10.
Plague
v
Facts About Plague
Plague is an infectious disease that affects
animals and humans. It is caused by the bacterium Yersinia Pestis. This
bacterium is found in rodents and their fleas and occurs in many areas of
the world, including the United States.
Y. pestis
is easily destroyed by sunlight and drying. Even so, when released into
air, the bacterium will survive for up to one hour, although this could
vary depending on conditions.
A plague vaccine is not currently available
for use in the United States.
v
Frequently Asked Questions (FAQ) About Plague
What is plague?
Plague is a disease caused by Yersinia
pestis ( Y. pestis), a bacterium found in rodents and their
fleas in many areas around the world.
Why are
we
concerned about pneumonic plague as a bio weapon?
Yersinia pestis used in an aerosol attack
could cause cases of the pneumonic form of plague. One to six days after
becoming infected with the bacteria, people would develop pneumonic plague.
Once people have the disease, the bacteria can spread to others who have
close contact with them. Because of the delay between being exposed to the
bacteria and becoming sick, people could travel over a large area before
becoming contagious and possibly infecting others. Controlling the disease
would then be more difficult. A bioweapon carrying Y. pestis is possible
because the bacterium occurs in nature and could be isolated and grown in
quantity in a laboratory. Even so, manufacturing an effective weapon using
Y. pestis would require advanced knowledge and technology.
|
How is plague transmitted?
By fleas that become infected with
bacteria Yersinia pestis that cause plague.
What is
the basic transmission cycle?
Fleas become infected by feeding
on rodents, such as the chipmunks, prairie dogs, ground squirrels,
mice, and other mammals that are infected with the bacteria Yersinia
pestis . Fleas transmit the plague bacteria to humans and other
mammals during the feeding process. The plague bacteria are maintained
in the blood systems of rodents.
|

|
What is
the
incubation period for plague?
A person usually becomes ill with bubonic
plague 2 to 6 days after being infected. When bubonic plague is left untreated,
plague bacteria invade the bloodstream. When plague bacteria multiply in
the bloodstream, they spread rapidly throughout the body and cause a severe
and often fatal condition. Infection of the lungs with the plague bacterium
causes the pneumonic form of plague, a severe respiratory illness. The infected
person may experience high fever, chills, cough, and breathing difficulty,
and expel bloody sputum. If plague patients are not given specific antibiotic
therapy, the disease can progress rapidly to death.
What is
the
mortality rate of plague?
About 14% (1 in 7) of all plague cases
in the United States are fatal.
How many
cases of plague occur in the U.S.?
Human plague in the United States has occurred
as mostly scattered cases in rural areas (an average of 10 to 20 persons
each year). Globally, the World Health Organization reports 1,000 to 3,000
cases of plague every year.
Pneumonic
plague is one of several forms of plague. Depending on circumstances,
these forms may occur separately or in combination:
-
Pneumonic plagueoccurs
when Y. pestis infects the lungs. This type of plague can spread
from person to person through the air. Transmission can take place
if someone breathes in aerosolized bacteria, which could happen
in a bioterrorist attack. Pneumonic plague is also spread by breathing
in Y. pestis suspended in respiratory droplets from a person
(or animal) with pneumonic plague. Becoming infected in this
way usually requires direct and close contact with the ill person
or animal. Pneumonic plague may also occur if a person with bubonic
or septicemic plague is untreated and the bacteria spread to the
lungs.
-
Bubonic plagueis the most common
form of plague. This occurs when an infected flea bites a person
or when materials contaminated with Y. pestis enter through a break
in a person’s skin. Patients develop swollen, tender lymph glands
(called buboes) and fever, headache, chills, and weakness. Bubonic
plague does not spread from person to person.
-
Septicemic plagueoccurs
when plague bacteria multiply in the blood. It can be a complication
of pneumonic or bubonic plague or it can occur by itself. When it
occurs alone, it is caused in the same ways as bubonic plague; however,
buboes do not develop. Patients have fever, chills, prostration,
abdominal pain, shock, and bleeding into skin and other organs. Septicemic
plague does not spread from person to person.
|
Is pneumonic plague different from bubonic plague?
Yes. Both are caused by Yersinia pestis
, but they are transmitted differently and their symptoms differ. Pneumonic
plague can be transmitted from person to person; bubonic plague cannot.
Pneumonic plague affects the lungs and is transmitted when a person breathes
in Y. pestis particles in the air. Bubonic plague is transmitted
through the bite of an infected flea or exposure to infected material through
a break in the skin. Symptoms include swollen, tender lymph glands called
buboes. Buboes are not present in pneumonic plague. If bubonic plague is
not treated, however, the bacteria can spread through the bloodstream and
infect the lungs, causing a secondary case of pneumonic plague.
What are
the signs and symptoms of pneumonic plague?
Patients usually have fever, weakness,
and rapidly developing pneumonia with shortness of breath, chest pain,
cough, and sometimes bloody or watery sputum. Nausea, vomiting, and abdominal
pain may also occur. Without early treatment, pneumonic plague usually
leads to respiratory failure, shock, and rapid death.
How do
people
become infected with pneumonic plague?
Pneumonic plague occurs when Yersinia
pestis infects the lungs. Transmission can take place if someone breathes
in Y. pestis particles, which could happen in an aerosol release
during a bioterrorism attack. Pneumonic plague is also transmitted by breathing
in Y. pestis suspended in respiratory droplets from a person (or
animal) with pneumonic plague. Respiratory droplets are spread most readily
by coughing or sneezing. Becoming infected in this way usually requires
direct and close (within 6 feet) contact with the ill person or animal. Pneumonic
plague may also occur if a person with bubonic or septicemic plague is untreated
and the bacteria spread to the lungs.
Does plague
occur
naturally?
Yes. The World Health Organization reports
1,000 to 3,000 cases of plague worldwide every year. An average of 5 to
15 cases occur each year in the western United States. These cases are usually
scattered and occur in rural to semi-rural areas. Most cases are of the bubonic
form of the disease. Naturally occurring pneumonic plague is uncommon, although
small outbreaks do occur. Both types of plague are readily controlled by
standard public health response measures.
Can
a person
exposed to pneumonic plague avoid becoming
sick?
Yes. People who have had close contact
with an infected person can greatly reduce the chance of becoming sick if
they begin treatment within 7 days of their exposure. Treatment consists
of taking antibiotics for at least 7 days.
How
quickly would some one get sick if exposed to plague bacteria through the air?
Someone exposed to Yersinia pestis
through the air—either from an intentional aerosol release or from close
and direct exposure to someone with plague pneumonia—would become ill within
1 to 6 days.
Can pneumonic
plague
be treated?
Yes. To prevent a high risk of death, antibiotics
should be given within 24 hours of the first symptoms. Several types of
antibiotics are effective for curing the disease and for preventing it.
Available oral medications are a tetracycline (such as doxycycline) or
a fluoroquinolone (such as ciprofloxacin). For injection or intravenous
use, streptomycin or gentamicin antibiotics are used. Early in the response
to a bioterrorism attack, these drugs would be tested to determine which
is most effective against the particular weapon that was used.
Would enough medication be available in the event of
a bioterrorism attack involving pneumonic plague?
National and state public health officials
have large supplies of drugs needed in the event of a bioterrorism attack.
These supplies can be sent anywhere in the United States within 12 hours.
What
should some one
do if they suspect they or others have been exposed to plague?
Get immediate medical attention: To prevent
illness, a person who has been exposed to pneumonic plague must receive antibiotic
treatment without delay. If an exposed person becomes ill, antibiotics
must be administered within 24 hours of their first symptoms to reduce the
risk of death. Notify authorities: Immediately notify local or state health
departments so they can begin to investigate and control the problem right
away. If bioterrorism is suspected, the health departments will notify the
CDC, FBI, and other appropriate authorities.
How
can some one
reduce the risk of getting pneumonic plague from another person or giving it to
some one else?
People having direct and close contact
with someone with pneumonic plague should wear tightly fitting disposable
surgical masks. Patients with the disease should be isolated and medically
supervised for at least the first 48 hours of antibiotic treatment. People
who have been exposed to a contagious person can be protected from developing
plague by receiving prompt antibiotic treatment.
How
is plague diagnosed?
The first step is evaluation by a health
worker. If the health worker suspects pneumonic plague, samples of the patient’s
blood, sputum, or lymph node aspirate are sent to a laboratory for testing.
Once the laboratory receives the sample, preliminary results can be ready
in less than two hours. Confirmation will take longer, usually 24 to 48 hours.
How long
can plague bacteria exist in the environment?
Yersinia pestis is easily destroyed by
sunlight and drying. Even so, when released into air, the bacterium will
survive for up to one hour, depending on conditions.
Is a vaccine available to prevent
pneumonic plague?
Currently, no plague vaccine is available
in the United States. Research is in progress, but we are not likely to
have vaccines for several years or more.
How Can People Get More Information
About Pneumonic Plague?
