12.
Viral Hemorrhagic Fever
What are viral
hemorrhagic fevers?
|
Viral hemorrhagic fevers (VHFs) outbreak refer to a group of illnesses that are caused by several
distinct families of viruses. In general, the term "viral hemorrhagic
fever" is used to describe a severe multisystem syndrome (multisystem
in that multiple organ systems in the body are affected). Characteristically,
the overall vascular system is damaged, and the body’s ability to regulate
itself is impaired. These symptoms are often accompanied by hemorrhage
(bleeding); however, the bleeding is itself rarely life-threatening.
While some types of hemorrhagic fever viruses can cause relatively
mild illnesses, many of these viruses cause severe, life-threatening
disease. |

|
The Special Pathogens Branch (SPB) primarily
works with hemorrhagic fever viruses that are classified as biosafety level
four (BSL-4) pathogens. A list of these viruses appears in the SPB disease
information index. The Division of Vector-Borne Infectious Diseases, also
in the National Center for Infectious Diseases, works with the non-BSL-4
viruses that cause two other hemorrhagic fevers, dengue hemorrhagic fever
and yellow fever.
How are hemorrhagic fever viruses
grouped?
VHFs are caused by viruses of four distinct
families: arenaviruses, filoviruses, bunyaviruses, and flaviviruses. Each
of these families share a number of features:
- They
are all RNA viruses, and all are covered, or enveloped, in a fatty (lipid)
coating.
-
The viruses are geographically restricted to the areas where their host
species live.
-
Humans are not the natural reservoir for any of these viruses. Humans
are infected when they come into contact with infected hosts. However,
with some viruses, after the accidental transmission from the host, humans
can transmit the virus to one another.
-
Human cases or outbreaks of hemorrhagic fevers caused by these viruses
occur sporadically and irregularly. The occurrence of outbreaks cannot
be easily predicted.
-
With a few noteworthy exceptions, there is no cure or established drug
treatment for VHFs.
In rare cases, other viral and bacterial
infections can cause a hemorrhagic fever; scrub typhus is a good example.
What carries viruses that cause viral hemorrhagic fevers?
Viruses associated with most VHFs are zoonotic.
This means that these viruses naturally reside in an animal reservoir host
or arthropod vector. They are totally dependent on their hosts for replication
and overall survival. For the most part, rodents and arthropods are the main
reservoirs for viruses causing VHFs. The multimammate rat, cotton rat, deer
mouse, house mouse, and other field rodents are examples of reservoir hosts.
Arthropod ticks and mosquitoes serve as vectors for some of the illnesses.
However, the hosts of some viruses remain unknown — Ebola and Marburg viruses
are well-known examples.
Where are cases
of viral hemorrhagic fever found?
Taken together, the viruses that cause
VHFs are distributed over much of the globe. However, because each virus
is associated with one or more particular host species, the virus and the
disease it causes are usually seen only where the host species live(s). Some
hosts, such as the rodent species carrying several of the New World arenaviruses,
live in geographically restricted areas. Therefore, the risk of getting VHFs
caused by these viruses is restricted to those areas. Other hosts range over
continents, such as the rodents that carry viruses which cause various forms
of hantavirus pulmonary syndrome (HPS) in North and South America, or the
different set of rodents that carry viruses which cause hemorrhagic fever
with renal syndrome (HFRS) in Europe and Asia. A few hosts are distributed
nearly worldwide, such as the common rat. It can carry Seoul virus, a cause
of HFRS; therefore, humans can get HFRS anywhere where the common rat is
found.
While people usually become infected only
in areas where the host lives, occasionally people become infected by a host
that has been exported from its native habitat. For example, the first outbreaks
of Marburg hemorrhagic fever, in Marburg and Frankfurt, Germany, and in Yugoslavia,
occurred when laboratory workers handled imported monkeys infected with Marburg
virus. Occasionally, a person becomes infected in an area where the virus
occurs naturally and then travels elsewhere. If the virus is a type that
can be transmitted further by person-to-person contact, the traveler could
infect other people. For instance, in 1996, a medical professional treating
patients with Ebola hemorrhagic fever (Ebola HF) in Gabon unknowingly became
infected. When he later traveled to South Africa and was treated for Ebola
HF in a hospital, the virus was transmitted to a nurse. She became ill and
died. Because more and more people travel each year, outbreaks of these diseases
are becoming an increasing threat in places where they rarely, if ever, have
been seen before.
How are hemorrhagic
fever viruses transmitted?
Viruses causing hemorrhagic fever are initially
transmitted to humans when the activities of infected reservoir hosts or
vectors and humans overlap. The viruses carried in rodent reservoirs are
transmitted when humans have contact with urine, fecal matter, saliva, or
other body excretions from infected rodents. The viruses associated with
arthropod vectors are spread most often when the vector mosquito or tick
bites a human, or when a human crushes a tick. However, some of these vectors
may spread virus to animals, livestock, for example. Humans then become infected
when they care for or slaughter the animals.
Some viruses that cause hemorrhagic fever
can spread from one person to another, once an initial person has become
infected. Ebola, Marburg, Lassa and Crimean-Congo hemorrhagic fever viruses
are examples. This type of secondary transmission of the virus can occur
directly, through close contact with infected people or their body fluids.
It can also occur indirectly, through contact with objects contaminated with
infected body fluids. For example, contaminated syringes and needles have
played an important role in spreading infection in outbreaks of Ebola hemorrhagic
fever and Lassa fever.
What
are the symptoms of viral hemorrhagic
fever illnesses?
Specific signs and symptoms vary by the
type of VHF, but initial signs and symptoms often include marked fever, fatigue,
dizziness, muscle aches, loss of strength, and exhaustion. Patients with
severe cases of VHF often show signs of bleeding under the skin, in internal
organs, or from body orifices like the mouth, eyes, or ears. However, although
they may bleed from many sites around the body, patients rarely die because
of blood loss. Severely ill patient cases may also show shock, nervous system
malfunction, coma, delirium, and seizures. Some types of VHF are associated
with renal (kidney) failure.
How are patients
with viral hemorrhagic fever treated?
Patients receive supportive therapy, but
generally speaking, there is no other treatment or established cure for VHFs.
Ribavirin, an anti-viral drug, has been effective in treating some individuals
with Lassa fever or HFRS. Treatment with convalescent-phase plasma has been
used with success in some patients with Argentine hemorrhagic fever.
How can cases
of viral hemorrhagic fever be prevented
and controlled?
With the exception of yellow fever and
Argentine hemorrhagic fever, for which vaccines have been developed, no vaccines
exist that can protect against these diseases. Therefore, prevention efforts
must concentrate on avoiding contact with host species. If prevention methods
fail and a case of VHF does occur, efforts should focus on preventing further
transmission from person to person, if the virus can be transmitted in this
way. Because many of the hosts that carry hemorrhagic fever viruses are rodents,
disease prevention efforts include
-
controlling rodent populations;
-
discouraging rodents from entering or living in homes or workplaces;
-
encouraging safe cleanup of rodent nests and droppings.
For hemorrhagic fever viruses spread by
arthropod vectors, prevention efforts often focus on community-wide insect
and arthropod control. In addition, people are encouraged to use insect repellant,
proper clothing, bednets, window screens, and other insect barriers to avoid
being bitten.
For those hemorrhagic fever viruses that
can be transmitted from one person to another, avoiding close physical contact
with infected people and their body fluids is the most important way of controlling
the spread of disease. Barrier nursing or infection control techniques include
isolating infected individuals and wearing protective clothing. Other infection
control recommendations include proper use, disinfection, and disposal of
instruments and equipment used in treating or caring for patients with VHF,
such as needles and thermometers.
In conjunction with the World Health Organization,
CDC has developed practical, hospital-based guidelines, titled Infection
Control for Viral Haemorrhagic Fevers In the African Health Care Setting.
The manual can help health-care facilities recognize cases and prevent further
hospital-based disease transmission using locally available materials and
few financial resources.
What needs to
be done to address the threat
of viral hemorrhagic fevers?
Scientists and researchers are challenged
with developing containment, treatment, and vaccine strategies for these
diseases. Another goal is to develop immunologic and molecular tools for
more rapid disease diagnosis, and to study how the viruses are transmitted
and exactly how the disease affects the body (pathogenesis). A third goal
is to understand the ecology of these viruses and their hosts in order to
offer preventive public health advice for avoiding infection.
v Management of Patients with Suspected Viral Hemorrhagic Fever
In 1988, CDC published guidelines for managing
patients with suspected viral hemorrhagic fever (VHF)(1). Pending a comprehensive
review of the 1988 guidelines, this notice provides interim recommendations
that update the 1988 guidelines for health-care settings in the United States.
This update applies to four viruses that cause syndromes of VHF: Lassa, Marburg,
Ebola, and Congo-Crimean hemorrhagic fever viruses; although the risk and/or
mode of nosocomial transmission differs for each of these viruses, the limited
data do not permit clear distinctions.
Background
In Africa, transmission of VHF has
been associated with reuse of unsterile needles and syringes and with provision
of patient care without appropriate barrier precautions to prevent exposure
to virus-containing blood and other body fluids (including vomitus, urine,
and stool). The risks associated with various body fluids have not been well
defined as most caregivers who acquired infection had multiple contacts with
multiple fluids. Epidemiologic studies of VHF in humans indicate that infection
is not readily transmitted from person to person by the airborne route
(1,2). Airborne transmission involving humans
has never been documented and is considered a possibility only in rare instances
from persons with advanced stages of disease (e.g., one patient with Lassa
fever who had extensive pulmonary involvement may have transmitted infection
by the airborne route)(3)
. In contrast, investigation of VHF in nonhuman primates (i.e., monkeys)
has suggested possible airborne spread among these species
(4-7). Despite uncertainties regarding the applicability
to humans of data regarding airborne transmission in nonhuman primates, such
information must be considered in the development of infection-control precautions
because information regarding exposure and transmission in humans is limited.
The risk for person-to-person transmission
of hemorrhagic fever viruses is highest during the latter stages of illness,
which are characterized by vomiting, diarrhea, shock, and often hemorrhage.
VHF infection has not been reported in persons whose contact with an infected
patient occurred only during the incubation period (i.e., before the patient
became febrile; the incubation period ranges from 2 days to 3 weeks, depending
on the etiology of the VHF{1}). In the 1995 Zaire outbreak, some instances
of Ebola virus transmission within a few days after onset of fever were reported;
however, other symptoms in the source patients and the level of exposure
to body fluids among these secondary cases were unknown (CDC, unpublished
data, 1995). In studies involving three monkeys experimentally infected with
Ebola virus (Reston strain), fever and other systemic signs of illness preceded
detection of infectious virus in the pharynx by 2-4 days, in the nares by
5-10 days, in the conjunctivae by 5-6 days, and on anal swabs by 5-6 days
(P. Jahrling, U.S. Army Medical Research Institute of Infectious Diseases,
unpublished data, 1995).
All suspected cases of infection with Ebola
virus and other hemorrhagic fever viruses should be reported immediately
to local and state health departments and to CDC (telephone (404) 639-1511;
from 4:30 p.m. to 8 a.m., telephone (404) 639-2888). Specimens for virus-specific
diagnostic tests should be sent to CDC as rapidly as possible according to
instructions provided when contact is made. General information regarding
Ebola virus infection is available through the CDC Ebola Hotline (telephone
(800) 900-0681).
Recommendations
The following recommendations apply to
patients who, within 3 weeks before onset of fever, have either 1) traveled
in the specific local area of a country where VHF has recently occurred;
2) had direct contact with blood, other body fluids, secretions, or excretions
of a person or animal with VHF; or 3) worked in a laboratory or animal facility
that handles hemorrhagic fever viruses. The likelihood of acquiring VHF is
considered extremely low in persons who do not meet any of these criteria.
The cause of fever in persons who have traveled in areas where VHF is endemic
is more likely to be a different infectious disease (e.g., malaria or typhoid
fever); evaluation for and treatment of these other potentially serious infections
should not be delayed.
-
Because most ill persons undergoing prehospital evaluation and transport
are in the early stages of disease and would not be expected to have
symptoms that increase the likelihood of contact with infectious body
fluids (e.g., vomiting, diarrhea, or hemorrhage), universal precautions
are generally sufficient(8)
. If a patient has respiratory symptoms (e.g., cough or rhinitis), face
shields or surgical masks and eye protection (e.g., goggles or eyeglasses
with side shields) should be worn by caregivers to prevent droplet contact
(8). Blood, urine, feces, or vomitus, if
present, should be handled as described in the following recommendations
for hospitalized patients.
-
Patients in a hospital outpatient or inpatient setting should be placed
in a private room. A negative pressure room is not required during the
early stages of illness, but should be considered at the time of hospitalization
to avoid the need for subsequent transfer of the patient. Nonessential
staff and visitors should be restricted from entering the room. Caretakers
should use barrier precautions to prevent skin or mucous membrane exposure
to blood and other body fluids, secretions, and excretions. All persons
entering the patient’s room should wear gloves and gowns to prevent contact
with items or environmental surfaces that may be soiled. In addition,
face shields or surgical masks and eye protection (e.g., goggles or eyeglasses
with side shields) should be worn by persons coming within approximately
3 feet of the patient to prevent contact with blood, other body fluids,
secretions (including respiratory droplets), or excretions. The need
for additional barriers depends on the potential for fluid contact, as
determined by the procedure performed and the presence of clinical symptoms
that increase the likelihood of contact with body fluids from the patient
(8). For example, if copious amounts of blood,
other body fluids, vomit, or feces are present in the environment, leg
and shoe coverings also may be needed. Before entering the hallway, all
protective barriers should be removed and shoes that are soiled with
body fluids should be cleaned and disinfected as described below (see
recommendation 6). An anteroom for putting on and removing protective
barriers and for storing supplies would be useful, if available
(1).
-
For patients with suspected VHF who have a prominent cough, vomiting,
diarrhea, or hemorrhage, additional precautions are indicated to prevent
possible exposure to airborne particles that may contain virus. Patients
with these symptoms should be placed in a negative-pressure room
(9). Persons entering the room should wear
personal protective respirators as recommended for care of patients with
active tuberculosis (high efficiency particulate air {HEPA} respirators
or more protective respirators)(9)
.
