advisory Committee on Immunization
Practices (ACIP). MMWR Morb Mortal Wkly Rep 2000;49(No. RR-15):12-14.
-
Centers for Disease Control and Prevention. Update: investigation of
anthrax associated with intentional exposure and interim public health
guidelines, October 2001. MMWR Morb Mortal Wkly Rep 2001;50:889-97.
-
Centers for Disease Control and Prevention. Updated recommendations
for antimicrobial prophylaxis among asymptomatic pregnant women after
exposure to Bacillus anthracis. MMWR Morb Mortal Wkly Rep 2001;50:960.
-
Centers for Disease Control and Prevention. Update: interim recommendations
for antimicrobial prophylaxis for children and breastfeeding mothers and
treatment of children with anthrax. MMWR Morb Mortal Wkly Rep
2001;50:1014-6.
-
Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis
of experimental pulmonary anthrax in the monkey. J Hyg 1956;54:28-36.
-
Meselson M, Guillemin J, Hugh-Jones M, et al. The Sverdlovsk anthrax
outbreak of 1979. Science 1994;266:1202-8.
-
Centers for Disease Control and Prevention. Additional options for preventive
treatment for persons exposed to inhalational anthrax. MMWR Morb
Mortal Wkly Rep 2001;50:1142,1151.
-
Centers for Disease Control and Prevention. Interim guidelines for investigation
of and response to Bacillus anthracis exposures. MMWR Morb Mortal Wkly
Rep 2001;50:987-90.
-
Jernigan JA, Stephens DS, Ashford DA, et al. Bioterrorism-related inhalational
anthrax: the first 10 cases reported in the United States. Emerg
Infect Dis 2001;7:933-44.
-
Centers for Disease Control and Prevention. Update: investigation of
bioterrorism-related anthrax and interim guidelines for exposure management
and antimicrobial therapy. MMWR Morb Mortal Wkly Rep 2001;50:909-19.
-
Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J Med
1999:341:815-26.
v Additional Options for Preventive Treatment For Those Exposed
to Inhalational Anthrax
Many
of those who were exposed to inhalational anthrax in the recent mail attacks
are presently concluding their 60-day course of preventive antibiotic treatment.
Some of these persons, especially those who may have been exposed to very
high levels of anthrax spores, may wish to take additional precautions. The
Department of Health and Human Services (HHS) is providing two additional
options beyond the 60-day antibiotic course, for those who may wish to pursue
them: an extended course of antibiotics, and investigational post-exposure
treatment with anthrax vaccine.
HHS
will make anthrax vaccine available to those who were exposed to inhalational
anthrax, who have concluded their antibiotic treatment and who wish to receive
the vaccine as an investigational product. The vaccine is being made available
in this investigational mode, under an investigational new drug application
(IND) at the option of the individual, in recognition of the limited nature
of the data now available concerning inhalation anthrax treatment and the
factors underlying development of the disease, as well as uncertainty concerning
the extent of exposure to spores that some persons may have received in the
recent anthrax incidents. The decision to use this vaccine is at the discretion
of the individual, in consultation with his or her physician.
Background
Existing
data, based especially on animal models, indicate that inhalational anthrax
is unlikely to occur after 60 days following exposure. This is the basis
of the recommendation for 60 days treatment with an effective antibiotic.
So far, no known cases have developed in individuals who were recently exposed
to inhalation anthrax and who were prescribed the 60-day antibiotic course.
HHS health agencies continue to recommend that those who were prescribed
the 60-day antibiotic course and who conclude this course of treatment, or
who stopped taking the medicine prior to 60 days, should remain watchful
of their health and be in close communication with a physician who is aware
of their exposure status. A number of individuals have already concluded
the 60-day course, or have stopped taking the antibiotics prior to the 60-day
conclusion, and no cases have been reported among them.
At
the same time, other animal data indicate that live spores may continue to
reside in the lungs beyond the 60-day period, even though these animals did
not develop disease. Traces of live spores have been detected in the lungs
up to 100 days following exposure. This raises the theoretical possibility
that the spores remaining in the lung area might still, after 60 days, result
in anthrax.
If
such a late infection were to occur, HHS scientists believe that the infection
could be successfully treated, as were cases of inhalation anthrax that were
identified early during the anthrax mail attacks. At the same time, HHS recognizes
that some individuals may wish to take extra precautions, especially those
whose exposure may have been especially high.
Options
There
are three options for individuals exposed to inhalational anthrax:
-
Current Recommendation-60 days of antibiotic treatment, accompanied
by careful monitoring for illness.
-
Additional Option 1-40 additional days of antibiotic treatment. This
course would be intended to provide protection against the theoretical
possibility that spores might cause infection up to 100 days after exposure.
It should be accompanied by monitoring for illness or adverse reactions.
-
Additional Option 2-40 additional days of antibiotic treatment, plus
anthrax vaccine as an investigational treatment. In addition to the 40
days of additional protection, this option would involve three doses of
anthrax vaccine over a four-week period, to provide immunity to infection
over a longer period of time. This is not currently an FDA–approved use
of the vaccine, however the vaccine may provide additional protection
by inducing an immune response to the anthrax organism. As an investigational
new drug, the vaccine would need to be administered with the full informed
consent of the individual as to possible risks. Individuals would also
be asked to take part in a follow-up study measuring the effect of the
vaccine when administered after exposure.
All
those who are concluding a 60-day course of antibiotic treatment should monitor
their health and be in close contact with their physician. Those who may
wish to continue taking antibiotics for an additional 40 days should consult
their physician about this course. Those who may wish to take part in the
investigational post-exposure use of the anthrax vaccine should consult their
physician or a physician at the site where vaccine is being administered.