People can contact one of the following:
o Public Response Hotline
(CDC)
§
English (888) 246-2675
§
Espańol (888) 246-2857
§
TTY (866) 874-2646
o Emergency Preparedness and
Response Web site
o E-mail inquiries: cdcresponse@ashastd.org
o Mail inquiries:
Public Inquiry c/o BPRP
Bioterrorism Preparedness and Response Planning
Centers for Disease Control and Prevention
Mailstop C-18
1600 Clifton Road
Atlanta, GA 30333
- Agency for Toxic Substances and Disease
Registry (ATSDR) (1-888-422-8737)
o E-mail inquiries: atsdric@cdc.gov
o Mail inquiries:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road NE, Mailstop E-29
Atlanta, GA 30333
Source: Centers for Disease Control and Prevention
v
Introduction
Plague is a zoonotic infection caused
by Yersinia pestis , a Gram-negative bacillus, which has been the
cause of three great pandemics of human disease in the common era: in the
6th, 14th, and 20th centuries. The naturally occurring disease in humans
is transmitted from rodents and is characterized by the abrupt onset of
high fever, painful local lymphadenopathy draining the exposure site (i.e.,
a bubo, the inflammatory swelling of one or more lymph nodes, usually
in the groin; the confluent mass of nodes, if untreated, may suppurate and
drain pus), and bacteremia. Septicemic plague can sometimes ensue from untreated
bubonic plague or, de novo, after a flea bite. Patients with the bubonic
form of the disease may develop secondary pneumonic plague (also called
plague pneumonia); this complication can lead to human-to-human spread by
the respiratory route and cause primary pneumonic plague, the most severe
and frequently fatal form of the disease.
During the last four millennia, plague
has played a role in many military campaigns. During the Vietnam War, plague
was endemic among the native population, but U.S. soldiers escaped relatively
unaffected. This excellent protection of troops was largely due to our understanding
of the rodent reservoirs and flea vectors of disease, the pathophysiology
of the various clinical forms of plague, the widespread use throughout the
war of a plague vaccine, and prompt treatment of plague victims with effective
antibiotics. Mortality from endemic plague continues at low rates throughout
the world despite the availability of effective antibiotics. People continue
to die of plague, not because the bacilli have become resistant but, most
often, because physicians do not include plague in their differential diagnosis
(in the United States) or because treatment is absent or delayed (in underdeveloped
countries).
To be best prepared to treat soldiers who
are plague victims of endemic or biological agent attack by an enemy, military
physicians must understand the natural mechanisms by which plague spreads
between species, the pathophysiology of disease in fleas and humans, the
minimal diagnostic information necessary to begin treatment with effective
antibiotics, and the proper use and capabilities of the presently available
plague vaccine.
The United States military’s concern with
plague is both as an endemic disease and as a biological warfare threat.
A better understanding of the preventive medicine aspects of the disease
will aid in the prompt diagnosis and effective treatment necessary to survive
an enemy attack of plague.
Key terms in this chapter include enzootic
and epizootic. These refer, respectively, to plague that is
normally present in an animal community at all times but that occurs in
only a small number of animals and in a mildly virulent form, and to widespread
plague infections leading to death within an animal community (i.e., equivalent
to an epidemic in a human population). The death of a rodent pressures
the living fleas to leave that host and seek other mammals, including humans.
Understanding these two simple concepts will help us to understand how and
when humans may be attacked, both in endemic and biological warfare scenarios.
v
History
The biblical book of I Samuel records what
may be the oldest reference to bubonic plague. In approximately 1320 BC,
the Philistines stole the Ark of the Covenant from the Israelites and returned
home. Then, I Samuel continues,
[t]he Lord’s hand was heavy upon the
people of Ashdod and its vicinity; he brought devastation upon them and
afflicted them with tumors. And rats appeared in their land, and death
and destruction were throughout the city... [T]he Lord’s hand was against
that city, throwing it into a great panic. He afflicted the people of
the city, both young and old, with an outbreak of tumors in the groin.
1
After this time, plague became established
in the countries bordering the eastern Mediterranean Sea.
2 In 430 BC, Sparta won the
Peloponnesian War partly because of the plague of Athens.
3 Some scholars believe that
this was the bubonic plague, but others suggest that it may have been due
to other bacterial or viral diseases.
4
The First Pandemic
Procopius gave us the first identifiable
description of epidemic plague in his account of the plague of the Byzantine
empire during the reign of Justinian (AD 541–542),
5 which we now consider to
be the first great pandemic of the common era. As many as 100 million Europeans,
including 40% of the population of Constantinople, died during this epidemic.
6,7 Repeated, smaller epidemics
followed this plague. 8
The Black Death (The Second
Pandemic)
The second plague pandemic, known as
the Black Death, thrust this dread disease into the collective memory of
western civilization. 8
Plague bacilli in fleas on the fur of marmots (a rodent of the genus
Marmota) probably entered Europe via the trans- Asian silk road during
the early 14th century. When bales of these furs were opened in Astrakhan
and Saray, hungry fleas jumped from the fur seeking the first available
blood meal, often a human leg.
8–10 In 1346, plague arrived
in Caffa (modern Feodosiya, Ukraine), on the Black Sea. The large rat population
there helped spread the disease as they stowed away on ships bound for major
European ports such as Pera, a suburb of Constantinople, and Messina, in
Sicily. By 1348, plague had already entered Britain at Weymouth.
5
The Black Death took the lives of 24
million people between the years 1346 and 1352 and claimed perhaps another
20 million by the end of the 14th century.6 However, the plague continued
through 1720, with a final foray into Marseilles. Thirty percent to 60% of
the populations of major cities such as Genoa, Milan, Padua, Lyons, and Venice
succumbed during the 15th to the 18th centuries.
10
Physicians of the time offered no effective
treatment because they did not understand the epidemiology of plague. At
the highly regarded University of Paris, physicians theorized that a conjunction
of the planets Saturn, Mars, and Jupiter at 1:00 PM on March 20, 1345, caused
a corruption of the surrounding atmosphere that led to the plague.
6 They recommended a simple
diet; avoidance of excessive sleep, exercise, and emotion; regular enemas;
and abstinence from sexual intercourse.
11 While some people killed
cats and dogs, thinking them to be carriers of disease, no one ever thought
to kill the rats .6
Christians blamed the disease on Muslims, Muslims on Christians, and both
Christians and Muslims on Jews or on witches.
8
In 1666, a church rector in Eyam, Derbyshire,
England, persuaded the whole community to quarantine itself when plague
erupted there. This was the worst possible solution, since the people then
stayed in close proximity to the infected rats. The city experienced virtually
a 100% attack rate with 72% mortality (the average mortality for the Black
Death was consistently 70%–80%).
8,12
Accurate clinical descriptions of the
Black Death were written by contemporary observers such as Boccaccio, who
wrote in his Decameron:
The symptoms were not the same as in
the East, where a gush of blood from the nose was a plain sign of inevitable
death, but it began both in men and women with certain swellings [buboes]
in the groin or under the armpit. They grew to the size of a small apple
or an egg, more or less, and were vulgarly called tumours. In a short space
of time these tumours spread from the two parts named all over the body.
Soon after this, the symptoms changed and black or purple spots appeared
on the arms or thighs or any other part of the body, sometimes a few large
ones, sometimes many little ones.
13(p646)
Guy de Chauliac in Avignon added his
own commentary, describing pneumonic plague and the axillary and groin
forms of bubonic plague:
Doctors dared not visit the sick
for fear of infection; or, when they did, they helped little and gained
nothing. 14(p646)
The disease is three fold in its
infection; that is to say, firstly, men suffer in their lungs and breathing
and whoever have these corrupted, or even slightly attacked,
cannot by any means escape nor live beyond two days...and it is found
that all those who have died thus suddenly have had their lungs infected
and have spat blood. There is another form of the sickness, however, at
present running its course concurrently with the first; that is, certain
aposthumes appear under both arms and by these also people quickly die.
A third form of the disease —like the two former, running its course at
the same time with them—is that from which people of both sexes suffer from
aposthumes in the groin. This is likewise quickly fatal.
Some writers described bizarre neurological
disorders, which gave rise to the term "Dance of Death," followed by anxiety
and terror, resignation, blackening of the skin, and death. The sick gave
off a terrible stench: "Their sweat, excrement, spittle, breath, [were]
so foetid as to be overpowering"[; in addition, their urine was] "turbid,
thick, black, or red." 6(p70)
The second great pandemic slowly
died out in Europe by 1720. Many reasons, including the following, have
been suggested to explain its decline:
-
The oriental rat flea, Xenopsylla cheopis, the main vector
of the plague bacillus, could no longer exist in the cool European climate.
5
-
The black rat, Rattus rattus, was replaced by the brown rat,
Rattus norvegicus, which was less likely to live in
close proximity to man.5
-
A new and less virulent species of Y pestis, or a related
Yersinia species such as Y pseudotuberculosis,
may have developed, causing natural immunization of infected rats and
humans. 8
-
The European population was generally iron deficient, and iron is
an essential factor for the bacterium’s virulence.
12
-
Flea density on humans decreased as the use of soap became more widespread.
5
The Third Pandemic
The third, or modern, plague pandemic
arose in 1894 in China and spread throughout the world via modern transportation.
12,16 It was also in 1894
that Alexandre J. E. Yersin discovered that Yersinia pestis satisfied
Koch’s postulates for bubonic plague.
17 The reservoir of plague
bacilli in the fleas of the Siberian marmot was likely responsible for the
Manchurian pneumonic plague epidemic of 1910– 1911, which caused 50,000 deaths.2
The modern pandemic arrived in Bombay in 1898, and during the next 50 years,
more than 13 million Indians died of plague.
2,18
The disease officially arrived in
the United States in March 1900, when the lifeless body of a Chinese laborer
was discovered in a hotel basement in San Francisco, California
19; the disease appeared in
New York City and Washington state the same year.