-
Measures to prevent percutaneous injuries associated with the use and
disposal of needles and other sharp instruments should be undertaken
as outlined in recommendations for universal precautions
(8). If surgical or obstetric procedures
are necessary, the state health department and CDC’s National Center
for Infectious Diseases, Hospital Infections Program (telephone {404}
639-6425) and Division of Viral and Rickettsial Diseases (telephone {404}
639-1511; from 4:30 p.m. to 8 a.m., telephone {404} 639-2888) should
be consulted regarding appropriate precautions for these procedures.
-
Because of the potential risks associated with handling infectious materials,
laboratory testing should be the minimum necessary for diagnostic evaluation
and patient care. Clinical laboratory specimens should be obtained using
precautions outlined above (see recommendations 1-4 above), placed in
plastic bags that are sealed, then transported in clearly labeled, durable,
leakproof containers directly to the specimen handling area of the
laboratory. Care should be taken not to contaminate the external surfaces
of the container. Laboratory staff should be alerted to the nature of
the specimens, which should remain in the custody of a designated person
until testing is done. Specimens in clinical laboratories should be handled
in a class II biological safety cabinet following biosafety level 3 practices
(10). Serum used in laboratory tests should
be pretreated with polyethylene glycol p-tert-octylphenyl ether (Triton(R)
X-100)* ; treatment with 10 uL of 10% Triton(R) X-100 per 1 mL of serum
for 1 hour reduces the titer of hemorrhagic fever viruses in serum, although
100% efficacy in inactivating these viruses should not be assumed. Blood
smears (e.g., for malaria) are not infectious after fixation in solvents.
Routine procedures can be used for automated analyzers; analyzers should
be disinfected as recommended by the manufacturer or with a 500 parts
per million solution of sodium hypochlorite (1:100 dilution of household
bleach: 1/4 cup to 1 gallon water) after use. Virus isolation or cultivation
must be done at biosafety level 4(10). The CDC mobile isolation laboratory
is no longer available(1)
.
-
Environmental surfaces or inanimate objects contaminated with blood,
other body fluids, secretions, or excretions should be cleaned and disinfected
using standard procedures (8)
. Disinfection can be accomplished using a U.S. Environmental Protection
Agency (EPA)-registered hospital disinfectant or a 1:100 dilution of
household bleach.
-
Soiled linens should be placed in clearly labeled leak-proof bags at
the site of use and transported directly to the decontamination area.
Linens can be decontaminated in a gravity displacement autoclave or incinerated.
Alternatively, linens can be laundered using a normal hot water cycle
with bleach if universal precautions to prevent exposures are precisely
followed (8) and
linens are placed directly into washing machines without sorting.
-
There is no evidence for transmission of hemorrhagic fever viruses to
humans or animals through exposure to contaminated sewage; the risk of
such transmission would be expected to be extremely low with sewage treatment
procedures in use in the United States. As an added precaution, however,
measures should be taken to eliminate or reduce the infectivity of bulk
blood, suctioned fluids, secretions, and excretions before disposal.
These fluids should be either autoclaved, processed in a chemical toilet,
or treated with several ounces of household bleach for greater than or
equal to 5 minutes (e.g., in a bedpan or commode) before flushing or
disposal in a drain connected to a sanitary sewer. Care should be taken
to avoid splashing when disposing of these materials. Potentially infectious
solid medical waste (e.g., contaminated needles, syringes, and tubing)
should either be incinerated or be decontaminated by autoclaving or immersion
in a suitable chemical germicide (i.e., an EPA-registered hospital disinfectant
or a 1:100 dilution of household bleach), then handled according to existing
local and state regulations for waste management.
-
If the patient dies, handling of the body should be minimal. The corpse
should be wrapped in sealed leakproof material, not embalmed, and cremated
or buried promptly in a sealed casket. If an autopsy is necessary, the
state health department and CDC should be consulted regarding appropriate
precautions(1).
-
Persons with percutaneous or mucocutaneous exposures to blood, body fluids,
secretions, or excretions from a patient with suspected VHF should immediately
wash the affected skin surfaces with soap and water. Application of an
antiseptic solution or handwashing product may be considered also, although
the efficacy of this supplemental measure is unknown. Mucous membranes
(e.g., conjunctiva) should be irrigated with copious amounts of water
or eyewash solution. Exposed persons should receive medical evaluation
and follow-up management(1)
.
Source: Hospital Infections
Program, Div of Viral and Rickettsial Diseases, and Div of Quarantine, National
Center for Infectious Diseases; Office of the Director, National Institute
for Occupational Safety and Health; Office of Health and Safety, CDC.
References
-
CDC. Management of patients with suspected viral hemorrhagic fever.
MMWR 1988;37 (no. S-3):1-15.
-
Baron RC, McCormick JB, Zubeir OA. Ebola virus disease in southern Sudan:
hospital dissemination and intrafamilial spread. Bull WHO 1983;61:997-1003.
-
Carey DE, Kemp GE, White HA, et al. Lassa fever: epidemiological aspects
of the 1970 epidemic, Jos, Nigeria. Trans R Soc Trop Med Hyg 1972;66:402-8.
-
Dalgard DW, Hardy RJ, Pearson SL, et al. Combined simian hemorrhagic
fever and Ebola virus infection in cynomolgus monkeys. Lab Anim Sci
1992;42:152-7.
-
CDC. Update: filovirus infections among persons with occupational exposure
to nonhuman primates. MMWR 1990;39:266-7.
-
Johnson E, Jaax N, White, Jahrling P. Lethal experimental infection of
rhesus monkeys by aerosolized Ebola virus. Int J Exp Pathol (in
press).
-
Pokhodynev VA, Gonch ar NI, Pshenichnov VA. Experimental study of Marburg
virus contact transmission. Vopr Virusol 1991;36:506-8.
-
CDC. Guidelines for prevention of transmission of human immunodeficiency
virus and hepatitis B virus to health-care and public safety workers.
MMWR 1989;38:(no. S-6):1-37.
-
CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis
in health-care facilities. MMWR 1994;43(no. RR-13):33-34, 71-81.
-
CDC/National Institutes of Health. Biosafety in microbiological and biomedical
laboratories. 3rd ed. Atlanta, Georgia: US Department of Health and Human
Services, Public Health Service, 1993; DHHS publication no. (CDC)93-8395.
Source: Public Health Service of the U.S. Department of Health and Human
Services.
v
Hemorrhagic Fever Viruses as Biological Weapons
Introduction
Hemorrhagic fever viruses (HFVs) are the
subject of the sixth article in a series on medical and public health management
of civilian populations following use of biological weapons.1-5 Historically,
the term viral hemorrhagic fever (VHF) has referred to a clinical
illness associated with fever and a bleeding diathesis caused by a virus
belonging to 1 of 4 distinct families: Filoviridae, Arenaviridae, Bunyaviridae,
and Flaviviridae (Table 1).
The HFVs are transmitted to humans via
contact with infected animal reservoirs or arthropod vectors (the natural
reservoirs and vectors of the Ebola and Marburg viruses are unknown). The
mode of transmission, clinical course, and mortality of these illnesses vary
with the specific virus, but each is capable of causing a hemorrhagic fever
syndrome. Clinical and epidemiological data are limited; outbreaks are sporadic
and unanticipated, and there are few case series or clinical trials involving
human subjects.
The Working Group on Civilian Biodefense
previously established a list of key features that characterize biological
agents that pose particularly serious risks if used as biological weapons
against civilian populations: (1)
high morbidity and mortality; (2)
potential for person-to-person transmission;
(3) low infective dose and highly infectious
by aerosol dissemination, with a commensurate ability to cause large outbreaks;
(4) effective vaccine
unavailable or available only in limited supply;
(5) potential to cause public and health care
worker anxiety; (6)
availability of pathogen or toxin; (7)
feasibility of large-scale production; (8)
environmental stability; and (9)
prior research and development as a biological weapon. Some HFVs exhibit
a significant number of these key characteristics and pose serious risk as
biological weapons, including Ebola and Marburg viruses (Filoviridae), Lassa
fever and New World arenaviruses (Arenaviridae), Rift Valley fever (Bunyaviridae),
and yellow fever, Omsk hemorrhagic fever, and Kyasanur Forest disease (Flaviviridae).
Several viruses that can cause VHF
will not be considered further in this analysis. Dengue is excluded because
it is not transmissible by small-particle aerosol,
7 and primary dengue causes VHF only rarely.
Crimean-Congo hemorrhagic fever (CCHF) and the agents of hemorrhagic fever
with renal syndrome (HFRS) also have been excluded after much deliberation.
Although these pathogens can cause VHF and may be transmissible by small-particle
aerosol, the working group noted that technical difficulties (i.e., barriers
to large-scale production) currently preclude their development as mass casualty
weapons. Crimean-Congo hemorrhagic fever and the agents of HFRS do not readily
replicate to high concentrations in cell cultures, a prerequisite for weaponization
of an infectious organism. However, CCHF, the agents of HFRS, and dengue
may carry great morbidity and mortality in naturally occurring outbreaks.
In particular, CCHF may be transmitted from person to person, has a high
case-fatality rate, and is endemic in central Asia and southern Africa. We
acknowledge that technical difficulties may be overcome with advances in
technology and science, and these excluded viruses may become a greater threat
in the future. Other sources provide information on the viruses not addressed
herein.8-12
The consequences of an unannounced aerosol
attack with an HFV are the primary focus of this analysis. A variety of attack
scenarios with these agents are possible. This analysis does not attempt
to forecast the most likely but focuses on perhaps the most serious scenario.
Understanding and planning for a covert aerosol attack with HFVs will improve
preparedness for other scenarios as well.
|
Table 1. Hemorrhagic Fever Viruses |
|
Family Genus Virus
Disease Vector in
Geographic
Nature Distribution
|
|
Filoviridae Filovirus Ebola†
Ebola hemorrhagic Unknown Africa
fever
Marburg
Marburg Unknown
Africa
hemorrhagic fever
|
| Arenaviridae
Arena Lassa
Lassa fever Rodent
West Africa
New World
New World
Rodent Americas
Arenaviridae‡
hemorrhagic fever
Bunyaviridae Nairovirus Crimean-Congo Crimean-Congo
Tick Africa, Central
hemorrhagic fever
hemorragic fever
Asia, Eastern
Europe, Middle
East
Phlebovirus Rift Valley fever Rift
valley fever Mosquito Africa, Saudi
Arabia, Yemen
Hantavirus Agents of Hemorrhagic
fever Rodent Asia, Balkans,
hemorrhagic fever
Europe,
with renal syndrome
Eurasias§
|
|
Flaviviridae Flavivirus Dengue Dengue fever, Dengue
Mosquito Asia, Africa,
hemorrhagic fever, and
Pacific,
Dengue shock syndrome
Americas
Yellow fever Yellow
fever Mosquito Africa, tropical
Americas
Omsk
hemorrhagic Omsk hemorrhagic
Tick Central asia
fever
fever
Kyasanur
Forest Kyasanur Forest
Tick India
disease disease
|
|
* Bold indicates hemorrhagic fever viruses that pose serious risk as biological
weapons (addressed in this consensus statement).
† There are 4 subtypes of Ebola: Zaire, Sudan, Ivory Coast, and Reston
‡ The New World Arenaviridae include Machupo, the cause of Bolivian hemorrhagic
fever: Junin; the cause of Argentine hemorrhagic fever; Guanarito, the
cause of Venezuelan hemorrhagic fever; and Sabia, the cause of Brazilian
hemorrhagic fever. An additional renavirus has been isolated following 3
fatal cases of hemorrhagic fever in California, 1999-2000.
§ Additionally, the agents of
hantavirus pulmonary syndrome have been isolated in North America.
|
Consensus Methods
The working group for this article was
composed of 26 professionals from academic medical centers, public health,
military services, governmental agencies, and emergency management institutions.
Medline databases were searched from January 1966 to January 2002 for the
Medical Subject Headings viral hemorrhagic fever, Ebola, Marburg, Lassa,
arenavirus, Junin, Guanarito, Machupo, Sabia, CCHF, Rift Valley fever, hantavirus,
dengue, yellow fever, Omsk hemorrhagic fever, Kyasanur Forest disease, biological
weapons, biological terrorism, biological warfare, and biowarfare
. The references were reviewed and relevant materials published prior to
1966 were identified. The working group also identified other published and
unpublished references for review.
A first draft resulted from the synthesis
of information obtained during the evidence-gathering process. Members of
the working group were convened to discuss the first draft of the formulated
guidelines on January 10, 2002. Subsequently, a second draft was produced
incorporating comments and judgments of the working group. They reviewed
the second draft and submitted comments, which were incorporated into a third
and final draft of the document.
History and Potential as Biological Weapons
Hemorrhagic fever viruses have been
weaponized by the former Soviet Union, Russia, and the United States.
13-15 There are reports that yellow fever may
have been weaponized by North Korea.14
The former Soviet Union and Russia produced large quantities of Marburg,
Ebola, Lassa, and New World arenaviruses (specifically, Junin and Machupo)
until 1992.13, 15
Soviet Union researchers quantified the aerosol infectivity of Marburg virus
for monkeys, determining that no more than a few virions are required to
cause infection.16
Yellow fever and Rift Valley fever viruses were developed as weapons by
the US offensive biological weapons program prior to its termination in 1969.
14 The Japanese terrorist cult Aum Shinrikyo
unsuccessfully attempted to obtain Ebola virus as part of an effort to create
biological weapons.17
Several studies have demonstrated
successful infection of nonhuman primates by aerosol preparations of Ebola,
18 Marburg,19
Lassa,20 and New
World arenaviruses.21
Arguments asserting that the absence of effective antiviral therapy and
vaccines would make these viruses too dangerous to develop as weapons are
not supported by the historical record.
In 1999, the Centers for Disease
Control and Prevention (CDC) classified the HFVs as category A bioweapon
agents, based on the potential to cause widespread illness and death, ease
of dissemination or person-to-person transmission, potential for major public
health impact, and requirement of special action for public health preparedness.
22
Epidemiology of Disease Transmission
In
nature, HFVs reside in animal hosts or arthropod vectors. The natural reservoir
of filoviruses is unknown. Humans are infected incidentally, acquiring the
disease by the bite of an infected arthropod, via aerosol generated from
infected rodent excreta, or by direct contact with infected animal carcasses.
23 With the exception of Rift Valley fever and
the diseases caused by flaviviruses (yellow fever, Omsk hemorrhagic fever,
and Kyasanur Forest disease), which are not transmissible from person to
person, infected humans can spread the disease to close contacts, which may
result in community outbreaks and nosocomial infections. Limited knowledge
exists about transmission because outbreaks of these diseases are sporadic
and unpredicted and often occur in areas without adequate medical and public
health infrastructure. Outbreaks are usually well under way or have subsided
by the time data gathering begins. The risks associated with various modes
of transmission are not well defined because most persons who acquire these
infections have a history of multiple contacts by multiple modes. Infections
acquired percutaneously are associated with the shortest incubation period
and highest mortality. Person-to-person airborne transmission appears to
be rare but cannot be ruled out.
Filoviridae: Ebola and Marburg
Since 1967, when the first outbreak
of VHF caused by Marburg virus occurred in Germany and Yugoslavia, there
have been 18 reports of human outbreaks of VHF secondary to Ebola or Marburg
viruses, resulting in approximately 1500 cases to date
.24 Most have occurred in Africa. Epidemiological
investigation indicates that most cases occurred after direct contact with
blood, secretions, or tissues of infected patients or nonhuman primates.
Several cases have followed needlestick
injuries. During the 1976 Ebola epidemic in Zaire (now Democratic Republic
of the Congo),85 (26.7%) of 318 cases occurred in individuals who had received
an injection, and every case of disease acquired by contaminated syringes
resulted in death.25
Mortality was substantially higher when the disease was acquired percutaneously.
Evidence suggests that percutaneous exposure to very low inocula can result
in infection.26
Filoviruses can also be transmitted
by mucosal exposure. Experiments in nonhuman primates have documented transmission
of infection after direct administration of Marburg virus into the mouths
and noses of experimental animals27
and after direct administration of Ebola virus into the mouths or conjunctiva
28 of experimental animals. Human infections
might occur through contact of contaminated fingers with oral mucosa or conjunctiva,
29 but direct evidence is lacking.
Copious numbers of Ebola viral particles
found in human skin and lumina of sweat glands have raised concern that disease
transmission may occur from touching an infected patient or corpse.
30 In the 1995 Ebola outbreak in Kikwit, Democratic
Republic of the Congo, several persons preparing bodies for burial acquired
the infection.31-33
According to local custom, burial practices may involve washing the body
and cutting the hair and nails of the corpse.34
However, a study using guinea pigs was unable to document Marburg virus transmission
through intact skin, while infection through skin lesions did occur.
35
A few cases of disease transmission
by uncertain mechanisms described in two recent Ebola outbreaks,
36-37 and findings from animal studies
16, 18, 38 and one outbreak of Ebola in nonhuman
primates,39 raise
concern about the potential for person-to-person transmission by way of small-droplet
airborne nuclei. However, to date, Ebola epidemics in Africa were ultimately
controlled and ended without use of specific airborne precautions. (HICPAC’s
definitions of standard, contact, droplet, and airborne precautions are at
http://www.cdc.gov/ncidod/hip/isolat/isopart2.htm.)
Airborne transmission of Marburg
virus was not observed in the 1967 outbreak in Germany and Yugoslavia following
the importation of infected African green monkeys from eastern Africa.
40 In 1975, only 1 of 35 health care workers
who cared for 2 patients with Marburg disease in South Africa without any
barrier precautions became ill.41
In 1979, an outbreak of Ebola in southern Sudan infected 34 people. Although
direct physical contact could not be established in 2 instances,
29 cases resulted from direct physical contact
with an infected person and there were no cases of illness among 103 persons
who were exposed to cases in confined spaces without any physical contact.
42 In 1994, only 1 of 70 contacts of a patient
with Ebola acquired the disease despite lack of airborne precautions.
43 In 1996, none of the 300 contacts of 2 patients
with Ebola acquired the disease44
despite involvement in numerous hazardous procedures prior to the patients’
diagnosis, protected only by standard blood and bodily fluid precautions.
In 1995, 316 people became ill with
Ebola in the Democratic Republic of the Congo; 25% of the cases involved
health care workers. When barrier precautions were instituted, only 3 health
care workers became infected. One was nonadherent to barrier precautions,
the second had a needlestick injury, and it is speculated that the third,
who always used protective equipment, became infected after touching her
eyes with a contaminated glove.45
None of the 78 household members who did not have direct physical contact
with an infected person developed disease.31 However, in this outbreak, the
only risk factor identified for 5 patients was visiting an infected patient
in the absence of physical contact. These few cases led researchers to conclude
that airborne transmission could not be ruled out
37 but seemed to be, at most, a minor mode of
transmission.
In 2000, 224 people died in Uganda
during an Ebola outbreak.37
Fourteen (64%) of 22 medical personnel were infected after institution of
isolation wards and infection control measures37 including donning gowns,
gloves, and shoe covers, standard surgical masks, and either goggles or eye
glasses.46 It is not
clear whether lack of adherence to guidelines contributed to nosocomial cases
in this outbreak, but airborne transmission could not be ruled out.
Although Marburg virus has been isolated
from healthy-appearing infected monkeys several days before clinical signs
appear,27 no transmission
has been observed in this stage.40
In humans, transmission of Ebola during the incubation period does not appear
to be common.31 Transmissibility
of Ebola increases with the duration of disease, and direct physical contact
with an ill person during the late phase of clinical illness confers an additional
risk.31 There has
been only one reported case, during the outbreak in Zaire in 1976, in which
the only possible source of infection was contact with an unconfirmed case
hours before the patient developed symptoms.25
The preponderance of evidence suggests that transmission of Ebola and Marburg
virus rarely, if ever, occurs before the onset of signs and symptoms.
In several studies after the 1995
Kikwit outbreak, Ebola was detected in the seminal fluid of convalescing
patients by reverse transcriptase polymerase chain reaction (RT-PCR) up to
101 days after disease onset,47-48
and virus was isolated 82 days after disease onset in the seminal fluid
of 1 patient.48 Marburg
has been isolated 83 days after disease onset from the seminal fluid of a
patient who may have sexually transmitted the disease to his spouse.
40
Arenaviridae: Lassa Fever and
New World Arenaviruses
In nature, arenaviruses are transmitted
to humans via inhalation of aerosols present in rodent urine and feces,
49 by ingestion of food contaminated with rodent
excreta, or by direct contact of rodent excreta with abraded skin and mucous
membranes.50 Like
filoviruses, person-to-person transmission of the arenaviruses occurs predominantly
by direct contact with infectious blood and bodily fluids. A number of nosocomial
outbreaks of Lassa fever51-53
and of New World arenaviruses 54
have occurred via this mechanism. As with filoviruses, person-to-person
airborne transmission has been suspected in a few instances.
In 1969, during a nosocomial outbreak
in Nigeria, an index patient with severe pulmonary involvement caused 16
secondary cases in persons who shared the same hospital ward with her. Airborne
transmission was believed to have contributed to this outbreak, as there
were no tertiary cases of Lassa fever in the hospital, despite the admission
of Lassa fever–infected patients to other hospital wards.
51 However, there is no definitive evidence of
airborne transmission and the exact mechanisms of disease transmission during
that outbreak remain unknown. Conversely, in the case of 1 Lassa fever–infected
individual who traveled from Sierra Leone to the United States, no cases
were detected in 522 contacts, even prior to initiating additional barrier
precautions beyond standard precautions.55
In another instance, in which an infected individual originated in Nigeria
and traveled to St Thomas in the US Virgin Islands, none of the 159 people
who had direct contact with the patient developed clinical or serological
evidence of infection, even though they attended to the patient, without
barrier precautions, during a 5-day period before the diagnosis.
56
Airborne transmission of Bolivian
hemorrhagic fever has been implicated after a student became infected after
watching a nursing instructor demonstrate the changing of bed linens of an
infected patient, although the student did not touch the patient or any objects
in the room and kept a distance of greater than 6 ft from the patient.
54 Conversely, approximately 80 involved health
care workers who did not use airborne precautions remained healthy. Definitive
evidence of person-to-person airborne transmission is lacking but, in these
rare instances, there have been no plausible alternative explanations. There
have been no reports documenting transmission of arenaviruses by infected
persons during the incubation period.54, 57
However, Lassa fever virus can be detected in semen up to 3 months after
acute infection58 and
in urine 32 days after disease onset,59
and Argentine hemorrhagic fever has been transmitted to spouses of convalescent
patients 7 to 22 days after onset of illness.60
Bunyaviridae: Rift Valley Fever
Humans acquire Rift Valley fever
from the bite of an infected mosquito, direct contact with infected animal
tissues, or aerosolization of virus from infected animal carcasses.
61 Ingestion of contaminated raw animal milk
has been implicated epidemiologically.62
Despite high levels of viremia and isolation of low titers of virus from
throat washings, there are no reported cases of person-to-person transmission
of Rift Valley fever.62
However, laboratory technicians are at risk of acquiring the disease by
inhalation of infectious aerosols generated from specimens.
61, 63
If Rift Valley fever were used as
a biological weapon, susceptible domestic livestock (sheep, cattle, buffalo,
and goats) could also be infected. Infected livestock develop high levels
of viremia, sufficient to infect susceptible mosquito vectors and lead to
establishment of the disease in the environment61 and large epizootic epidemics,
as occurred in Egypt in 197764
and the Arabian peninsula in 2000.65
Several genera of mosquitoes (e.g., Aedes, Anopheles, and Culex) in the
United States have the capacity to act as vectors of Rift Valley fever.
66-67
Flaviviridae: Yellow Fever,
Omsk Hemorrhagic Fever, and Kyasanur Forest Disease
Humans acquire yellow fever virus
from the bite of an infected mosquito68
and acquire Omsk hemorrhagic fever and Kyasanur Forest disease viruses from
the bite of an infected tick.69
There are no reported cases of person-to-person transmission or nosocomial
spread of flaviviruses. Infection of laboratory personnel via inhalation
of aerosols during cultivation of these viruses has been reported.
69-70 As with Rift Valley fever, there is a theoretical
risk of flaviviruses becoming established in an environment following infection
of susceptible arthropod vectors.
Microbiology and Pathogenesis
All of the HFVs are small RNA viruses with
lipid envelopes. Specific microbiological characteristics of these viruses
are listed in Table 2.
Information regarding the pathogenesis
of these agents following infection in humans is incomplete. Most data have
been derived from clinical observations and experimentally induced disease
in nonhuman primates. Interpretation of data derived from animal studies
may be confounded by a series of factors, such as the species of the animal,
the route of inoculation, and the virus dose.40
All of the viruses of concern may
lead to thrombocytopenia, and data suggest that platelet dysfunction is present
in Ebola, Lassa fever, and Argentine hemorrhagic fever.
72 Reduced levels of coagulation factors may
be secondary to hepatic dysfunction and/or disseminated intravascular coagulation
and are most prominent in Rift Valley fever and yellow fever.
72 In addition, Ebola and Marburg viruses may
lead to a hemorrhagic diathesis through direct damage of cells involved in
hemostasis (such as platelets and endothelial cells) and/or indirectly through
immunological and inflammatory pathways.72
Filoviruses are extremely virulent
in nonhuman primates and humans.73
Necrosis of visceral organs (such as liver, spleen, and kidneys) has been
associated with both direct viral-induced cellular damage and impairment
of the microcirculation. Filoviruses are cytotoxic to cells. In general,
inflammatory infiltration is absent in the affected visceral organs.
74 Even when viral titers in the lungs of monkeys
are elevated, the virus is not apparent in the alveoli or airways, occurring
primarily in the vascular structures.28
All experimentally infected monkeys develop disseminated intravascular coagulation.
Ebola, but not Marburg virus, makes a secreted form of its glycoprotein that
has been suggested to have a role in virulence.
73 Endothelial cells support Marburg virus replication,
and their destruction may contribute to the associated hemorrhagic diathesis
and shock.75
Infection with arenaviruses is initiated
in nasopharyngeal mucosa.76
Arenaviruses produce carrier states in rodents, their natural hosts, and
viral multiplication is not associated with extensive cell damage. In vitro
infections with Arenaviridae show that virus spreads throughout a variety
of different cellular monolayers, with little or absent cytopathic effects
77; hence, it is believed that these viruses
may exert their effects (at least in part) by inducing the secretion of inflammatory
mediators from macrophages. Following experimental infection of nonhuman
primates with arenaviruses, virtually all tissues become infected, with little
histologic evidence of damage.78
Hemorrhage following arenavirus infection appears to be associated with
the presence of a circulating inhibitor of platelet aggregation and thrombocytopenia.
However, disseminated intravascular coagulation does not appear to be a central
pathogenic mechanism.79
Lassa fever appears to be terminated by a cellular, not humoral, immune response,
77 whereas in New World arenaviruses, recovery
is preceded by cellular and humoral immune responses.
80
In contrast with arenaviruses, Rift
Valley fever virus leads to destruction of infected cells.
77 The hemostatic derangements in Rift Valley
fever are poorly understood, and a combination of vasculitis and hepatic
necrosis has been postulated.81-82
Interferon alfa given shortly before or after experimental infection with
Rift Valley fever virus has been shown to protect rhesus monkeys from viremia
and hepatocellular damage.83
Clinical recovery is associated with appearance of neutralizing antibodies,
and passive immunization prevented development of viremia in nonhuman primates
inoculated with the virus.83
Like Rift Valley fever, yellow fever
virus leads to destruction of infected cells. Hepatocyte infection and degeneration
is a late event in the course of infection,84
associated with virtually no inflammation.68
Neutralizing antibodies correlate with clearance of viremia, and paradoxically,
with the second phase of illness, when patients may develop hemorrhage and
shock.68
Little is known about the pathogenesis
of Omsk hemorrhagic fever and Kyasanur Forest disease viruses. Findings from
postmortem examinations of 3 individuals who died of Kyasanur Forest disease
showed degeneration of the larger visceral organs (especially liver and spleen)
and hemorrhagic pneumonia.85
Clinical Manifestations
Information on the clinical manifestations
of these diseases is derived from naturally occurring outbreaks. Although
data derived from experimentally infected animals do not support marked differences
in the clinical presentation according to route of exposure (parenteral vs
aerosol),18, 21 it
is not possible to be certain that the same manifestations would follow bioweapons
attacks on humans.