20 New Orleans, Louisiana,
was infected in 1924 and 1926.
20 Rodents throughout the
western United States were probably infected from the San Francisco focus,
leading to more infected rodents in the western United States than existed
in Europe at the time of the Black Death.
12 Therefore, human plague
was initially a result of urban rat epizootics until 1925. After general
rat control and hygiene measures were instituted in various port cities,
urban plague vanished—only to spread into rural areas, where virtually all
cases in the United States have been acquired since 1925.
21
vPlague as a Biological Warfare Agent
The first attempt at what we now
call "biological warfare" is purported to have occurred at the Crimean
port city of Caffa on the Black Sea during the years 1346–1347.
2,6 During the conflict between
Christian Genoese sailors and Muslim Tatars, the Tatar army was struck with
plague. The Tatar leader catapulted corpses of Tatar plague victims at the
Genoese sailors. The Genoese became infected with plague and fled to Italy.
However, the disease was most likely spread by the local population of infected
rats, not by the corpses, since an infected flea leaves its host as soon
as the corpse cools. 6
The 20th-century use of plague as
a potential biological warfare weapon is the immediate concern of this
chapter. Medical officers need to keep this use of plague in mind, particularly
when the disease appears in an unlikely setting.
World War
II
During World War II, the Japanese
army established a secret biological warfare research unit (Unit 731) in
Manchuria, where epidemics of pneumonic plague had occurred in 1910–1911,
1920–1921, and 1927, and a cholera epidemic had spread in 1919. General Shiro
Ishii, the physician leader of Unit 731, was fascinated by plague because
it could create casualties out of proportion to the number of bacteria disseminated,
the most dangerous strains could be used to make a very dangerous weapon,
and its origins could be concealed to appear as a natural occurrence. Early
experiments, however, demonstrated that dropping bacteria out of aerial
bombs had little effect because air pressure and high temperatures that
were created by the exploding bombs killed nearly 100% of the bacteria.
30
One of Ishii’s greatest achievements
was his use of the human flea, Pulex irritans, as a stratagem to simultaneously
protect the bacteria and target humans. This flea is resistant to air drag,
naturally targets humans, and could also infect a local rat population to
prolong an epidemic. Infected fleas may regurgitate up to 24,000 organisms
in a single feeding. Spraying fleas out of compressed-air containers was not
successful since aircraft had to fly too low for safety. High flying meant
too much dispersion. Clay bombs solved these problems and resulted in an
80% survival rate of fleas.30
The Japanese apparently used plague
as a biological warfare agent in China several times during World War II.
At 0500 hours on a November morning in 1941, a lone Japanese plane made
three low passes over the business center of Changteh, a city in the Hunan
province. Although no bombs were dropped, a strange mixture of wheat and
rice grains, pieces of paper, cotton wadding, and other unidentified particles
were. Within 2 weeks, individuals in Changteh started dying of plague. This
miniepidemic was thought to be of human origin for the following reasons
30:
-
Changteh and the whole surrounding area of China had never been afflicted
by plague.
-
Plague usually spreads with rice (because rats infest the grain) along
shipping routes, but the nearest epidemic center was 2,000 km away by
land or river. Changteh exported, not imported, rice. No individual who
contracted plague had recently traveled outside the city.
-
All reported instances of human plague occurred in the area of the
city where the strange particles were dropped.
-
No evidence of excessive rat mortality occurred until 2 months after
the people began dying.
-
The first six human cases occurred within 15 days of the aerial incident.
Applying the concepts implicit in
these five points will help medical officers differentiate endemic plague
from plague used as a biological warfare agent. In fact, these concepts are
important in making a diagnosis of most forms of biological warfare. In
another incident, on October 4, 1940, a Japanese plane dropped rice and wheat
grains mixed with fleas over the city of Chuhsien, in Chekiang province.
A month later, bubonic plague appeared for the first time there, in the area
where the particles had been dropped. There were 21 plague deaths in 24 days.
Again, on October 27, 1940, a Japanese plane was seen releasing similar particles
over the city of Ningpo, in Chekiang province. Two days later, bubonic plague
occurred for the first time in that city, producing 99 deaths in 34 days.
No epizootic or excessive mortality was found in the rat population.
30
Since World
War II
An article
31 published in the popular
press in 1993 stated that in the 1970s and 1980s the Soviet Union created
lethal diseases that defied cures. This included a genetically engineered,
dry, antibiotic resistant form of plague. In this article, a defecting
Soviet microbiologist was quoted as saying that producing this form of
plague had been a top priority of the Soviets in the 5-year plan that started
in 1984.
During the Korean War, allied forces
were accused of dropping on North Korea insects that were capable of spreading
plague, typhus, malaria, Japanese B encephalitis, and other diseases. No
evidence exists to support such claims.
32
vEpidemiology
| During
the modern pandemic, W. G. Liston, a member of the Indian Plague
Commission (1898-1914), made the association of plague with rats
and incriminated the rat flea as a vector.2 Subsequently, more than
200 species of animals and 80 species of fleas have been implicated
in maintaining Y pestis endemic foci throughout
the world. 21
Throughout history, the oriental
rat flea ( Xenopsylla cheopis) has been largely responsible
for spreading bubonic plague.5 After the flea ingests a blood meal
on a bacteremic animal, bacilli can multiply and eventually block
the flea’s foregut, or proventriculus, with a fibrinoid mass of
bacteria (Figure 3). 2
When an infected flea with a blocked foregut attempts to feed again,
it regurgitates clotted blood and bacteria into the victim’s bloodstream,
and so passes the infection on to the next mammal–whether rat or
human. As many as 24,000 organisms may be inoculated into the mammalian
host. 2
This flea desiccates rapidly in very hot and dry weather when away
from its hosts, but flourishes at humidity just above 65% and temperatures
between 20°C and 26°C,
2 and can survive
6 months without a feeding.21
|
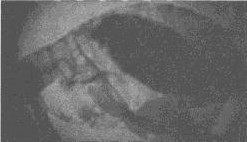
Figure 1. The oriental
rat flea ( Xenopsylla cheopis) has historically been most
responsible for the spread of plague to humans. This flea has a
blocked proventriculus, equivalent to a human’s gastroesophageal
region. In nature, this flea would develop a avenous hunger because
of its inability to digest the fibrinoid mass of blood and bacteria.
The ensuing biting of the nearest mammal will clear the proventriculus
through regurgitation of thousands of bacteria into the bite wound,
thereby inoculating the mammal with the plague bacillus. Photomicrograph:
Courtesy of Ken Gage, Ph.D., Centers for Disease Control and Prevention,
Fort Collins, Colo.
|
Although the largest outbreaks of
plague have been associated with X cheopis, all fleas should be considered
dangerous in plague endemic areas.
2 During the Black Death,
the human flea, Pulex irritans, may have aided in human-to-human
spread of plague; and during other epidemics, bedbugs (Cimex lectularius
), lice, and flies have been found to contain Y pestis.
5 The presence of plague bacilli
in these latter insects is associated with ingestion of contaminated blood
from plague victims, however, and plays little or no role as a vector for
the disease. The most important vector of human plague in the United States
is Diamanus montanus, the most common flea on rock squirrels and
California ground squirrels.
21
|
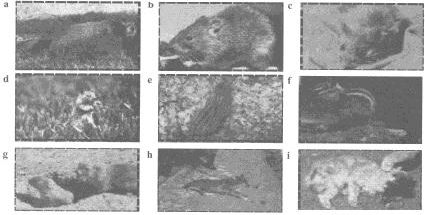
|
Figure 2. Known mammalian reservoirs of plague in the United
States (noninclusive). The common North American marmot (a) and
the brown rat (Rattus norvegicus) (b), which has largely
replaced the black rat, are considered to be reservoirs of plague
(i.e., hosts to infected fleas). Other reservoirs of plague during
enzootics are thought to include the deer mouse (c), the California
ground squirrel (d), and the 13-lined ground squirrel (e). Other
infective mammals that can spread plague to humans include the chipmunk
(f), prairie dogs (g), and the coyote (h). Domestic and nondomestic
cats are also reservoirs of plague. This cat (i), which died of
pneumonic plague, demonstrates a necrotic head. Photographs a, h:
Courtesy of Denver Zoological Society, Denver, Colo. Photographs b-g,
i: Courtesy of Centers for Disease Control and Prevention, Fort
Collins, Colo. |
Throughout history, the black rat,
Rattus rattus , has been most responsible worldwide for the
persistence and spread of plague in urban epidemics. R rattus is
a nocturnal, climbing animal that does not burrow. Instead, it nests overhead
and lives in close proximity to humans.
5 In the United Kingdom and
much of Europe, the brown rat, R norvegicus, has replaced R rattus
as the dominant city rat.
44 Unlike R rattus, R norvegicus
is essentially a burrowing animal that lives under farm buildings
and in ditches. However, R norvegicus may be involved in both
rural and urban outbreaks of plague.
5
Most carnivores, except cats, are
resistant to plague infection, but animals such as domestic dogs, all rodents,
and even burrowing owls may mechanically transmit fleas. Mammals that are
partially resistant to plague infection serve as continuous reservoirs of
plague. In the United States, deer mice (Peromyscus species) and
ground squirrels (Spermophilus species) are thought to serve
as the main reservoirs. Some susceptible mammals are only occasionally infected:
chipmunks, tree squirrels, cottontail rabbits, and domestic cats (Figure
2).