There are a variety of potential clinical
manifestations following infection with these viruses, and not all patients
develop the classic VHF syndrome. Clinical manifestations are nonspecific
and may include fever, myalgias, rash, and encephalitis. The propensity to
cause the classic VHF syndrome also differs among agents. Therefore, in the
event of a bioterrorist attack with one of these agents, infected patients
may have a variety of clinical presentations, complicating early detection
and management. It may not be possible to differentiate among these diseases
on clinical grounds alone, although a number of specific clinical features
may be useful clues to diagnosis (Table 3).
The overall incubation period for
HFVs ranges from 2 to 21 days. Patients initially exhibit a nonspecific prodrome,
which typically lasts less than 1 week. Symptoms typically include high fever,
headache, malaise, arthralgias, myalgias, nausea, abdominal pain, and nonbloody
diarrhea. Filoviruses, Rift Valley fever, and flaviviruses are characterized
by an abrupt onset, while arenaviruses have a more insidious onset.
40, 54, 61, 68-69,99-100
Early signs typically include fever, hypotension,
relative bradycardia, tachypnea, conjunctivitis, and pharyngitis. Most diseases
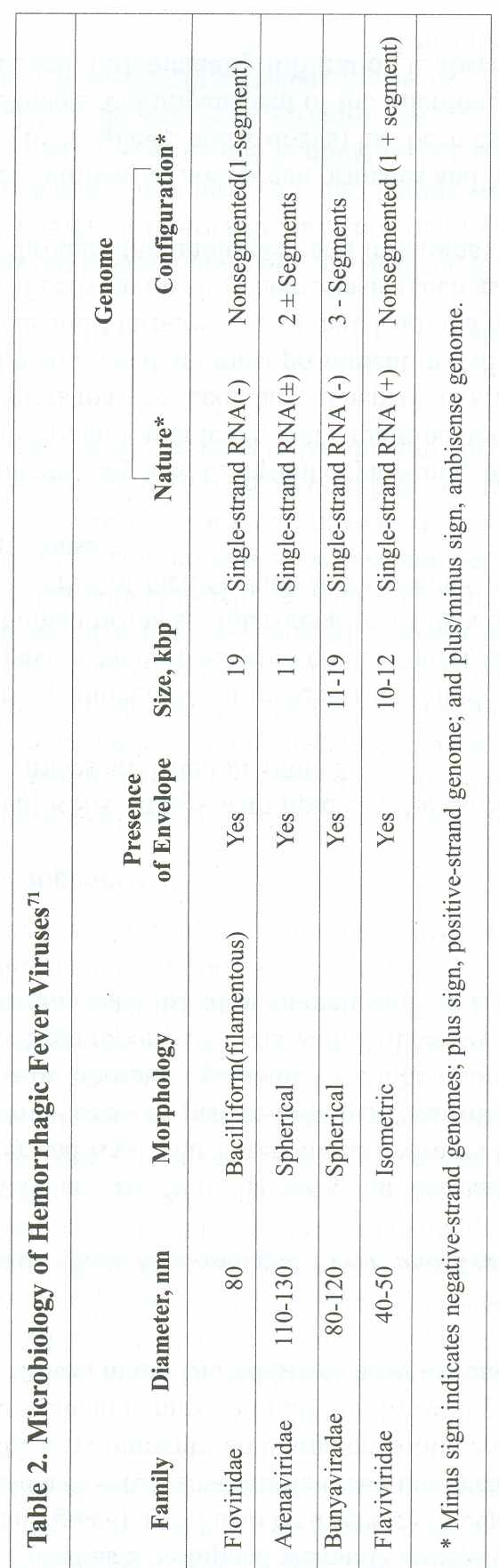
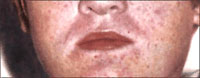
are associated with cutaneous flushing or a skin rash (Figure 1 and Figure
2), but the specific characteristics of the rash vary with each disease (Table
3). Later, patients may show signs of progressive hemorrhagic diathesis,
such as petechiae, mucous membrane and conjunctival hemorrhage (Figure 3);
hematuria; hematemesis; and melena. Disseminated intravascular coagulation
and circulatory shock may ensue. Central nervous system dysfunction may be
present and manifested by delirium, convulsions, cerebellar signs, or coma
and imparts a poor prognosis.
|
Figure 1
. Maculopapular Rash in Marburg Disease
A nonpruritic maculopapular rash (resembling the
rash of measles) may occur in up to 50% of patients infected with
the Ebola or Marburg viruses within the first week of illness. The
rash is more common in light-colored skin and desquamates on resolution.
Reprinted with permission from Thieme (Martini GA, Knauff HG, Schmidt
HA, et al. A hitherto unknown infectious disease contracted from
monkeys. Ger Med Mon. 1968;13:457-470).
|
|
Table 3. Clinical Characteristics of Hemorrhagic Fever Viruses Noted
in Past Case Outbreaks |
|
Virus
Distinctive Clinical Features
Person-to-Person
Incubation Mortality
Transmission Period, d
|
|
Ebola 25, 42,
44-47, 85-99
High fever and severe prostration. A
Yes 2-21 50-90
diffuse maculopapular
rash may occur
by day 5 of ilness.
Bleeding and
disseminated intravascular
coagulation are
common.
|
|
Marburg40, 41,
87-102
High fever, myalgias. Nonpruritic
Yes 2-14
28-70
maculopapular
rash of the face, neck,
trunk, and arms
may develop.
Bleeding and
disseminated intravascular
coagulation are
common.
|
|
Lassa fevers 52, 66-91,
Gradual
onsets of fever, nausea,
Yes 5-16
15-20
100, 101, 110
abdominal pain, severe sore throat,
cough, conjunctivitis
ulceration of
buccal mucosa,
exudative pharyngitis,
and cervical
lymphadenopathy. Late signs
include severe
swelling of head and neck;
pleural and
pericardial effusions.
Hemorrhagic
complications less common.
|
|
New World Gradual
onsets of fever, myalgias, nausea, No
7-14 15-30
Arenaviruses
54,92,126
abdominal pain, conjunctivitis, flushing of
and trunk,
and generalized lymphadenopathy.
May develop
petechiae, bleeding, and central
nervous system
dysfunction (tremors of the
tongue and
upper extremities, myoclonic
movements,
dysarthria, and generalized
seizures).
|
|
Rift Valley
Fever, headache, retro-orbital pain,
No 2-6
<1
fever6l,
11-96
photophobia, and jaundice. Less than 1%
develops
hemorrhagic fever or encephalitis.
Retinitis
affects approximately 10% which
may occur
at time of acute febrile illness or
up to 4 weeks
later.
|
|
Yellow fever
66,97
Fever, myalgias, facial flushing, and
No 3-6
20
conjunctival
injection. Patients either recover
or enter
a short remission followed by fever,
relative
bradycardia, jaundice, renal failure,
and hemorrhagic
complications.
|
|
Omsk hemorrhagic
Fever, cough, conjunctivitis, papulov-
No 2-9
0.5-10
fever69+
esicular eruption
on the soft palate, marked
hyperemia
of the face and trunk (but no rash),
generalized
lymphadenopathy, and
splenomegaly.
Some patients may develop
pneumonia
and central nervous system
dysfunction.
|
|
Kyasanur Forest
Similar to Omsk but biphasic illness: first
No 2-9
3-10
of diseases
59,98
phase lasts 6-11 days and is followed
by
an
afebrile afebrile period of 9-21 days.
Up
to 50% of patients relapse and develop
meningoencephalitis.
|
|
*Reported Ebola data are for Sudan (50%) and Zaire
(90%) subtypes. The Ivory Coast subtype has an
indeterminate case-fatality rate, as there has
been a single no The Reston subtype causes subclinical
infection in humans.
+Mortality
ranges from 23% in the 1967 outbreak in Germany
to 70% in the largest outbreak of 1999 in the Democratic
Republican of the Congo.
� Also
Sergery Netesov, MID, written communication, February
27, 2002.
|
|
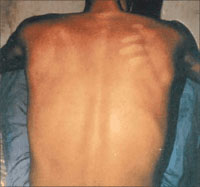
Figure 2
. Erythematous Rash in Bolivian Hemorrhagic Fever
This macular, flushed, erythematous rash that blanches
with pressure may be associated with infections caused by arenaviruses.
The rash most commonly involves the face and thorax and may desquamate
on convalescence. Reprinted with permission from Current Science/Current
Medicine (Peters CJ, Zaki SR, Rollin PE. Viral hemorrhagic fevers.
In: Fekety R, vol ed. Atlas of Infectious Diseases, Volume VIII.
Philadelphia, Pa: Churchill Livingstone; 1997:10.1-10.26).
|
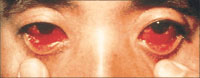
|
Figure 3
. Ocular Manifestations in Bolivian Hemorrhagic Fever
Ocular manifestations associated with hemorrhagic
fever viruses range from conjunctival injection to subconjunctival
hemorrhage, as seen in this patient. Reprinted with permission from
Current Science/Current Medicine (Peters CJ, Zaki SR, Rollin PE.
Viral hemorrhagic fevers. In: Fekety R, vol ed. Atlas of Infectious
Diseases, Volume VIII. Philadelphia, Pa: Churchill, Livingstone;
1997:10.1-10.26).
|
The differential diagnosis includes a variety
of viral and bacterial diseases: influenza, viral hepatitis, staphylococcal
or gram-negative sepsis, toxic shock syndrome, meningococcemia, salmonellosis
and shigellosis, rickettsial diseases (such as Rocky Mountain spotted fever),
leptospirosis, borreliosis, psittacosis, dengue, hantavirus pulmonary syndrome,
malaria, trypanosomiasis, septicemic plague, rubella, measles, and hemorrhagic
smallpox. Noninfectious processes associated with bleeding diathesis that
should be included in the differential diagnosis include idiopathic or thrombotic
thrombocytopenic purpura, hemolytic uremic syndrome, acute leukemia, and collagen-vascular
diseases.
Laboratory abnormalities include
leukopenia (except in some cases of Lassa fever, in which leukocytosis occurs),
anemia or hemoconcentration, thrombocytopenia, and elevated liver enzymes.
Jaundice is typical in Rift Valley fever and yellow fever.
61, 68 In addition, coagulation abnormalities
may include prolonged bleeding time, prothrombin time, and activated partial
thromboplastin time; elevated fibrin degradation products; and decreased
fibrinogen. Urinalysis may reveal proteinuria and hematuria, and patients
may develop oliguria and azotemia.26, 40, 54,
61, 68, 100-101
Convalescence may be prolonged and
complicated by weakness, fatigue, anorexia, cachexia, alopecia, and arthralgias.
43, 45 Reported clinical sequelae include hearing
or vision loss, impaired motor coordination, transverse myelitis, uveitis,
pericarditis, orchitis, parotitis, and pancreatitis.
36, 40, 52, 54, 61, 102
The case-fatality rate varies markedly
among these agents, ranging from as low as 0.5% for Omsk hemorrhagic fever
69 to as high as 90% for Ebola (subtype Zaire).
33 Death is typically preceded by hemorrhagic
diathesis, shock, and multiorgan system failure 1 to 2 weeks following onset
of symptoms.
Diagnosis
A high index of suspicion will be required
to diagnose VHF among persons exposed to a covert bioterrorist attack. In
naturally occurring cases, patients are likely to have risk factors such
as travel to Africa or Asia, handling of animal carcasses, contact with sick
animals or people, or arthropod bites within 21 days of onset of symptoms.
No such risk factors would be associated with a bioterrorist attack. The
variable clinical presentation of these diseases presents a major diagnostic
challenge. Clinical microbiology and public health laboratories are not currently
equipped to make a rapid diagnosis of any of these viruses, and clinical
specimens would need to be sent to the CDC or the US Army Medical Research
Institute of Infectious Diseases (USAMRIID; Frederick, Md), the only 2 level
D laboratories in the Laboratory Response Network. There are future plans
to decentralize the process required for the laboratory confirmation of these
viruses by equipping selected US public health laboratories in the Laboratory
Response Network with standard diagnostic reagents. This would likely expedite
laboratory confirmation of suspected cases in the event of an outbreak (Michael
Ascher, MD, written communication, February 26, 2002).
All suspected cases of HFV disease
should be immediately reported to local and/or state health departments (BOX
1), who would then notify the CDC. The World Health Organization has developed
surveillance standards for acute VHF syndrome with the aim of early detection
of naturally occurring outbreaks and notification of cases, even before identification
of the causal agent.103
This includes prompt reporting to public health authorities of any patient
with acute onset of fever of less than 3 weeks’ duration who is severely
ill, has no known predisposing host factors for hemorrhagic manifestations,
and has any 2 of the following: hemorrhagic or purpuric rash, epistaxis,
hematemesis, hemoptysis, blood in stool, or other hemorrhagic symptom. This
broad definition may be useful in the early period following a confirmed
bioterrorist-related case of VHF as well. Public health authorities may develop
more specific case definitions after the etiologic agent is identified.
Public health authorities, in consultation
with the CDC, should provide assistance and detailed instructions to clinical
laboratories and to clinicians for processing and transport of laboratory
specimens required for diagnosis of these agents. (See "Packaging Protocols
for Biological Agents/Diseases" at http://www.bt.cdc.gov/Agent/VHF/VHF.asp.)
|
Box 1. Key Medical and Public Health Interventions After Identification
of Suspected Index Case of VHF
Identification
Identify suspected index case using
these clinical criteria:* temperature 101°F (38.3°C) of <3 weeks’
duration; severe illness, and no predisposing factors for hemorrhagic
manifestations; and at least 2 of the following hemorrhagic symptoms:
hemorrhagic or purple rash, epistaxis, hematemesis, hemoptysis, blood
in stools, other, and no established alternative diagnosis.
Reporting
-
Report immediately to local and/or state health department.
-
Report immediately to infection control professional and laboratory
personnel.
Treatment
-
Initiate supportive and ribavirin therapy (see Table 4) immediately
while diagnostic confirmation is pending.
-
If infection with arenavirus or bunyavirus is confirmed, continue
10-day course of ribavirin.
-
If infection with filovirus or flavivirus is confirmed, or if
the diagnosis of VHF is excluded or an alternative diagnosis
is established, discontinue ribavirin.
Infection Control Measures
-
Initiate VHF-specific
barrier precautions.
-
Initiate airborne
precautions, with negative-pressure rooms if resources are
available.
Public Health Measures
-
Confirm or exclude
diagnosis via laboratory Response Network.
-
Designated public
health authority begins epidemiologic invetigation.
-
Identify close
and high-risk contacts and place under medical surveillance for
21 days from day of suspected/known exposure.
-
If contact does
not have temperature 101°F (38.3°C) or signs or symptoms of VHF
by the end of 21 days, discontinue medical surveillance.
-
If contact has
temperature 101°F (38.3°C) or signs or symptoms consistent with
VHF, initiate diagnostic workup and treatment, infection control,
and public health interventions described for index case.
*Criteria are adapted from World Health Organization's surveillance
standards for hemorrhagic fever syndroms.
103
|
Methods of diagnosis at specialized laboratories
include antigen detection by antigen-capture enzyme-linked immunosorbent
assay (ELISA), IgM antibody detection by antibody-capture ELISA, RT-PCR,
and viral isolation. Antigen detection (by ELISA) and RT-PCR are the most
useful diagnostic techniques in the acute clinical setting. Viral isolation
is of limited value because it requires a biosafety level 4 (BSL-4) laboratory.
(A full description of BSL-4 criteria is available at http://www.cdc.gov/od/ohs/biosfty/bmbl4/bmbl4s3.htm.)
There are only 2 BSL-4 facilities in the United States, located at the CDC
and the USAMRIID, with in-depth diagnostic capability. Either the presence
of IgM or a 4-fold rise in titer of IgG antibody between acute- and convalescent-phase
serum samples are diagnostic of these viral illnesses, but antibody-capture
ELISA is of limited value in early diagnosis because antibodies to these
viruses usually do not appear until onset of recovery, approximately at the
second week of illness. The CDC requires approximately 1 working day (with
prior notification of arrival) to offer a preliminary laboratory diagnosis
following receipt of patient specimens.
The diagnosis of VHF should be based initially
on clinical criteria and judgment, with laboratory testing used to confirm
or exclude this clinical diagnosis. Laboratory testing will require time
and, in the event of a large attack, may be delayed or perhaps not possible
given current laboratory capacities.
Treatment
The mainstay of treatment of VHF
is supportive, with careful maintenance of fluid and electrolyte balance,
circulatory volume, and blood pressure. Because in some cases intravenous
fluids have not reversed hypotension and may have contributed to pulmonary
edema,104 consideration
should be given to early vasopressor support with hemodynamic monitoring.
Mechanical ventilation, renal dialysis, and antiseizure therapy may be required.
Intramuscular injections, aspirin, nonsteroidal anti-inflammatory drugs,
and anticoagulant therapies are contraindicated. Steroids are not indicated.
9
Drug Therapy
There are no antiviral drugs approved
by the US Food and Drug Administration (FDA) for treatment of HFVs. Ribavirin,
a nucleoside analog, has some in vitro and in vivo activity against Arenaviridae
and Bunyaviridae (including CCHF) but no utility against Filoviridae or Flaviviridae.
Oral ribavirin, in combination with interferon alfa, is FDA-approved for treatment
of chronic hepatitis C virus infection. Intravenous ribavirin is of limited
availability in the United States. It is produced by ICN Pharmaceuticals Inc
(Costa Mesa, Calif) for compassionate use under an investigational new drug
(IND) application. Although a risk of human teratogenicity has not been demonstrated
for ribavirin, its pharmacologic action and its teratogenicity and embryolethality
in several animal species raise concern that such a risk may exist with maternal
therapy during pregnancy. Therefore, ribavirin is classified as a pregnancy
category X drug, and is contraindicated in pregnancy.
105 The primary adverse effect caused by ribavirin
is a dose-related, reversible, hemolytic anemia. However, a range of cardiac
and pulmonary events associated with anemia occurred in approximately 10%
of patients treated with combination ribavirin-interferon therapy for hepatitis
C.105
Small trials have shown that ribavirin
may reduce mortality after infection with Lassa fever
106 and select New World arenaviruses.
57, 107 Ribavirin does not penetrate the brain
well; therefore, it is not expected to be particularly effective against
the neurological effects of these pathogens.57,
108 Intravenous ribavirin given within the first
6 days of fever to patients with Lassa fever who had high levels of viremia
decreased mortality from 76% to 9%.107
A controlled trial of 18 patients with Argentine hemorrhagic fever resulted
in 12.5% mortality in treated patients compared with 40% in untreated patients.
108
Recommendations for drug therapy by the
working group are not approved by the FDA for any of these indications and
should always be administered under an IND protocol. In a mass casualty situation,
these requirements may need to be modified to permit timely administration
of the drug. In addition, treatment of other suspected possible causes, such
as bacterial sepsis, should not be withheld while awaiting confirmation or
exclusion of the diagnosis of VHF.
In a contained casualty situation
(in which a modest number of patients require therapy), the working group
recommends that an intravenous regimen of ribavirin be given as described
in Table 4, in accordance with CDC’s recommendations for treating patients
with suspected VHF of unknown cause, pending identification of the agent.
109 A similar dose has been used in the treatment
of Lassa fever.106
In a mass casualty situation (in
which the number of persons requiring therapy is sufficiently high that delivery
of intravenous therapy is no longer possible), an oral regimen of ribavirin
as described in Table 4 is recommended. This dose is currently licensed for
treatment of chronic hepatitis C infection in the United States.
105 Although it is substantially lower than that
in the intravenous regimen, a similar dose has been used to treat a few patients
with Lassa fever,106
and there are no available studies on tolerability or efficacy of higher
doses of oral ribavirin.
Ribavirin is contraindicated in pregnancy.
However, in the context of infection with VHF of unknown cause or secondary
to an arenavirus or Rift Valley fever, the working group believes that the
benefits appear likely to outweigh any fetal risk of ribavirin therapy, and
ribavirin is therefore recommended. The associated mortality of VHF tends
to be higher in pregnancy.110
|
Table 4. Recommendations for Ribavirin Therapy in Patients With Clinically
Viral Hemorrhagic Fever of Unknown Etiology or Secondary to Arenaviruses
or Bunyaviruses* |
|
Contained Casualty Setting
Mass Casualty Setting± |
|
Adults Loading dose of 30 mg/kg intravenously
(IV) Loading dose of 2000 mg orally once,
(maximum, 2 g) once, followed
by 16 mg/kg IV followed by 1200 mg/d orally in 2
(maximum, I g per
dose) every 6 hours for 4 divided doses (if weight >75 kg),
or
days followed by
8 mg/kg IV (maximum, 500 1000 mg/d orally in 2 doses (400 mg
mg per dose) every
8 hours for 6 days in AM and 600 mg in PM)
(if weight
_<75 kg) for 10 days#
|
|
Pregnant women§ Same as
for adults
Same as for adults |
|
Children Same as for adults, dosed according
to Loading dose of 30 m&/kg orally
weight
in 2 divided doses for 10 days.
|
|
* Recommendations are not approved by the US Food and Drug Administration
for any of these indictions and should always be administered under an investigational
new drug protocol. However, in a mass casualty setting, these requirements
may need to be modified to permit timely administration of the drug.
±
The threshold number of cases at which parenteral therapy becomes impossible
depends on a variety of factors including local health care resources.
# Although
a similar dosage (1000 mg/d in 3 divided doses) has been used in a small
number of patients with Lassa fever, 106 this regimen would be impractical
because the current formulation of oral ribavirin in the United States consists
of 200 mg capsules, and ribavirin capsules may not be broken open.
§ Refer to the
section in the text on treatment of pregnant women for details.
|
The use of oral or intravenous ribavirin
is not approved by the FDA for children, and proper doses have not been established.
Only aerosolized ribavirin has been approved by the FDA for children, to
treat respiratory syncytial virus infection. However, in the context of infection
with VHF of unknown cause or secondary to an arenavirus or Rift Valley fever,
the working group believes that the benefits likely outweigh the risks of
ribavirin therapy, and it is therefore recommended as described in Table
4. Similar doses have been used to treat children with adenovirus pneumonia
111 and hepatitis C
112 and were well tolerated. Ribavirin capsules
may not be broken open and are only available in 200-mg doses. However, Schering-Plough
Corp (Kenilworth, NJ) produces a pediatric syrup formulation (which is not
commercially available) for use under an IND application. For infections
caused by filoviruses or flaviviruses, the working group recommends supportive
medical care only. Ribavirin has been shown to have no clinical utility against
these groups of viruses.
Passive Immunization
Studies and case reports evaluating
convalescent plasma as therapy (or prophylaxis) of the diseases caused by
HFVs have yielded mixed results depending on the disease, with some reports
suggesting clinical utility26, 80, 82, 101, 113-117
and other studies showing no benefit.52, 106,
118 Passive immunization has also been associated
with enhanced viral replication in experimentally infected animals.
119 The logistics of collection, testing, and
storing immune convalescent plasma are formidable. In the United States,
the paucity of survivors of these diseases and the lack of a national program
that collects and stores HFV immune plasma preclude its use in the initial
response to a bioterrorist attack. Development of methods to manufacture
monoclonal antibodies and recent advances in selecting highly effective human-derived
or humanized products may provide new approaches to therapy in the future.
Postexposure Prophylaxis
Effective prophylaxis following exposure
to an HFV is hampered by the absence of effective vaccines and antiviral
medications. The working group does not recommend preemptive administration
of ribavirin in the absence of signs of infection to persons with known or
suspected exposures to the HFVs. Ribavirin has no utility against filoviruses
or flaviviruses. For arenaviruses, there is limited experimental evidence
that postexposure prophylaxis with ribavirin will delay onset of disease
but not prevent it.120-121
Furthermore, the effectiveness of ribavirin as postexposure prophylaxis
for arenaviruses or Rift Valley fever virus has never been studied in humans.
While 1995 CDC guidelines recommend ribavirin to high-risk contacts of patients
with Lassa fever,109
a review and possible revision of these recommendations is to be shortly
undertaken (James Hughes, MD, oral communication, January 10, 2002). However,
public health professionals suggest that stratification of risk groups into
high-risk and close contacts may facilitate counseling and outbreak investigation.
High-risk contacts are those who have had mucous membrane contact with a
patient (such as during kissing or sexual intercourse) or have had a percutaneous
injury involving contact with the patient’s secretions, excretions, or blood.
Close contacts are those who live with, shake hands with, hug, process laboratory
specimens from, or care for a patient with clinical evidence of VHF prior
to initiation of appropriate precautions.
Persons considered potentially exposed
to HFVs in a bioterrorist attack and all known high-risk and close contacts
of patients diagnosed with VHF should be placed under medical surveillance.
All such individuals should be instructed to record their temperatures twice
daily and report any temperature of 101°F (38.3°C) or higher (or any symptom
noted in Table 3) to a clinician, hospital epidemiologist, or public health
authority designated with surveillance. Surveillance should be continued
for 21 days after the person’s deemed potential exposure or last contact
with the ill patient.
If a temperature of 101°F (38.3°C) or higher
develops, ribavirin therapy should be initiated promptly as presumptive treatment
of VHF, as described in Table 4, unless an alternative diagnosis is established
or the etiologic agent is known to be a filovirus or a flavivirus. In the
case of close and high-risk contacts of patients diagnosed with Rift Valley
fever or a flavivirus, only those who process laboratory specimens from a
patient prior to initiation of appropriate precautions require medical surveillance,
as these specific viruses are not transmitted from person to person but may
be transmitted in the laboratory setting.
Vaccine
With the exception of yellow fever
live attenuated 17D vaccine, which is highly effective when administered
to travelers to endemic areas,68
there is no licensed vaccine for any of the HFVs. The yellow fever vaccine
is produced in limited supply, and world stocks are not sufficient to meet
a surge.122 This vaccine
would not be useful in preventing disease if given in the postexposure setting
because yellow fever has a short incubation period of 3 to 6 days, and neutralizing
antibodies take longer to appear following vaccination.
68
Infection Control
Given the lack of licensed or effective
therapies and vaccines against the HFVs, efforts to prevent transmission
of infection must rely on the meticulous implementation of and compliance
with strict infection control measures. Filoviruses and arenaviruses are
highly infectious after direct contact with infected blood and bodily secretions.
A suspected case of VHF must be immediately reported to the hospital epidemiologist
(or infection control professional) and to the local or state health department.
The epidemiologist (or infection control professional) should, in turn, notify
the clinical laboratory (so that additional precautions are put in place)
as well as other clinicians and public health authorities.
Isolation Precautions
Direct contact with infected blood and
bodily fluids has accounted for the majority of person-to-person transmission
of filoviruses and arenaviruses. Therefore, we recommend that in the case
of any patient with suspected or documented VHF, VHF-specific barrier precautions
should be implemented immediately (Box 2). These precautions do not reflect
HICPAC’s isolation guidelines terminology and are defined here as strict
hand hygiene plus use of double gloves, impermeable gowns, face shields,
eye protection, and leg and shoe coverings (given the copious amounts of
infected material, such as vomitus and liquid stool, that may be present
in the environment).