Highly susceptible animals amplify
both fleas and bacilli. Such epizootics occur in chipmunks, ground squirrels,
and wood rats, but especially in prairie dogs, rock squirrels (Spermophilus
variegatus ), and California ground squirrels (Spermophilus beechyi
). Although prairie dog fleas rarely bite humans, the infectious rodents
can transmit plague to humans via direct contact (e.g., handling a live
or dead animal; stumbling into a nest while walking; or dissecting specimens
[primarily laboratory personnel]). Rock squirrels and California ground
squirrels both infect humans via direct contact and fleas.
5,21,45,46
Many mammals in the United States
harbor plague (Exhibit 1). Knowledge of this widespread harborage is important,
because certain mammal–flea complexes found in the United States are dangerous:
they contain both a susceptible mammal and a flea known to bite humans. These
pairings include the following:
21
-
the rock squirrel (S variegatus) or California ground squirrel
(S beechyi) and the fleas Diamanus montanus or Hoplopsyllus
anomalus;
-
the prairie dog (Cynomys species) and the flea Opisochrostis
hirsutus; and
-
Richardson’s ground squirrel (Spermophilus richardsoni) or
the golden-mantled ground squirrel (S lateralis) and the fleas
Oropsylla labis, O idahoensis, or Thrassus
bacchi .
|
Exhibit 1: Mammals Known to Harbor Plague in the United States.
Carnivores
Black bears, cats (including bobcats and mountain lions), coyotes,
dogs, foxes, martens, raccoons, skunks, weasels, wolverines, wolves
Rodents
Chipmunks, gophers, marmots, mice, prairie dogs, rats, squirrels,
voles
Lagomorphs
Hares, rabbits
Hooved Stock
Pigs, mule deer, pronghorn antelope
Adapted from Harrison
FJ. Prevention and Control of Plague. Aurora, Colo: US Army
Center for Health Promotion and Preventive Medicine, Fitzsimons
Army Medical Center; September 1995: 25–28. Technical Guide 103.
|
Plague exists in one of two
states in nature, enzootic or epizootic. An enzootic is the state of a stable
rodent–flea infection cycle in a relatively resistant host population, without
excessive rodent mortality. Importantly for humans, when the disease is
in an enzootic state, the fleas have no need to seek less desirable hosts—such
as ourselves. During an epizootic, on the other hand, plague bacilli have
been introduced into moderately or highly susceptible mammals. High mortality
occurs, most conspicuously in larger colonial rodents such as prairie dogs.
47
Man is an accidental host
in the plague cycle and is not necessary for the persistence of the organism
in nature. Humans usually acquire plague from
-
fleas whose usual host is another mammal (e.g., from flea bites, flea
feces inoculated into skin with bites, and by directly biting the fleas
[during the grooming behavior practiced in some cultures]);
-
fleas whose usual host is a human;
-
infected animals (e.g., from aerosols, draining abscesses, eating
infected tissue, and handling infected pelts); and
-
other humans, via aerosol or direct contact with infected body substances.
The greatest risk to humans
occurs when large concentrations of people live under unsanitary conditions
in close proximity to large commensal or wild rodent populations that are
infested with fleas that bite both humans and rodents.
2
Human-to-human transmission
of plague can occur from patients with pulmonary infection. However, understanding
of the epidemiology of pneumonic plague is incomplete. Most epidemics have
occurred in cool climates with moderate humidity and close contact between
susceptible individuals. Outbreaks of pneumonic plague have been rare tropical
climates even during epidemics of bubonic disease. Respiratory transmission
may occur more efficiently via larger droplets or fomites rather than via
small-particle aerosols.48
vClinical Manifestations
In the United States, most
patients (85%-90%) with human plague present clinically with the bubonic
form, 10% to 15% with the primary septicemic form, and 1% with the pneumonic
form. Secondary septicemic plague occurs in 23% of patients who present
with bubonic plague, and secondary pneumonic plague occurs in 9%.46 If
Y pestis were used as a biological warfare agent,
the clinical manifestations of plague would be (a) epidemic pneumonia with
blood-tinged sputum if aerosolized bacteria were used or (b) bubonic or
septicemic plague, or both, if fleas were used as carriers.
Bubonic Plague
Buboes manifest after a 1-
to 8-day incubation period, with the regular onset of symptoms of sudden
fever, chills, and headache often followed several hours later by nausea
and vomiting. Presenting symptoms include prostration or severe malaise
(75%), headache (20%-85%), vomiting (25%-49%), chills (40%), altered mentation
(26%-38%), cough (25%), abdominal pain (18%), and chest pain (13%).2 Six
to 8 hours after onset of symptoms, buboes, heralded by severe pain, occur
in the groin (90%, with femoral more frequent than inguinal), axillary,
or cervical lymph nodes–depending on the site of bacterial inoculation (Figure
3). Buboes become visible within 24 hours; they are so intensely painful
that even nearly comatose patients will attempt to shield them from trauma
and will abduct their extremities to decrease pressure. Other manifestations
of bubonic plague include bladder distention, apathy, confusion, fright,
anxiety, oliguria, and anuria. Tachycardia, hypotension, leukocytosis, and
fever are frequently encountered. Untreated, septicemia will develop in
2 to 6 days.55
Approximately 5% to 15% of bubonic plague patients will develop secondary
pneumonic plague and, as a result, the potential for airborne transmission.
56
Septicemic
Plague
Septicemic plague may occur
primarily, or secondarily as a complication of hematogenous dissemination
of bubonic plague. Presenting signs and symptoms of primary septicemic plague
are essentially the same as those for any Gram-negative septicemia: fever,
chills, nausea, vomiting, and diarrhea. Later, purpura (Figure 4), disseminated
intravascular coagulation (DIC), and acral cyanosis and necrosis (Figure
5) may be seen.
In New Mexico between 1980
and 1984, plague was suspected in 69% of patients who had bubonic plague,
but in only 17% of patients who had the septicemic form. The mortality was
33.3% for septicemic plague versus 11.5% for bubonic, thus highlighting
the difficulty of diagnosing septicemic plague. Diagnosis of septicemic
plague took longer (5 vs 4 d) after onset, although patients sought physicians
earlier (1.7 vs. 2.1 d) and were hospitalized sooner (5.3 vs 6.0 d) than
patients with bubonic plague. The only symptom present significantly more
frequently in septicemic than in bubonic plague was abdominal pain (40% vs
< 10%), probably due to hepatosplenomegaly.
57
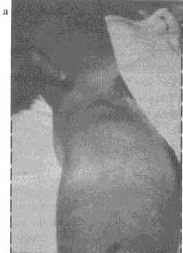
|
|
|
Figure 3. A femoral
bubo (a), the most common site of an erythematous, tender,
swollen, lymph node in patients with plague. This painful lesion
may be aspirated in a sterile fashion to relieve pain and pressure;
it should not be incised and drained. The next most common lymph
node regions involved are the inguinal, axillary (
b ), and cervical
areas. Bubo location is a function of the region of the body in
which an infected flea inoculates the plague bacilli. Photographs:
Courtesy of Ken Gage, Ph.D., Centers for Disease Control and Prevention,
Fort Collins, Colo. |
|
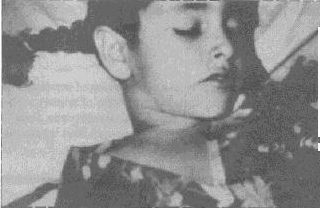
Figure 4. Purpuric
lesions can be seen on the upper chest of this girl with plague. The
bandage on her neck indicates that a bubo has been aspirated.
Photograph: Courtesy Ken Gage, Ph.D., Centers of Disease Control
and Prevention, Fort Collins, Colo. |
|
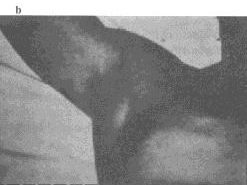
|
|
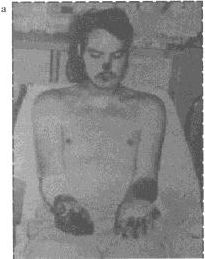
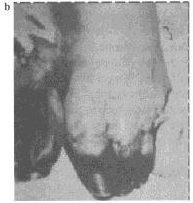
Figure 5. This patient is recovering from
bubonic plague that disseminated to the blood (septicemic form)
and the lungs (pneumonic form). Note the dressing over the tracheostomy
site. At one point, the patient’s entire body was purpuric. Note
the acral necrosis of (a) the patient’s nose and fingers
and (b ) the toes. Photographs: Courtesy Ken Gage, Ph.D.,
Centers of Disease Control and Prevention, Fort Collins, Colo. |
|
|
|
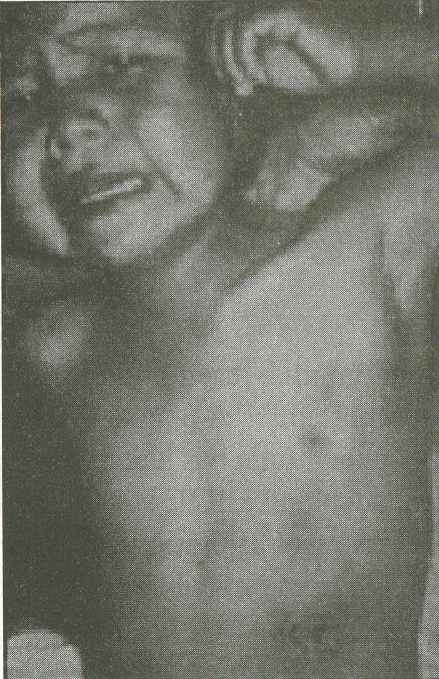
Figure 7. This child has left axillary bubonic plague. The erythematous,
eroded, crusting, necrotic ulcer on the child’s left upper quadrant
is located at the presumed primary inoculation site. Photograph:
Courtesy of Ken Gage, Ph.D., Centers for Disease Control and Prevention,
Fort Collins, Colo. |
Figure 6. This chest roentgenogram shows right middle and lower-lobe
involvement in a patient with pneumonic plague. Photograph: Courtesy
Ken Gage, Ph.D., Centers for Disease Control and Prevention, Fort
Collins, Colo. |
The risk of developing
septicemic plague is higher for individuals older than
40 years of age, although the risk of dying from septicemic
plague is higher for those younger than 30 years. This difference is most
likely due to older undiagnosed patients being treated empirically with
antibiotics that kill Y pestis , and younger undiagnosed patients
being treated with antibiotics (such as penicillin) that do not affect
Y pestis. Earlier diagnosis and appropriate therapy, not newer antibiotics,
will have the greatest effect on reducing mortality from septicemic plague.