Airborne transmission of HFVs appears
to be a rare event but cannot be conclusively excluded. Given the inability
to completely exclude this potential, the lack of preventive vaccines, and,
in the case of filoviruses, the lack of effective drug therapy, we recommend
that in addition to VHF-specific barrier precautions, airborne precautions
also be instituted. Airborne precautions entail the use of a high-efficiency
particulate respirator for any person entering the room and, as required
by HICPAC standards,123
the patient should be placed in a room with negative air pressure, 6 to
12 air changes per hour, air exhausted directly to the outdoors or passage
through a high-efficiency particulate air (HEPA) filter before recirculation,
and doors kept closed. There are many circumstances in which the use of negative-pressure
rooms may not be possible, including mass casualty situations. In such conditions,
all other infection control measures should be taken (i.e., VHF-specific
barrier precautions and a HEPA respirator for any person entering the room),
which would, in combination, substantially reduce the risk of nosocomial
transmission. Available evidence suggests that in the great preponderance
of historical cases, these measures were sufficient to prevent transmission
of disease to health care workers, family members, and other patients. Nonessential
staff and visitors should have restricted access to patients’ rooms. If there
are multiple patients with VHF in a health care facility, they should be
cared for in the same part of the hospital to minimize exposure to other
persons.
All persons, including health care workers
and laboratory personnel who have had a close or high-risk contact with a
patient infected with a filovirus or an arenavirus within 21 days of the
patient’s onset of symptoms, prior to the institution of appropriate infection
control precautions, should be placed under medical surveillance and managed
as described in the section on postexposure prophylaxis. Laboratory personnel
who have processed laboratory specimens from a patient with any HFVs (including
Rift Valley fever and the flaviviruses) within 21 days of the patient’s onset
of symptoms, prior to the institution of appropriate infection control precautions,
should also be placed under medical surveillance.
Because some of these viruses may
remain present in bodily fluids for long periods following clinical recovery,
convalescent patients continue to pose a risk of disease transmission.
40, 60 Therefore, patients convalescing from
a filoviral or an arenaviral infection should refrain from sexual activity
for 3 months after clinical recovery.
|
Box 2. Recommendations for Protective Measures Against Nosocomial
Transmission of Hemorrhagic Fever Viruses
Strict
adherence to hand hygiene:
Health
care workers should clean their hands prior to donning personal protective
equipment for patient contact. After patient contact, health care workers
should remove gown, leg and shoe coverings, and gloves and immediately clean
their hands. Hands should be clean prior to the removal of facial
protective equipment (i.e., personal respirators,
face shields, and goggles) to minimize exposure of mucous membranes with
potentially contaminated hands, and once again after the removal of all personal
protective equipment.
-
Double gloves
-
Impermeable gowns
-
N-95 masks or powered air-purifying respirators, and a negative isolation
room with 6-12 air changes per hour, as required by Healthcare Infection
Control Practices Advisory Committee standards for airborne precautions*
-
Leg and shoe coverings
-
Face shields±
-
Goggles for eye protection
-
Restricted access of nonessential staff and visitors to patient's room
-
Dedicated medical equipment, such as stethoscopes, glucose monitors, and,
if available, point-of- care analyzers
-
Environmental disinfection with an Environmental Protection Agency-registered
hospital disinfectant or a 1: 100 dilution of household bleach
-
If there are multiple patients with viral hemorrhagic fever in one health
care facility, they should be cared for in the same part of the hospital
to minimize exposures to other patients and health care workers.
*These resources
may not be possible in many health care facilities or in a mass casualty
situation. In this case, all other measures should be taken and would, in
combination, be expected to substantially diminish the risk of nosocomial
spread.
±Face shields
and eye protection may be already incorporated in certain personal protective
equipment, such as powered air-purifying respirators.
|
Personal
Protective Equipment
Powered air-purifying respirators
(PAPRs) are theoretically more efficacious than N-95 disposable masks in
providing respiratory protection from small-particle aerosols, mostly due
to issues related to proper fitting of the masks.
124 However, no data exist to support higher
efficacy of PAPRs over N-95 masks in preventing airborne transmission of
infection in the health care setting.125
PAPRs are more expensive ($300-$600 vs less than $1 for disposable N-95
masks), are bulky, require maintenance, and impair voice communication to
a higher degree than disposable N-95 masks.126
One study has shown that PAPRs are associated with a higher incidence of
needlestick injuries.127
Disadvantages of the N-95 masks include the difficulty in ensuring a reliable
face-mask seal with each use and impossibility of effective use by bearded
individuals. The theoretical advantage of PAPRs over N-95 masks may be offset
by the danger of increased needlestick or sharp injuries to those using PAPRs
in these settings. The N-95 masks (in combination with face shields and goggles)
are likely equivalent in protection to PAPRs in the health care setting.
Therefore, we recommend that clinicians
caring for patients with a VHF use either N-95 masks or PAPRs, depending
on their familiarity with one or the other, the suitability for the individual,
and availability at a given institution. Some experts have advocated that
PAPRs be used during cough-inducing procedures (i.e., endotracheal intubations,
bronchoscopies), autopsies, and centrifugation or pipetting of laboratory
specimens. While there are no data to support this recommendation, we would
concur as long as the health care workers are familiar with the use of PAPRs
and are not subjecting themselves to the risk of inadvertent needlestick
injury.
Laboratory Testing
The HFVs described herein (including
Rift Valley fever and the flaviviruses) are highly infectious in the laboratory
setting and may be transmitted to laboratory personnel via small-particle
aerosols. The risk is especially high during aerosol-generating procedures,
such as centrifugation. To minimize the possibility of small-particle aerosol
generation, all laboratory staff must be alerted to any suspected diagnosis
of VHF. Designated laboratory workers should receive training in handling
specimens from any suspected VHF patients in advance of such an event. Laboratory
workers should wear personal protective equipment that ensures VHF-specific
barrier and airborne precautions (Box 2). All specimens should be handled,
at a minimum, in a class 2 biological safety cabinet following BSL-3 practices.
127 (A detailed description of class 2 biological
safety cabinets is available at http://www.cdc.gov/od/ohs/biosfty/bmbl4/b4aa.htm,
and a detailed description of BSL-3 practices is available at http://www.cdc.gov/od/ohs/biosfty/bmbl4/bmbl4s3.htm.)
Most clinical facilities are not equipped with a BSL-3 laboratory. Virus isolation
should only be attempted in a BSL-4 laboratory.
Potential hazards associated with handling
of clinical specimens from patients infected with an HFV pose great problems
in hospital facilities. Laboratory tests should be limited to critical diagnostic
tests. If adequate resources are available, point-of-care analyzers for routine
laboratory analysis of infected patients should be used. Point-of-care analyzers
are small, portable devices that may be used at the bedside, require only
a few drops of fresh whole blood, display test results in a few minutes,
limit the exposure of laboratory personnel to infectious clinical specimens,
do not disrupt the clinical laboratory routine, and do not contaminate clinical
laboratory equipment.
If point-of-care analyzers are not
available, clinical specimens need to be processed in a clinical laboratory.
Precautions that parallel those of a US hospital’s successful efforts to
care for a patient infected with a New World arenavirus should be followed.
128 Laboratory specimens should be clearly identified,
double bagged, and hand carried to the laboratory at prescheduled times,
preferably prior to equipment maintenance to enable decontamination of instruments
after testing. Specimens should never be transported in pneumatic tube systems.
Only dedicated, trained laboratory personnel should process clinical specimens
from patients with VHF, wearing protective equipment to ensure airborne and
VHF-specific barrier precautions. Serum should be pretreated with the detergent
Triton X-100 (10 µL of 10% Triton X-100 per 1 mL of serum for 1 hour). Pretreatment
with Triton X-100 may reduce the titers of these enveloped viruses, but efficacy
has not been tested.109
Pretreatment with Triton X-100 does not significantly alter serum electrolytes,
urea nitrogen, creatinine, and glucose or liver function test results.
128 Additional guidelines for clinical specimen
transport, processing, and disposal have been described by Armstrong et al.
128
Postmortem Practices
In the event of an outbreak of VHF,
special provisions will be required for burial practices. Contact with cadavers
has been implicated as a source of transmission in the Kikwit Ebola outbreak
of 199536 and in Uganda
in 2000.37 We recommend
that trained personnel, using the same infection control precautions as those
used to transport ill patients, handle the bodies of patients who die of
VHF. Autopsies should be performed only by specially trained persons using
VHF-specific barrier precautions and HEPA-filtered respirators (N-95 masks
or PAPRs) and negative-pressure rooms, as would be customary in cases in
which contagious biological aerosols, such as Mycobacterium tuberculosis,
are deemed a possible risk.129
We recommend prompt burial or cremation of the deceased, with minimal handling.
Specifically, no embalming should be done. Surgery or postmortem examinations
are associated with increased risks of transmission and should be done only
when absolutely indicated and after consultation with experts.
Environmental
Decontamination
Linen handlers and workers involved
in environmental decontamination should wear personal protective equipment
that ensures VHF-specific barrier precautions (Box 2). We recommend that
contaminated linens be placed in double bags and washed without sorting in
a normal hot water cycle with bleach. Alternatively, they may be autoclaved
or incinerated.109
Detailed instructions on handling and disinfection of contaminated linens
are available from the CDC.109 Environmental surfaces in patients’ rooms
and contaminated medical equipment should be disinfected with an Environmental
Protection Agency–registered hospital disinfectant or a 1:100 dilution of
household bleach.109
It has been suggested that excreta
should be disinfected with 0.6% sodium hypochlorite before disposal.
130 Although a theoretical concern remains that
the disposal of contaminated human excreta may contaminate sewage systems,
the working group does not recommend the addition of disinfectants to human
excreta prior to disposal. Disinfectants are not effective in sterilizing
solid waste, the indiscriminate addition of hypochlorite may damage septic
tanks, and these viruses are not likely to survive standard sewage treatment
in the United States.
In general, in their natural state,
these lipid-enveloped viruses are not environmentally stable and are not
expected to persist in the environment for prolonged periods.
7 Decisions regarding the need for and methods
of decontamination following an attack with an HFV should be made following
expert analysis of the contaminated environment and the weapons used in the
attack, in consultation with experts in environmental remediation.
Ongoing Research and Proposed Agenda
Mechanisms of disease transmission in human
outbreaks of HFVs are still poorly understood. Clarification of the role
of airborne transmission is vital. Rapid diagnostic methods need to be developed
for all of the HFVs, including those that have been excluded from this article
and made available to selected state health departments for the expedient
diagnosis of suspected cases. Methods to safely handle potentially infected
specimens in a clinical laboratory should be developed.
The diagnostic and therapeutic armamentarium
urgently needs to be augmented. There also is an urgent need to develop vaccines
and drug therapy. A live attenuated vaccine against Argentine hemorrhagic
fever (candid No. 1) developed at the USAMRIID131 is available as an IND.
This vaccine has been shown to be safe and effective in protecting agricultural
workers in South America132
and may provide cross-protection against Bolivian hemorrhagic fever.
9 There are 2 vaccines against Rift Valley fever
also available as INDs. One is formalin inactivated and appears to be safe
and effective when administered to laboratory workers. However, it is available
only in limited supply, and the manufacturing capacity for producing additional
vaccine no longer exists in the United States.
133-134 Lastly, a formalin-inactivated Kyasanur
Forest disease vaccine exists and has been shown to be protective in field
trials in India.135
There are several promising vaccines in development for prevention of filoviruses
and Lassa fever, some in nonhuman primate models.
136-139 Passive immunization strategies using
recombinant human monoclonal antibodies should be pursued, given the potential
benefit of passive immunization in a series of reports.
80, 114, 116-117,140 Research with these agents
is hampered by the requirement of conducting experiments in BSL-4 laboratories.
More BSL-4 laboratories would expand research opportunities.
Ribavirin is the only potentially effective
drug available for selected hemorrhagic fever because it is approved by the
FDA for another indication. However, it is not effective against all of the
HFVs and it is not widely available. The supply of ribavirin should be rapidly
augmented, and studies to demonstrate its efficacy and safety against selected
HFVs should be conducted to support an FDA approval for those indications.
We also recommend the addition of intravenous and oral formulations of ribavirin
to the US National Pharmaceutical Stockpile (a repository of antibiotics,
chemical antidotes, and other medical supplies managed by the CDC that may
be emergently sent to the site of a disaster anywhere in the United States).
New antiviral therapies should be pursued for the treatment of all HFVs,
including those excluded from this article. The effects of any developed
therapy in pediatric populations should also be evaluated.
PAGE 333 USE OMNI PAGE PRO
ADDITIONAL ARTICLES
This article is the sixth in a series entitled Medical and Public Health
Management Following the Use of a Biological Weapon: Consensus Statements
of the Working Group on Civilian Biodefense. See references 1 through 5.
ACKNOWLEDGEMENT
We thank those who generously provided invaluable assistance in the preparation
of this article: Julie Gerberding, MD, MPH, Jim LeDuc, PhD, Steve Ostroff,
MD, Duane Gubler, ScD, Denise Cardo, MD, and Lynn Steele, MS, CDC; Anthony
Suffredini, MD, National Institutes of Health; Elin Gursky, ScD, and Paul
Pham, PharmD, Johns Hopkins University; and Lauren Iacono-Connors, PhD, Karen
Midthun, MD, Joanne Holmes, MBA, Jerry Weir, PhD, Kathleen Uhl, MD, Diane
Kennedy, RPh, MPH, Dianne Murphy, MD, Harry Haverkos, MD, Brad Leissa, MD,
Sandra Folkendt, Lewis Markoff, MD, and Karen Oliver, MSN, FDA.
CORRESPONDING AUTHOR AND REPRINTS
Luciana Borio, MD, Johns Hopkins Center for Civilian Biodefense Strategies,
Johns Hopkins Schools of Medicine and Public Health, 111 Market Pl, Suite
830, Baltimore, MD 21202 (e-mail: Lborio@jhsph.edu).
AUTHOR AFFILIATIONS
Johns Hopkins Center for Civilian Biodefense
Strategies (Drs Borio, Inglesby, O’Toole, Henderson, and Kwik) and Departments
of Microbiology and Neuroscience (Dr K. M. Johnson), Johns Hopkins Schools
of Medicine and Public Health, and Division of Infectious Diseases, Johns
Hopkins School of Medicine (Drs Bartlett and Perl), Baltimore, Md; Critical
Care Medicine Department, Clinical Center (Dr Borio), Fogarty International
Center (Dr Breman), and Vaccine Research Center (Dr Nabel), National Institutes
of Health, Bethesda, Md; Center for Biodefense, University of Texas Medical
Branch, Galveston (Dr Peters); US Army Medical Research Institute of Infectious
Diseases, Frederick, Md (Drs Schmaljohn, Jahrling, and Eitzen); National
Center for Infectious Diseases, Centers for Disease Control and Prevention,
Atlanta, Ga (Drs Hughes and Ksiazek); Departments of Biology and Medicine,
University of New Mexico, Albuquerque (Dr R. T. Johnson); Office of the Commissioner,
US Food and Drug Administration (Dr Meyerhoff), and Office of Emergency Preparedness,
Department of Health and Human Services (Dr Tonat), Rockville, Md; Office
of Public Health Preparedness, Department of Health and Human Services (Drs
Ascher, Lillibridge, and Russell and Mr Hauer), and Nuclear Threat Initiative
(Dr Hamburg), Washington, DC; Bureau of Communicable Diseases, New York City
Health Department, New York, NY (Dr Layton); and Center for Infectious Disease
Research and Policy, University of Minnesota, Minneapolis (Dr Osterholm).
References
-
Inglesby TV, Henderson DA, Bartlett JG, et al for the Working Group on
Civilian Biodefense. Anthrax as a biological weapon. JAMA. 1999;281:1735-1745.
-
Henderson DA, Inglesby TV, Bartlett JG, et al for the Working Group on
Civilian Biodefense. Smallpox as a biological weapon. JAMA.
1999;281:2127-2137.
-
Inglesby TV, Dennis DT, Henderson DA, et al for the Working Group on
Civilian Biodefense. Plague as a biological weapon. JAMA. 2000;283:2281-2290.
-
Arnon SS, Schechter R, Inglesby TV, et al for the Working Group on Civilian
Biodefense. Botulinum toxin as a biological weapon. JAMA.
2001;285:1059-1070.
-
Dennis DT, Inglesby TV, Henderson DA, et al for the Working Group on
Civilian Biodefense. Tularemia as a biological weapon. JAMA.
2001;285:2763-2773.
-
Fatal illnesses associated with a new world arenavirus—California, 1999-2000.
MMWR Morb Mortal Wkly Rep. 2000;49:709-711.
-
Peters CJ, Jahrling PB, Khan AS. Patients infected with high-hazard viruses.
Arch Virol Suppl. 1996;11:141-168.
-
Solomon T, Mallewa M. Dengue and other emerging flaviviruses. J Infect.
2001;42:104-115.
-
Jahrling P. Viral hemorrhagic fevers. In: Textbook of Military Medicine.
Vol 1. Falls Church, Va: Office of the Surgeon General; 1989.
-
Mandell GL, Douglas RG, Bennett JE, Dolin R. Mandell, Douglas, and
Bennett’s Principles and Practice of Infectious Diseases. 5th ed.
Philadelphia, Pa: Churchill Livingstone; 2000.
-
Swanepoel R, Shepherd AJ, Leman PA, et al. Epidemiologic and clinical
features of Crimean-Congo hemorrhagic fever in southern Africa. Am
J Trop Med Hyg. 1987;36:120-132.
-
Peters CJ, Simpson GL, Levy H. Spectrum of hantavirus infection: hemorrhagic
fever with renal syndrome and hantavirus pulmonary syndrome. Annu
Rev Med. 1999;50:531-545.
-
Alibek K, Handelman S. Biohazard: The Chilling True Story of the Largest
Covert Biological Weapons Program in the World, Told From the Inside
by the Man Who Ran It. New York, NY: Random House; 1999.
-
Center for Nonproliferation Studies. Chemical and biological weapons:
possession and programs past and present. November 2000. Available at:
http://cns.miis.edu/research/cbw/possess.htm. Accessed January 10, 2002.
-
Miller J, Engelberg S, Broad WJ. Germs: Biological Weapons and America’s
Secret War. Waterville, Me: GK Hall; 2002.
-
Bazhutin NB, Belanov EF, Spiridonov VA, et al. The effect of the methods
for producing an experimental Marburg virus infection on the characteristics
of the course of the disease in green monkeys [in Russian]. Vopr Virusol.
1992;37:153-156.
-
Global Proliferation of Weapons of Mass Destruction: Hearings Before
the Permanent Subcommittee on Investigations of the Committee on Governmental
Affairs, United States Senate, 104th Cong, 1st-2nd Sess (1996).
-
Johnson E, Jaax N, White J, Jahrling P. Lethal experimental infections
of rhesus monkeys by aerosolized Ebola virus. Int J Exp Pathol.
1995;76:227-236.
-
Lub M, Sergeev AN, P’Iankov OV, P’Iankova OG, Petrishchenko VA, Kotliarov
LA. Certain pathogenetic characteristics of a disease in monkeys in infected
with the Marburg virus by an airborne route [in Russian]. Vopr Virusol.
1995;40:158-161.
-
Stephenson EH, Larson EW, Dominik JW. Effect of environmental factors
on aerosol-induced Lassa virus infection. J Med Virol. 1984;14:295-303.
-
Kenyon RH, McKee KT Jr, Zack PM, et al. Aerosol infection of rhesus macaques
with Junin virus. Intervirology. 1992;33:23-31.
-
Centers for Disease Control and Prevention. Category A agents. Available
at: http://www.bt.cdc.gov/Agent/Agentlist.asp. Accessed January 10, 2002.
-
LeDuc JW. Epidemiology of hemorrhagic fever viruses. Rev Infect Dis.
1989;11(suppl 4):S730-S735.
-
Schou S, Hansen AK. Marburg and Ebola virus infections in laboratory
nonhuman primates: a literature review. Comp Med. 2000;50:108-123.
-
Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ.
1978;56:271-293.
-
Emond RT, Evans B, Bowen ET, Lloyd G. A case of Ebola virus infection.
BMJ. 1977;2:541-544.
-
Simpson DI. Marburg agent disease. Trans R Soc Trop Med Hyg. 1969;63:303-309.
Cited by: Schou S, Hansen AK. Marburg and Ebola virus infections in
laboratory nonhuman primates. Comp Med. 2000;50:2108-2123.
-
Jaax NK, Davis KJ, Geisbert TJ, et al. Lethal experimental infection
of rhesus monkeys with Ebola-Zaire (Mayinga) virus by the oral and conjunctival
route of exposure. Arch Pathol Lab Med. 1996;120:140-155.
-
Colebunders R, Borchert M. Ebola haemorrhagic fever—a review. J Infect.
2000;40:16-20.
-
Zaki SR, Shieh WJ, Greer PW, et al. A novel immunohistochemical assay
for the detection of Ebola virus in skin. J Infect Dis. 1999;179(suppl
1):S36-S47.
-
Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission
of Ebola hemorrhagic fever: a study of risk factors in family members,
Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis.
1999;179(suppl 1):S87-S91.
-
Khan AS, Tshioko FK, Heymann DL, et al. The reemergence of Ebola hemorrhagic
fever, Democratic Republic of the Congo, 1995. J Infect Dis.
1999;179(suppl 1):S76-S86.
-
Muyembe-Tamfum JJ, Kipasa M, Kiyungu C, Colebunders R. Ebola outbreak
in Kikwit, Democratic Republic of the Congo: discovery and control measures.
J Infect Dis. 1999;179(suppl 1):S259-S262.
-
Butler JC, Kilmarx PH, Jernigan DB, Ostroff SM. Perspectives in fatal
epidemics. Infect Dis Clin North Am. 1996;10:917-937.
-
Shu HL, Siegert R, Slenczka W. The pathogenesis and epidemiology of the
"Marburg-virus" infection. Ger Med Mon. 1969;14:7-10.Cited by:
Schou S, Hansen AK. Marburg and Ebola virus infections in laboratory
nonhuman primates. Comp Med. 2000;50:2108-2123.
-
Roels TH, Bloom AS, Buffington J, et al. Ebola hemorrhagic fever, Kikwit,
Democratic Republic of the Congo, 1995: risk factors for patients without
a reported exposure. J Infect Dis. 1999;179(suppl 1):S92-S97.
-
Outbreak of Ebola hemorrhagic fever, Uganda, August 2000–January 2001.
MMWR Morb Mortal Wkly Rep. 2001;50:73-77.
-
Jaax N, Jahrling P, Geisbert T, et al. Transmission of Ebola virus (Zaire
strain) to uninfected control monkeys in a biocontainment laboratory.
Lancet. 1995;346:1669-1671.
-
Dalgard DW, Hardy RJ, Pearson SL, et al. Combined simian hemorrhagic
fever and Ebola virus infection in cynomolgus monkeys. Lab Anim Sci.
1992;42:152-157.
-
Slenczka WG. The Marburg virus outbreak of 1967 and subsequent episodes.
Curr Top Microbiol Immunol. 1999;235:49-75.
-
Gear JS, Cassel GA, Gear AJ, et al. Outbreak of Marburg virus disease
in Johannesburg. BMJ. 1975;4:489-493.
-
Baron RC, McCormick JB, Zubeir OA. Ebola virus disease in southern Sudan.
Bull World Health Organ. 1983;61:997-1003.
-
Formenty P, Hatz C, Le Guenno B, Stoll A, Rogenmoser P, Widmer A. Human
infection due to Ebola virus, subtype Cote d’Ivoire. J Infect Dis.
1999;179(suppl 1):S48-S53.
-
Richards GA, Murphy S, Jobson R, et al. Unexpected Ebola virus in a tertiary
setting. Crit Care Med. 2000;28:240-244.
-
Guimard Y, Bwaka MA, Colebunders R, et al. Organization of patient care
during the Ebola hemorrhagic fever epidemic in Kikwit, Democratic Republic
of the Congo, 1995. J Infect Dis. 1999;179(suppl 1):S268-S273.
-
Centers for Disease Control and Prevention and World Health Organization.
Infection control for viral hemorrhagic fevers in the African health
care setting. Atlanta, Ga: Centers for Disease Control and Prevention.
Available at: http://www.cdc.gov/ncidod/dvrd/spb/mnpages/vhfmanual.htm.
Accessed January 10, 2002.
-
Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic
follow-up of convalescent Ebola hemorrhagic fever patients and their
household contacts, Kikwit, Democratic Republic of the Congo. J Infect
Dis. 1999;179(suppl 1):S28-S35.
-
Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability
of Ebola virus during the outbreak in Kikwit, Democratic Republic of
the Congo, 1995. J Infect Dis. 1999;179(suppl 1):S170-S176.
-
Johnson KM, Mackenzie RB, Webb PA, Kuns ML. Chronic infection of rodents
by Machupo virus. Science. 1965;150:1618-1619.
-
Johnson KM, Kuns ML, Mackenzie RB, Webb PA, Yunker CE. Isolation of Machupo
virus from wild rodent Calomys callosus. Am J Trop Med Hyg.
1966;15:103-106.
-
Carey DE, Kemp GE, White HA, et al. Lassa fever: epidemiological aspects
of the 1970 epidemic, Jos, Nigeria. Trans R Soc Trop Med Hyg.
1972;66:402-408.
-
White HA. Lassa fever: a study of 23 hospital cases. Trans R Soc Trop
Med Hyg. 1972;66:390-401.
-
Monath TP, Mertens PE, Patton R, et al. A hospital epidemic of Lassa
fever in Zorzor, Liberia, March-April 1972. Am J Trop Med Hyg.
1973;22:773-779.
-
Peters CJ, Kuehne RW, Mercado RR, Le Bow RH, Spertzel RO, Webb PA. Hemorrhagic
fever in Cochabamba, Bolivia, 1971. Am J Epidemiol. 1974;99:425-433.
-
Zweighaft RM, Fraser DW, Hattwick MA, et al. Lassa fever: response to
an imported case. N Engl J Med. 1977;297:803-807. ABSTRACT
-
Cooper CB, Gransden WR, Webster M, et al. A case of Lassa fever: experience
at St Thomas’s Hospital. Br Med J (Clin Res Ed). 1982;285:1003-1005.
-
Kilgore PE, Ksiazek TG, Rollin PE, et al. Treatment of Bolivian hemorrhagic
fever with intravenous ribavirin. Clin Infect Dis. 1997;24:718-722.
-
World Health Organization. Fact sheet 179: Lassa fever. April 2000. Available
at: http://www.who.int/inf-fs/en/fact179.html. Accessed January 10, 2002.
-
Buckley SM, Casals J. Lassa fever, a new virus disease of man from West
Africa, III: isolation and characterization of the virus. Am J Trop
Med Hyg. 1970;19:680-691.
-
Briggiler AM, Enria DA, Feuillade MR, Maiztegui JI. Contagio interhumano
e infeccion clinical con virus Junin en matrimonios residentes en el
area endemica de fiebre hemorragica Argentina. Medicina (B Aires).
1987;47:565.
-
Swanapoel R, Coetzer JA. Rift Valley fever. In: Infectious Diseases
of Livestock With Special Reference to Southern Africa. Vol 1. New
York, NY: Oxford University Press; 1994.
-
Jouan A, Coulibaly I, Adam F, et al. Analytical study of a Rift Valley
fever epidemic. Res Virol. 1989;140:175-186.
-
Smithburn KC, Mahaffy AF, Haddow AJ, Kitchen SF, Smith JF. Rift Valley
fever: accidental infections among laboratory workers. J Immunol.
1949;62:213-227.
-
Meegan JM. The Rift Valley fever epizootic in Egypt 1977-78, I: description
of the epizootic and virological studies. Trans R Soc Trop Med Hyg.
1979;73:618-623.
-
Shawky S. Rift valley fever. Saudi Med J. 2000;21:1109-1115.
-
Turell MJ, Kay BH. Susceptibility of selected strains of Australian mosquitoes
(Diptera: Culicidae) to Rift Valley fever virus. J Med Entomol.
1998;35:132-135.
-
Gargan II TP, Clark GG, Dohm DJ, Turell MJ, Bailey CL. Vector potential
of selected North American mosquito species for Rift Valley fever virus.
Am J Trop Med Hyg. 1988;38:440-446.
-
Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1:11-20.
-
Cunha BA. Tickborne Infectious Diseases: Diagnosis and Management
. New York, NY: Marcel Dekker; 2000.
-
Banerjee K, Gupta NP, Goverdhan MK. Viral infections in laboratory personnel.
Indian J Med Res. 1979;69:363-373.