57
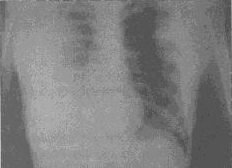
Pneumonic Plague
Pneumonic plague may occur
primarily, from inhalation of aerosols, or secondarily, from hematogenous
dissemination. Patients typically have a productive cough with blood-tinged
sputum within 24 hours after onset of symptoms.
2 The findings on chest roentgenography
may be variable, but bilateral alveolar infiltrates appear to be the most
common finding in pneumonic plague (Figure 6).
58,59
Plague
Meningitis
Plague meningitis is seen
in 6% to 7% of cases. The condition manifests itself most often in children
after 9 to 14 days of ineffective treatment. Symptoms are similar to those
of other forms of acute bacterial meningitis.
60
Pharyngeal
Plague
Asymptomatic pharyngeal carriage
has been reported to occur in contacts of plague patients.
53,54
Rarely, pharyngitis–resembling
tonsillitis and associated with cervical lymphadenopathy–has been reported.
17,55 A plague syndrome of
cervical buboes, peritonsillar abscesses, and fulminant pneumonia has also
been reported to occur among Indians of Ecuador, who are known to catch
and kill fleas and lice with their teeth. It is thought, although not proven,
that endobronchial aspiration from peritonsillar abscesses leads to fulminant
pneumonia. A similar syndrome may have occurred in Vietnam.
55
Cutaneous
Manifestations
Approximately 4% to 10% of
plague patients are said to have an ulcer or pustule at the inoculation
site (Figure 6). 59,61
The flea typically bites the lower extremities; therefore, femoral and
inguinal buboes are the most common. Infection arising from the skinning
of infected animals typically produces axillary buboes. Buboes may point
and drain spontaneously or, rarely, they may require incision and drainage
because of pronounced necrosis. Petechiae and ecchymoses may occur during
hematogenous spread to such an extent that the signs mimic severe meningococcemia,
and the microscopic lesions are almost indistinguishable. The pathogenesis
of these lesions is probably that of a generalized Shwartzman reaction (DIC
secondary to the Y pestis endotoxin). Purpura and acral gangrene
may also be due to the activities of the plasminogen activator/coagulase
enzyme, and prognosis is poor when these signs occur.
2,62 Patients in the terminal
stages of pneumonic and septicemic plague often develop large ecchymoses
on the back. Lesions like these are likely to have given rise to the medieval
epithet "the Black Death."
Ecthyma gangrenosum has been
reported in several patients.53,62
The only case cultured grew Y pestis, which suggests that the skin
lesions were the result of septicemic seeding of the organism.
62
vDiagnosis
Signs and Symptoms
A patient with a typical
presentation of bubonic plague (e.g., with a painful bubo in the setting
of fever, prostration, and possible exposure to rodents or fleas in an endemic
area) should readily suggest the diagnosis of plague. However, if the medical
officer is not familiar with the disease or if the patient presents in a
nonendemic area or without a bubo, then the diagnosis can be most difficult.
When a bubo is present, the differential diagnosis should include tularemia,
cat scratch disease, lymphogranuloma venereum, chancroid, tuberculosis, streptococcal
adenitis, and scrub typhus (Figure 8). In both tularemia and cat scratch
disease, the inoculation site will usually be more evident and the patient
will usually not be septic. In chancroid and scrofula, the patient has less
local pain, the course is more indolent, and there is no sepsis. Patients
with chancroid and lymphogranuloma venereum will have a recent history of
sexual contact and genital lesions. Those with the latter disease may be
as sick as patients with plague. Streptococcal adenitis may be difficult
to distinguish initially, but the patient is usually not septic, and the
node is more tender when plague is present.
The implications of the absence
of a bubo were clearly demonstrated in a review of 27 cases of plague seen
in New Mexico.59 There were no deaths among 10 patients with typical bubonic
plague. However, 3 of 5 patients died who presented with an upper respiratory
infection syndrome of fever, sore throat, and headache. Similarly, 3 of
5 patients died who presented with fever, chills, and anorexia. The other
7 patients presented with nonspecific gastrointestinal and urinary tract
symptoms without a bubo. Thus, other causes of lymphadenitis, upper respiratory
tract infection, gastrointestinal disease including appendicitis, and nonspecific
febrile illnesses, must all be considered.
The differential diagnosis
of septicemic plague also includes meningococcemia, Gram-negative sepsis,
and the rickettsioses. The patient with pneumonic plague who presents with
systemic toxicity, a productive cough, and bloody sputum suggests a large
differential diagnosis. However, demonstration of Gram-negative rods in the
sputum should readily suggest the correct diagnosis, because Y pestis
is perhaps the only Gram-negative bacterium that can cause
extensive, fulminant pneumonia with bloody sputum in an otherwise healthy,
immunocompetent host.
Laboratory
Confirmation
In patients with lymphadenopathy,
a bubo aspirate should be obtained by inserting a 20-gauge needle attached
to a 10-mL syringe containing 1 mL of sterile saline. Saline is injected
and withdrawn several times until it is tinged with blood. Repeated, sterile
bubo aspiration may also be done
to decompress buboes and relieve pain. Drops of the aspirate should be air-dried
on a slide for one of the following stains: Gram’s, Wright-Giemsa, or Wayson’s.
If available, a direct fluorescent antibody (DFA) stain of bubo aspirate
for the presence of Y pestis capsular antigen should be performed;
a positive DFA result is more specific for Y pestis than are the
other listed stains (Figure 10).
63,64
Both Wright-Giemsa stain
and DFA stain for Y pestis should also be performed
on peripheral blood smears and sputum specimens, when applicable. Although
a bipolar, safety-pin staining morphology has been reported to be specific
for Y pestis , it is not. Other bacteria such as Pasteurella
species, Escherichia coli, Klebsiella species, and diplococci (
Streptococcus) may also exhibit this morphology. None of the listed
stains is better than any other for demonstrating the bipolar, safety-pin
morphology. In fact, even Y pestis will
sometimes not exhibit this morphology.
64
Cultures of blood, bubo aspirate,
sputum, and cerebrospinal fluid (if indicated) should be performed. Tiny,
1- to 3-mm "beaten-copper" colonies will appear on blood agar by 48 hours,
but it is important to remember that cultures may be negative at 24 hours.
In a recent study, 24 (96%) of 25 blood cultures of patients with bubonic
plague were positive on standard supplemented peptone broth.
59
Complete blood counts often
reveal leukocytosis with a left shift. Leukemoid reactions with up to 100,000
white blood cells per microliter may be seen, especially in children. Platelet
counts may be normal or low, and partial thromboplastin times are often
increased. When DIC is present, fibrin degradation products will be elevated.
Because of liver involvement, alanine aminotransferase, aspartate aminotransferase,
and bilirubin levels are often increased.
Serologic assays measuring
the immune response to plague infection are mainly of value retrospectively,
since patients present clinically before they develop a significant antibody
response. Enzymelinked immunosorbent assay (ELISA) tests and the older,
less-sensitive passive hemagglutination assay (PHA) both measure antibodies
to the fraction 1 capsule. They are available from the Centers for Disease
Control and Prevention, Fort Collins, Colorado, and the U.S. Army Medical
Research Institute of Infectious Diseases, Fort Detrick, Frederick, Maryland.
Rapid diagnostic tests are available on an investigative basis.
An immunological assay to
detect circulating fraction 1 antigen in the serum of acutely infected patients
can detect levels as low as 0.4 ng/mL serum.
65 During plague infection,
fraction 1 antigenemia may reach levels of 4 to 8 µg/mL serum. During a
plague outbreak in Namibia, 38 cases of plague were confirmed: 50% by culture,
34% by antibody response, and 16% by antigenemia.