-
International Committee on Taxonomy of Viruses. Seventh Report of
the International Committee on Taxonomy of Viruses. San Diego, Calif:
Academic Press; 2000.
-
Chen JP, Cosgriff TM. Hemorrhagic fever virus-induced changes in hemostasis
and vascular biology. Blood Coagul Fibrinolysis. 2000;11:461-483.
-
Feldmann H, Volchkov VE, Volchkova VA, Klenk HD. The glycoproteins of
Marburg and Ebola virus and their potential roles in pathogenesis.
Arch Virol Suppl. 1999;15:159-169.
-
Ryabchikova EI, Kolesnikova LV, Luchko SV. An analysis of features of
pathogenesis in 2 animal models of Ebola virus infection. J Infect
Dis. 1999;179(suppl 1):S199-S202.
-
Schnittler HJ, Mahner F, Drenckhahn D, Klenk HD, Feldmann H. Replication
of Marburg virus in human endothelial cells. J Clin Invest. 1993;91:1301-1309.
-
Samoilovich SR, Carballal G, Weissenbacher MC. Protection against a pathogenic
strain of Junin virus by mucosal infection with an attenuated strain.
Am J Trop Med Hyg. 1983;32:825-828.
-
Peters CJ, Liu CT, Anderson GW Jr, Morrill JC, Jahrling PB. Pathogenesis
of viral hemorrhagic fevers. Rev Infect Dis. 1989;11(suppl 4):S743-S749.
-
Jahrling PB, Hesse RA, Eddy GA, Johnson KM, Callis RT, Stephen EL. Lassa
virus infection of rhesus monkeys. J Infect Dis. 1980;141:580-589.
-
Cummins D, Molinas FC, Lerer G, Maiztegui JI, Faint R, Machin SJ. A plasma
inhibitor of platelet aggregation in patients with Argentine hemorrhagic
fever. Am J Trop Med Hyg. 1990;42:470-475.
-
Enria DA, Briggiler AM, Fernandez NJ, Levis SC, Maiztegui JI. Importance
of dose of neutralising antibodies in treatment of Argentine haemorrhagic
fever with immune plasma. Lancet. 1984;2:255-256.
-
Cosgriff TM, Morrill JC, Jennings GB, et al. Hemostatic derangement produced
by Rift Valley fever virus in rhesus monkeys. Rev Infect Dis.
1989;11(suppl 4):S807-S814.
-
Peters CJ, Jones D, Trotter R, et al. Experimental Rift Valley fever
in rhesus macaques. Arch Virol. 1988;99:31-44.
-
Morrill JC, Jennings GB, Cosgriff TM, Gibbs PH, Peters CJ. Prevention
of Rift Valley fever in rhesus monkeys with interferon-alpha. Rev
Infect Dis. 1989;11(suppl 4):S815-S825.
-
Monath TP, Brinker KR, Chandler FW, Kemp GE, Cropp CB. Pathophysiologic
correlations in a rhesus monkey model of yellow fever with special
observations on the acute necrosis of B cell areas of lymphoid tissues.
Am J Trop Med Hyg. 1981;30:431-443.
-
Iyer CG, Laxmana Rao R, Work TH, Narasimha Murthy DP. Pathological findings
in 3 fatal human cases of Kyasanur Forest disease. Indian J Med Sci.
1959;13:1011-1022. Cited by: Pavri K. Clinical, clinicopathologic,
and hematologic features of Kyasanur Forest disease. Rev Infect Dis
. 1989;11(suppl 4):S854-S859.
-
Ebola haemorrhagic fever in Sudan, 1976. Bull World Health Organ.
1978;56:247-270.
-
Smith DH, Johnson BK, Isaacson M, et al. Marburg-virus disease in Kenya.
Lancet. 1982;1:816-820.
-
Monson MH, Frame JD, Jahrling PB, Alexander K. Endemic Lassa fever in
Liberia, I: clinical and epidemiological aspects at Curran Lutheran Hospital,
Zorzor, Liberia. Trans R Soc Trop Med Hyg. 1984;78:549-553.
-
Keane E, Gilles HM. Lassa fever in Panguma Hospital, Sierra Leone, 1973-6.
BMJ. 1977;1:1399-1402.
-
Mertens PE, Patton R, Baum JJ, Monath TP. Clinical presentation of Lassa
fever cases during the hospital epidemic at Zorzor, Liberia, March-April
1972. Am J Trop Med Hyg. 1973;22:780-784.
-
McCormick JB, King IJ, Webb PA, et al. A case-control study of the clinical
diagnosis and course of Lassa fever. J Infect Dis. 1987;155:445-455.
-
Re-emergence of Bolivian hemorrhagic fever. Epidemiol Bull. 1994;15:4-5.
-
Gear JH. Haemorrhagic fevers of Africa: an account of 2 recent outbreaks.
J S Afr Vet Assoc. 1977;48:5-8. As cited in: Gear JH. Clinical
aspects of African viral hemorrhagic fevers. Rev Infect Dis. 1989;11(suppl
4):S777-S782.
-
Abd el-Rahim IH, Abd el-Hakim U, Hussein M. An epizootic of Rift Valley
fever in Egypt in 1997. Rev Sci Tech. 1999;18:741-748.
-
Laughlin LW, Meegan JM, Strausbaugh LJ, Morens DM, Watten RH. Epidemic
Rift Valley fever in Egypt. Trans R Soc Trop Med Hyg. 1979;73:630-633.
-
Strausbaugh LJ, Laughlin LW, Meegan JM, Watten RH. Clinical studies on
Rift Valley fever, I: acute febrile and hemorrhagic-like diseases.
J Egypt Public Health Assoc. 1978;53:181-182.
-
Craven RB. Flaviviruses. In: Textbook of Human Virology. 2nd ed.
St Louis, Mo: Mosby–Year Book Medical; 1991.
-
Adhikari Prabha MR, Prabhu MG, Raghuveer CV, Bai M, Mala MA. Clinical
study of 100 cases of Kyasanur Forest disease with clinicopathological
correlation. Indian J Med Sci. 1993;47:124-130.
-
Bwaka MA, Bonnet MJ, Calain P, et al. Ebola hemorrhagic fever in Kikwit,
Democratic Republic of the Congo: clinical observations in 103 patients.
J Infect Dis. 1999;179(suppl 1):S1-S7.
-
Frame JD, Baldwin JM Jr, Gocke DJ, Troup JM. Lassa fever, a new virus
disease of man from West Africa, I: clinical description and pathological
findings. Am J Trop Med Hyg. 1970;19:670-676.
-
Monath TP, Maher M, Casals J, Kissling RE, Cacciapuoti A. Lassa fever
in the Eastern Province of Sierra Leone, 1970-1972, II: clinical observations
and virological studies on selected hospital cases. Am J Trop Med
Hyg. 1974;23:1140-1149.
-
Kuming BS, Kokoris N. Uveal involvement in Marburg virus disease.
Br J Ophthalmol. 1977;61:265-266.
-
World Health Organization. Acute haemorrhagic fever syndrome. Available
at: http://www. who.int/emc-documents/surveillance/docs/whocdscsrisr992.html/41acutete%20haemorrhagic%20fever%20syndrome.htm.
Accessed February 10, 2002.
-
Franz DR, Jahrling PB, Friedlander AM, et al. Clinical recognition and
management of patients exposed to biological warfare agents. JAMA.
1997;278:399-411.
-
Rebetol product information. Available at: http://www.hepatitisinnovations.com/pro/rebetol/rebetol_pi.html.
Accessed January 10, 2002.
-
McCormick JB, King IJ, Webb PA, et al. Lassa fever: effective therapy
with ribavirin. N Engl J Med. 1986;314:20-26.
-
Enria DA, Maiztegui JI. Antiviral treatment of Argentine hemorrhagic
fever. Antiviral Res. 1994;23:23-31.
-
Huggins JW. Prospects for treatment of viral hemorrhagic fevers with
ribavirin, a broad-spectrum antiviral drug. Rev Infect Dis. 1989;11(suppl
4):S750-S761.
-
Update: management of patients with suspected viral hemorrhagic fever—United
States. MMWR Morb Mortal Wkly Rep. 1995;44:475-479.
-
Frame JD. Clinical features of Lassa fever in Liberia. Rev Infect
Dis. 1989;11(suppl 4):S783-S789.
-
Shetty AK, Gans HA, So S, Millan MT, Arvin AM, Gutierrez KM. Intravenous
ribavirin therapy for adenovirus pneumonia. Pediatr Pulmonol.
2000;29:69-73.
-
Kelly DA, Bunn SK, Apelian D, et al. Safety, efficacy, and pharmacokinetics
of interferon alfa-2b plus ribavirin in children with chronic hepatitis
C. Hepatology. 2001;342A:680.
-
Mupapa K, Massamba M, Kibadi K, et al. Treatment of Ebola hemorrhagic
fever with blood transfusions from convalescent patients. J Infect
Dis. 1999;179(suppl 1):S18-S23.
-
Stille W, Bohle E, Helm E, van Rey W, Siede W. On an infectious disease
transmitted by Cercopithecus aethiops ("green monkey disease")
[in German]. Dtsch Med Wochenschr. 1968;93:572-582. Cited by:
Slenczka WG. The Marburg virus outbreak of 1967 and subsequent episodes.
Curr Top Microbiol Immunol. 1999;235:49-75.
-
Leifer E, Gocke DJ, Bourne H. Lassa fever, a new virus disease of man
from West Africa, II: report of a laboratory-acquired infection treated
with plasma from a person recently recovered from the disease. Am
J Trop Med Hyg. 1970;19:677-679.
-
Frame JD, Verbrugge GP, Gill RG, Pinneo L. The use of Lassa fever convalescent
plasma in Nigeria. Trans R Soc Trop Med Hyg. 1984;78:319-324.
-
Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma
in treatment of Argentine haemorrhagic fever and association between
treatment and a late neurological syndrome. Lancet. 1979;2:1216-1217.
-
Clayton AJ. Lassa immune serum. Bull World Health Organ. 1977;55:435-439.
-
Halstead SB. In vivo enhancement of dengue virus infection in rhesus
monkeys by passively transferred antibody. J Infect Dis. 1979;140:527-533.
-
Kenyon RH, Canonico PG, Green DE, Peters CJ. Effect of ribavirin and
tributylribavirin on argentine hemorrhagic fever (Junin virus) in guinea
pigs. Antimicrob Agents Chemother. 1986;29:521-523.
-
Seiler P, Senn BM, Klenerman P, Kalinke U, Hengartner H, Zinkernagel
RM. Additive effect of neutralizing antibody and antiviral drug treatment
in preventing virus escape and persistence. J Virol. 2000;74:5896-5901.
-
Nathan N, Barry M, Van Herp M, Zeller H. Shortage of vaccines during
a yellow fever outbreak in Guinea. Lancet. 2001;358:2129-2130.
-
Garner JS. Guideline for isolation precautions in hospitals. Infect
Control Hosp Epidemiol. 1996;17:53-80.
-
National Institute for Occupational Safety and Health. NIOSH Guide
to the Selection and Use of Particulate Respirators. Cincinnati,
Ohio: National Institute for Occupational Safety and Health; January
1996. Publication 96-101.
-
Fennelly KP. Personal respiratory protection against Mycobacterium
tuberculosis. Clin Chest Med. 1997;18:1-17.
-
Eck EK, Vannier A. The effect of high-efficiency particulate air
respirator design on occupational health. Infect Control Hosp Epidemiol.
1997;18:122-127.
-
Richmond JY, McKinney RW. Biosafety in Microbiological and Biomedical
Laboratories. 4th ed. Washington, DC: US Government Printing Office;
1999.
-
Armstrong LR, Dembry LM, Rainey PM, et al. Management of a Sabia virus-infected
patients in a US hospital. Infect Control Hosp Epidemiol. 1999;20:176-182.
-
Gershon RR, Vlahov D, Escamilla-Cejudo JA, et al. Tuberculosis risk in
funeral home employees. J Occup Environ Med. 1998;40:497-503.
-
Management of patients with suspected viral hemorrhagic fever. MMWR
Morb Mortal Wkly Rep. 1988;37(suppl 3):1-16.
-
McKee KT Jr, Oro JG, Kuehne AI, Spisso JA, Mahlandt BG. Candid No. 1
Argentine hemorrhagic fever vaccine protects against lethal Junin virus
challenge in rhesus macaques. Intervirology. 1992;34:154-163.
-
Maiztegui JI, McKee KT Jr, Barrera Oro JG, et al. Protective efficacy
of a live attenuated vaccine against Argentine hemorrhagic fever.
J Infect Dis. 1998;177:277-283.
-
Niklasson B, Peters CJ, Bengtsson E, Norrby E. Rift Valley fever virus
vaccine trial. Vaccine. 1985;3:123-127.
-
ittman PR, Liu CT, Cannon TL, et al. Immunogenicity of an inactivated
Rift Valley fever vaccine in humans. Vaccine. 1999;18:181-189.
-
Dandawate CN, Desai GB, Achar TR, Banerjee K. Field evaluation of formalin
inactivated Kyasanur forest disease virus tissue culture vaccine in 3
districts of Karnataka state. Indian J Med Res. 1994;99:152-158.
-
Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of
a preventive vaccine for Ebola virus infection in primates. Nature.
2000;408:605-609.
-
Vanderzanden L, Bray M, Fuller D, et al. DNA vaccines expressing either
the GP or NP genes of Ebola virus protect mice from lethal challenge.
Virology. 1998;246:134-144.
-
Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines
based upon alphavirus replicons protect guinea pigs and nonhuman primates.
Virology. 1998;251:28-37.
-
Fisher-Hoch SP, Hutwagner L, Brown B, McCormick JB. Effective vaccine
for Lassa fever. J Virol. 2000;74:6777-6783.
-
Gupta M, Mahanty S, Bray M, Ahmed R, Rollin PE. Passive transfer of antibodies
protects immunocompetent and immunodeficient mice against lethal Ebola
virus infection without complete inhibition of viral replication.
J Virol. 2001;75:4649-4654.
Reprinted with permission.
The Journal of the American Medical Association, Vol. 287, No. 18, May 8,
2002