66 Because fraction 1 antigen
and antibody do not occur simultaneously in serum, and because neither may
be present early in infection, titers for both should be performed on several
sequential blood specimens.
a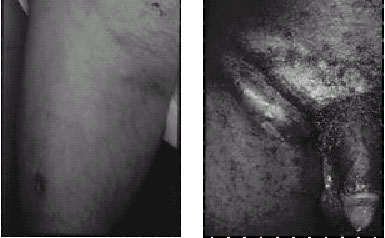 c
c |
b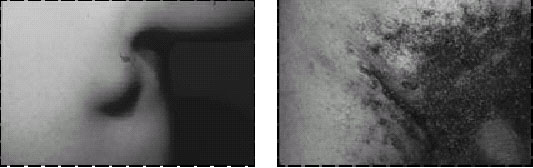 d
d |
|
Figure 9. (a) Small femoral bubo and presumed inoculation site
(on the inferior thigh) in a patient with tularemia. This Gram-negative
bacterial infection (with Francisella tularensis) may closely mimic
bubonic plague and is successfully treated with the same antibiotics.
(b) Axillary bubo seen in child with cat scratch disease. (c) Greenblatt’s
sign of ipsilateral femoral and inguinal buboes with intervening
depression over the inguinal ligament, seen in a patient with lymphogranuloma
venereum caused by Chlamydia trachomatis. (d) Large inguinal bubo
seen in a patient with chancroid caused by Haemophilusducreyi
. Photographs: Courtesy of Dermatology Service, Fitzsimons Army
Medical Center, Aurora, Colo. |
|
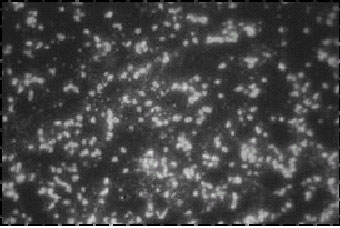
Figure 10. These
Yersinia pestis fluorescent cells are from infected
mouse spleen. Notice how the outlines of the coccobacilli "light
up" in this direct fluorescent antibody (DFA) test. The DFA test
is specific and therefore better than the other stains discussed
in this chapter (original magnification x 1,000). Photograph: Courtesy
of M. C. Chu, Centers for Disease Control and Prevention, Fort Collins,
Colo. |
A polymerase chain reaction
(PCR) test, using primers for the plasminogen activator gene, can detect
as few as 10 Y pestis organisms, even in the presence
of flea tissue. This test may be useful in surveillance of rats and could
be adapted to aid in the diagnosis of human infection.
67
v
Treatment
Isolation
All patients with plague
should be isolated for the first 48 hours after the initiation of treatment.
Special care must be taken in handling blood and bubo discharge. If pneumonic
plague is present, then strict, rigidly enforced respiratory isolation procedures
must be followed, including the use of gowns, gloves, and eye protection.
Patients with pneumonia must be isolated until they have completed at least
4 days of antibiotic therapy. If patients have no pneumonia or draining lesions
at 48 hours, they may be taken out of strict isolation.
Antibiotics
Since 1948, streptomycin
has remained the treatment of choice for bubonic, septicemic, and pneumonic
plague. It should be given intramuscularly in a dose of 30 mg/kg/d in two
divided doses. In cases of suspected meningitis or in patients who are hemodynamically
unstable, intravenous chloramphenicol (50–75 mg/kg/d in four divided doses)
should be added. Gentamicin has had much less clinical usage but can be
used as an alternative to streptomycin or given together with chloramphenicol.
Treatment should be continued for a minimum of 10 days or 3 to 4 days after
clinical recovery. If clinically indicated, oral tetracycline can be used
to complete a 10-day course of treatment after at least 5 days of systemic
therapy. In patients with very mild bubonic plague who are not septic, tetracycline
can be used orally at a dose of 2 g/d in 4 divided doses for 10 days. Doxycycline
should be an acceptable alternative, although there are no published data
on its efficacy in humans. Doxycycline, ofloxacin, and ceftriaxone have all
been shown to be effective in experimental animal models of septicemic plague.
68
In pregnant women, streptomycin
or gentamicin should be used unless chloramphenicol is specifically indicated.
Streptomycin is also the treatment of choice in newborns.
If treated with antibiotics,
buboes typically recede in 10 to 14 days and do not require drainage. Patients
are unlikely to survive primary pneumonic plague if antibiotic therapy is
not initiated within 18 hours of the onset of symptoms. Without treatment,
mortality is 60% for bubonic plague and 100% for the pneumonic and septicemic
forms. 53
vPrevention
All plague-control measures
must include insecticide use, public health education, and reduction of rodent
populations with chemicals such as cholecalciferol.
2,25 Fleas must always
be targeted before rodents, because killing rodents may
release massive amounts of infected fleas.
56 Use of insecticides in
rodent areas is effective because rodents pick up dust on their feet and
carry it back to their nests, where they distribute it over their bodies
via constant preening. 2
Plague must be reported to the World Health Organization as an internationally
quarantinable disease for which travelers may be detained up to 6 days.
Post exposure
Prophylaxis
Not only contacts of patients
with pneumonic plague but also individuals who have been exposed to aerosols
(e.g., in a biological warfare attack) should be treated with tetracycline
15 to 30 mg/kg/d (1–2 g/d) administered in four divided doses for 7 days.
Doxycycline 100 mg administered twice daily is probably an effective alternative
if tetracycline is not available. Pregnant women and children under 8 years
of age should receive trimethoprim/ sulfamethoxazole (40 mg sulfa/kg/d)
administered orally in two divided doses for 7 days.
Hospital personnel who are
observing recommended isolation procedures do not require prophylactic therapy,
nor do contacts of patients with bubonic plague. However, people who were
in the same environment and who were potentially exposed to the same source
of infection as the contact case should be given prophylactic antibiotics.
In addition, previously vaccinated individuals should receive prophylactic
antibiotics if they have been exposed to a plague aerosol.
Immunization
The first plague vaccine,
consisting of killed whole cells, was developed by Russian physician Waldemar
M. W. Haffkine, working in India in 1897. In 1942, Karl F. Meyer, D.V.M.,
began developing an immunogenic and less-reactogenic vaccine for the U.S.
Army from an agar-grown, formalin-killed, suspension of virulent plague bacilli.
With minor modifications, this is the same procedure used to prepare the
licensed vaccine we have available today. Live-attenuated vaccines have
been unsuccessful, since they are much more reactogenic than the present
killed vaccine. 23
Only individuals at high
risk for plague should be immunized—such as military troops and other field
personnel working in plague endemic areas in which exposure to rats and fleas
cannot be controlled. Laboratory personnel working with Y pestis
, people who reside in enzootic or epidemic plague areas, and those whose
vocations bring them into regular contact with wild animals, particularly
rodents and rabbits, should also be vaccinated.
69
The dose schedule for adults
is 1.0 mL initially, with 0.2 mL at 1 to 3 months, followed by a third dose
5 to 6 months later. Booster doses of 0.2 mL are given every 6 months for
1.5 years, and then every 1 to 2 years thereafter if risk for exposure continues.
If an accelerated schedule is essential, then 0.5 mL at 0, 7, and 14 days
has been recommended, although no supporting data exist.
69
Approximately 92% to 93%
of vaccinees will produce antibody titers after the initial series of three
injections. 69-71
Local side effects include erythema, soreness, or swelling, in any combination,
in 11% of vaccinees and 6% of injections. Systemic side effects include
headache, malaise, and myalgias in 4% of vaccinees and 1% of injections.
Rarely, sterile abscesses, necrotic lesions, or anaphylaxis may occur.
72
Data from animal and human
investigations suggest that the killed plague vaccine is effective for preventing
or ameliorating bubonic but not pneumonic plague.
50,51,73-75 A recombinant
vaccine candidate that protects laboratory animals from inhalational challenge
is being studied.
vSummary
Plague is a zoonotic infection
caused by the Gram-negative bacillus Yersinia pestis. Three great
human pandemics have been responsible for more deaths than any other infectious
agent in history. Plague is maintained in nature, predominantly in urban
and sylvatic rodents, by a flea vector. Humans are not necessary for persistence
of the organism, and we acquire the disease from animal fleas, contact with
infected animals, or, rarely, from other humans, via aerosol or direct contact
with infected secretions.
To be able to differentiate
endemic disease from plague used in biological warfare, medical officers
must understand the typical way in which humans contract plague in nature.
First, a dieoff of animals in the mammalian reservoir that harbors bacteria-infected
fleas will occur. Second, troops who have been in close proximity to such
infected mammals will become infected. By contrast, in the most likely biological
warfare scenario, plague would be spread via aerosol. A rapid, person-to-person
spread of fulminant pneumonia, characterized by blood-tinged sputum, would
then ensue. If, on the other hand, an enemy force were to release fleas infected
with Y pestis, then soldiers would present with classic bubonic plague
before a die-off in the local mammalian reservoir occurred.
The most common form of the
disease is bubonic plague, characterized by painful lymphadenopathy and
severe constitutional symptoms of fever, chills, and headache. Septicemic
plague without localized lymphadenopathy occurs less commonly and is difficult
to diagnose. Secondary pneumonia may follow either the bubonic or the septicemic
form. Primary pneumonic plague is spread by airborne transmission, when
aerosols from an infected human or animal are inhaled.
Diagnosis is established
by isolating the organism from blood or other tissues. Rapid diagnosis may
be made with fluorescent antibody stains of sputum or tissue specimens.
Patients should be isolated and treated with aminoglycosides, preferably
streptomycin, plus chloramphenicol when meningitis is suspected or shock
is present. A licensed, killed, whole-cell vaccine is available to protect
humans against bubonic, but not against primary pneumonic, plague.
|
Working Group Recommendation for Treatment of Patients With Pneumonic
Plague in the Contained and Mass Casualty Settings and for Post-exposure
Prophylaxis*
|
|
Patient Category |
Recommended Therapy
|
|
Contained Casualty Setting |
|
Adults |
Preferred choices:
Streptomycin, 1g IM twice daily
Gentamicin, 5 mg/kg IM or IV once daily or 2 mg/kg loading dose
followed by 1.7 mg/kg IM or IV three times daily
†
Alternative choices:
Doxycycline, 100 mg IV twice daily or 200 mg IV once daily
Ciprofloxacin, 400 mg IV twice daily
‡
Chloramphenicol, 25 mg/kg IV 4 times daily
§
|
|
Children|| |
Preferred choices:
Streptomycin, 15 mg/kg IM twice daily (maximum daily dose 2 g)
Gentamicin, 2.5 mg/kg IM or IV 3 times daily
†
Alternative choices:
Doxycycline, If >= 45 kg, give adult dosage
If < 45 kg, give 2.2 mg/kg IV twice daily (maximum 200 mg/dl)
Ciprofloxacin, 15 mg/kg IV twice daily
‡
Chloramphenicol, 25 mg/kg IV 4 times daily
§
|
|
Pregnant Women |
Preferred choice:
Gentamicin, 5 mg/kg
IM or IV once daily or 2 mg/kg loading dose followed by 1.7 mg/kg
IM or IV three times daily
†
Alternative choices:
Doxycycline, 100 mg IV twice daily or 200 mg IV once daily
Ciprofloxacin, 400 mg IV twice daily
‡
|
|
Mass Casualty
Setting and Post-exposure Prophylaxis#
|
|
Adults |
Preferred
choices:
|
|
Doxycycline, 100 mg orally twice daily**
Ciprofloxacin, 500 mg orally twice daily‡
Alternative
choices:
Chloramphenicol, 25 mg/kg orally
4 times daily §
,††
|
|
Children || |
Preferred
choices:
Doxycycline, **
If >=45kg give adult dosage
If <45 kg then give 2.2 mg/kg orally twice daily
Ciprofloxacin, 20 mg/kg orally twice daily
Alternative
choices:
Chloramphenicol, 25 mg/kg orally
4 times daily §
,††
|
|
Pregnant Women |
Preferred
choices:
Doxycycline, 100 mg orally twice daily and
Ciprofloxacin, 500 mg orally twice daily
Alternative
choices:
Chloramphenicol, 25 mg/kg orally
4 times daily §
,††
|
* These are consensus recommendations of the Working Group on Civilian
Biodefense and are not necessarily approved by the U.S. Food and Drug Administration.
See "Therapy" section for explanations. One antimicrobial agent should be
selected. Therapy should continue for 10 days. Oral therapy should be substituted
when the patient’s condition improves. IM indicates intramuscularly; IV indicates
intravenously.
† Aminoglycosides
must be adjusted according to renal function. Evidence suggests that gentamicin,
5 mg/kg IM or IV once daily, would be efficacious in children, although this
is not yet widely accepted clinical practice. Neonates up to 1 week of age
and premature infants should receive gentamicin, 2.5 mg/kg IV twice a day.
‡ Other fluoroquinolones can be substituted at doses appropriate for age.
Ciprofloxacin dosage should not exceed 1 g/d in children.
§ Concentration should be maintained between 5 and 20 µg/mL. Concentrations
greater than 25 µl/mL can cause reversible bone marrow suppression.
|| Refer to "Management of Special Groups" for details. In children, ciprofloxacin
dose should not exceed 1g/d, and chloramphenicol should not exceed 4g/d.
Children younger then 2 years should not receive chloramphenicol.
¶ Refer to "Management of Special Groups" for details and discussion of
breastfeeding women; in neonates, gentamicin-loading dose of 4 mg/kg should
be given initially.
# Duration of treatment of plague in mass casualty setting is 10 days.
Duration of postexposure prophylaxis to prevent plague infection is 7 days.
** Tetracycline could be substituted for doxycycline.
†† Children younger than 2 years should not receive chloramphenicol. Oral
formulation available only outside the U.S
Post-exposure
Prophylaxis
-
Once plague is confirmed or strongly suspected in a particular area,
anyone in that area with fever (of 38.5°C or higher) or cough should
immediately be treated with antimicrobials for presumptive pneumonic plague.
Delaying therapy until tests confirm plague will greatly decrease the
person’s chance of survival.
-
Doxycycline is the first-choice antibiotic for postexposure prophylaxis;
other recommended antibiotics are included in the Table.
-
Asymptomatic persons who have had household, hospital, or other close
contact (2 meters or less) with persons with untreated pneumonic plague
should receive postexposure prophylaxis for 7 days and be monitored for
fever and cough. Tetracycline, doxycycline, sulfonamides, and chloramphenicol
have been recommended for these individuals. On the basis of mice studies,
fluoroquinolones might also be protective.
-
Persons refusing prophylaxis should be closely monitored for the development
of fever or cough for the first 7 days after exposure and should be treated
immediately if either occurs.
-
Clinical deterioration of patients despite early presumptive therapy
could indicate antimicrobial resistance and should be promptly evaluated.
-
Special measures should be taken for treatment or prophylaxis of those
unaware of the outbreak or those requiring special assistance, such as
persons who are homeless or who have cognitive disorders.
Infection
Control and Decontamination of the Environment
-
National infection control guidelines recommend the use of disposable
surgical masks to prevent transmission via respiratory droplets.
-
Other respiratory droplet precautions (gown, gloves, and eye protection)
also should be used by persons caring for pneumonic plague cases.
-
Patients with pneumonic plague should be isolated until they have
had at least 48 hours of antibiotic therapy and shown clinical improvement.
-
If large numbers of patients make isolation impractical, pneumonic
plague patients may be cohorted. Patients should wear surgical masks
while they are being transported.
-
Hospital rooms should receive terminal cleaning consistent with standard
precautions; clothing and linens contaminated with the body fluids of
pneumonic plague patients should be disinfected per hospital protocol.
-
Laboratories should observe biosafety level 2 conditions. Activities
with a high potential for aerosol or droplet production (centrifuging,
grinding, vigorous shaking, animal studies) require biosafety level 3
conditions.
-
Bodies of patients who have died should be handled with routine strict
precautions. Aerosol-generating procedures (bone-sawing associated with
surgery or post-mortem examinations) should be avoided.
-
There is no evidence to suggest that environmental decontamination
following an aerosol release is warranted. Y. pestis is very sensitive
to sunlight and heating and does not survive long outside its host.
According to the WHO analysis, a plague aerosol would be viable for
1 hour after release, long before the first cases would alert health
personnel to a clandestine attack.
Additional
Research Needs
-
Additional knowledge about the organism, its genetics, and its pathogenesis
will improve the ability to respond to a bioterrorist attack.
-
Improved rapid diagnostic and standard laboratory microbiology techniques
are necessary.
-
Improved understanding of prophylactic and therapeutic regimens is
needed.
REFERENCES
-
I Samuel 5:6, 9 (NIV).
-
Cavanaugh DC, Cadigan FC, Williams JE, Marshall JD. Plague. In: Ognibene
AJ, Barrett O’N. General Medicineand Infectious Diseases. Vol
2. In: Ognibene AJ, Barrett O’N. Internal Medicine in Vietnam
. Washington, DC: Office of The Surgeon General and Center of Military
History; 1982: Chap 8, Sec 1.
-
Doyle RJ, Lee NC. Microbes, warfare, religion, and human institutions.
Can J Microbiol. 1985;32:193-200.
-
Langmuir DA, Worthen TD, Solomon J, et al. The Thucydides syndrome:
A new hypothesis for the cause of the plague at Athens. N Engl
J Med. 1985;313:1027-1030.
-
Bayliss JH. The extinction of bubonic plague in Britain. Endeavour
. 1980;4(2):58-66.
-
Mee C. How a mysterious disease laid low Europe’s masses. Smithsonian
. 1990;20(Feb):66-79.
-
Gibbon E. The History of the Decline and Fall of the Roman Empire
. London, England: W Allason; 1781; Chap 43.
-
McEvedy C. The bubonic plague. Sci Am. 1988;Feb:118-123.
-
Lederberg J. Biological warfare: A global threat. American Scientist
. 1971;59(2):195-197.
-
Slack P. The black death past and present, II: Some historical problems.
Trans Roy Soc Trop Med Hyg. 1989;83:461-463.
-
Sloan AW. The black death in England. SA Mediese Tydskrif.
1981;59:646-650.
-
Ampel NM. Plagues-What’s past is present: Thoughts on the origin
and history of new infectious diseases. Rev Infect Dis.
1991;13(Jul-Aug):658-665.
-
Boccaccio G (ca 1350); Aldington C, trans. The Decameron.
London, England: Folio Society; 1954: 24-28. Quoted by: Sloan AW.
The black death in England. SA Mediese Tydskrif. 1981;59:646-650.
-
Coulton GG. The Black Death. London, England: Benn; 1929:
37. Quoted by: Sloan AW. The black death in England. SA Mediese
Tydskrif . 1981;59:646-650.
-
Gasquet FA. The Great Pestilence. London, England: Simpson,
Marshall, Hamilton, Kent; 1893. Quoted by: Sloan AW. The black death
in England. SA Mediese Tydskrif. 1981;59:646-650.
-
Plague in Vietnam. Lancet. 1968;13 Apr:799-800.
-
Butler T. Plague and Other Yersinia Infections. New York,
NY: Plenum Press; 1983.
-
Cavanaugh DC. KF Meyer’s work on plague. J Infect Dis.
1974;129(suppl):S10-S12.
-
Risse GB. A long pull, a strong pull and all together: San Francisco
and bubonic plague, 1907-1908. Bull HistMed. 1992;66(2):260-286.
-
Caten JL, Kartman L. Human plague in the United States: 1900-1966.
JAMA. 1968;205(6):81-84.
-
Harrison FJ. Prevention and Control of Plague. Aurora, Colo:
US Army Center for Health Promotion and Preventive Medicine, Fitzsimons
Army Medical Center; September 1995. Technical Guide 103.
-
Mason VR. Central pacific area. In: Coates JB, ed. Activities
of Medical Consultants. Vol 1. In: Havens WP. Internal
Medicine in World War II. Washington, DC: US Department of the
Army, Medical Department, Office of The Surgeon General; 1961: Chap
7: 647, 667.
-
Meyer KF, Cavanaugh DC, Bartelloni PJ, Marshall JD Jr. Plague immunization,
I: Past and present trends. J Infect Dis. 1974;129(suppl):S13-S18.
-
Trong P, Nhu TQ, Marshall JD. A mixed pneumonic bubonic plague outbreak
in Vietnam. Milit Med. 1967;Feb:93-97.
-
Butler T. The black death past and present, I: Plague in the 1980s.
Trans Roy Soc Trop Med Hyg. 1989;83:458-460.
-
Marshall JD, Joy RJT, AI NV, Quy DV, Stockard JL, Gibson FL. Plague
in Vietnam 1965-1966. Am J Epidemiol.1967;86(2):603-616.
-
Meyer KF. Effectiveness of live or killed plague vaccines in man.
Bull WHO. 1970;42:653-666.
-
Reiley CG, Kates ED. The clinical spectrum of plague in Vietnam.
Arch Intern Med. 1970;126(12):990-994.
-
Engelman RC, Joy RJT. Two hundred years of military medicine. Fort
Detrick, Frederick, Md: US Army Medical Department, Historical Unit;
1975.
-
Williams P, Wallace D. Unit 731: Japan’s Secret Biological Warfare
in World War II. New York, NY: The Free Press; 1989.
-
Barry J. Planning a plague? Newsweek. 1993;(Feb 1):40-41.
-
Cowdrey AE. "Germ warfare" and public health in the Korean conflict.
J Hist Med All Sci. 1984;39:153-172.
-
Brubaker RR. Factors promoting acute and chronic diseases caused
by Yersiniae. Clin Microbiol Rev. 1991;4(3):309-324.
-
Lindler LE, Klempner MS, Straley SC. Yersinia pestis pH 6
antigen: Genetic, biochemical, and virulence characterization of a
protein involved in the pathogenesis of bubonic plague. Infect
Immun. 1990;58:2569-2577.
-
Straley SC, Skrzypek E, Plano GV, Bliska JB. Yops of Yersinia
spp pathogenic for humans. Infect Immun
. 1993;61:3105-3110.
-
Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers
expression and polarized transfer of Yersinia Yop E cytotoxin
into mammalian cells. EMBO J. 1994;13:964-972.
-
Price SB, Leung KY, Barve SS, Straley SC. The Yersinia pestis
V antigen is a regulatory protein necessary
for Ca2+-dependent growth and maximal expression of low Ca2+ response
virulence genes. J Bacteriol . 1991;173:2649-2657.
-
Reisner BS, Straley SC. Yersinia pestis Yop M: Thrombin binding
and overexpression. Infect Immun. 1992;60:5242-5252.
-
Straley SC. The plasmid-encoded outer-membrane proteins of Yersinia
pestis. Rev Infect Dis. 1988;10:S323-S326.
-
Guan K, Dixon JE. Protein tyrosine phosphatase activity of an essential
virulence determinant in Yersinia . Science 1990;249:553-556.
-
Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the
Yersinia Yop E cytotoxin in mammalian cells
induces actin microfilament disruption. Infect Immun. 1991;59:4562-4569.
-
Sodeinde OA, Subrahmanyam YVBK, Stark K, Quan T, Bao Y, Goguen JD.
A surface protease and the invasive character of plague. Science
. 1992;258:1004-1007.
-
Burrows TW. Virulence of Pasteurella pestis and immunity to
plague. Ergebn Mikrobiol. 1963;37:59-113.
-
Hirst LF. The Conquest of Plague: A Study of the Evolution of
Epidemiology . Oxford, England: Clarendon Press; 1953.
-
Craven RB, Maupin GO, Beard ML, Quan TJ, Barnes AM. Reported cases
of human plague infections in the United States, 1970-1991. J Med
Entomol. 1993;30(4):758-761.
-
Gage KL, Lance SE, Dennis DT, Montenieri JA. Human plague in the
United States: A review of cases from 1988-1992 with comments on the
likelihood of increased plague activity. Border Epidemiological
Bulletin . 1992;19(6):1-10.
-
Poland JD. Plague. In: Hoeprich PD, Jordan MC, eds. Infectious
Diseases: A Modern Treatise of Infectious Processes. Philadelphia,
Pa: JB Lippincott; 1989: Chap 151.
-
Cavanaugh DC, Williams JE. Plague: Some ecological interrelationships.
In: Traub R, Starcke H, eds. Fleas. Rotterdam, Netherlands:
A A Balkema; 1980: 245-256.
-
Centers for Disease Control and Prevention. Update: Human Plague-India,
1994. MMWR. 1994;43(41):761-762.
-
Ehrenkranz NF, Meyer KF. Studies on immunization against plague,
VIII: Study of three immunizing preparations in protecting primates
against pneumonic plague. J Infect Dis. 1955;96:138-144.
-
Speck RS, Wolochow H. Studies on the experimental epidemiology of
respiratory infections, VIII: Experimental pneumonic plague in
Macacus rhesus . J Infect Dis. 1957;100:58-68.
-
Cavanaugh DC, Randall R. The role of multiplication of P pestis
in mononuclear phagocytes in the pathogenesis
of flea-borne plague. J Immunol. 1959;83:348-363.
-
Legters LJ, Cottingham AJ Jr, Hunter DH. Clinical and epidemiologic
notes on a defined outbreak of plague in Vietnam. Am J Trop Med
Hyg . 1970;19(4):639-652.
-
Marshall JD, Quy DV, Gibson FL. Asymptomatic pharyngeal plague infection
in Vietnam. Am J Trop Med Hyg. 1967;16(2):175-177.
-
Conrad FG, LeCocq FR, Krain R. A recent epidemic of plague in Vietnam.
Arch Intern Med. 1968;122(3):193-198.
-
Poland JD. Plague. In: Hoeprich PD, ed. Infectious Diseases: A
Guide to the Understanding and Management of Infectious Processes
. New York, NY: Harper & Row; 1972.
-
Hull HF, Montes JM, Mann JM. Septicemic plague in New Mexico.
J Infect Dis. 1987;155(1):113-118.
-
Alsofrom DJ, Mettler FA Jr, Mann JM. Radiographic manifestations
of plague in New Mexico, 1975–1980: A review of 42 proved cases.
Radiology . 1981;139:561–565.
-
Crook LD, Tempest B. Plague: A clinical review of 27 cases. Arch
Intern Med. 1992;152(June):1253–1256.
-
Becker TM, Poland JD, Quan TJ, White ME, Mann JM, Barnes AM. Plague
meningitis—A retrospective analysis of cases reported in the United
States, 1970–1979. West J Med. 1987;147:554–557.
-
Welty TK. Plague. Am Fam Phys. 1986;33(6):159–164.
-
Welty TK, Grabman J, Kompare E, et al. Nineteen cases of plague in
Arizona: A spectrum including ecthyma gangrenosum due to plague and
plague in pregnancy. West J Med. 1985;142(May):641-646.
-
Tikomirov EV, Gratz NA, eds. WHO Plague Manual. Rev 3rd ed.
Fort Collins, Colo: Centers for Disease Control and Prevention; 1996.
-
Chu MC. Acting Chief, Diagnostic and Reference Section, National
Center for Infectious Diseases, Centers for Disease Control and Prevention,
Division of Vector-Borne Infectious Diseases, Fort Collins, Colo.
Personal communication, 7 February 1996.
-
Williams JE, Gentry MK, Braden CA, Leister F, Yolken RH. Use of an
enzyme-linked immunosorbent assay to measure antigenaemia during acute
plague. Bull WHO. 1984;62(3):463-466.
-
Williams JE, Arntzen L, Tyndal GL, Isaacson M. Application of enzyme
immunoassays for the confirmation of clinically suspect plague in
Namibia, 1982. Bull WHO. 1986;64(5):745-752.
-
Hinnebusch J, Schwan TG. New method for plague surveillance using
polymerase chain reaction to detect Yersinia pestis in fleas.
J Clin Microbiol. 1993;31(6):1511-1514.
-
Bonacorsi SP, Scavizzi MR, Guiyoule A, Amouroux JH, Carniel E. Assessment
of a fluoroquinolone, three betalactams, two aminoglycosides, and
a tetracycline in treatment of murine Yersinia pestis infection.
Antimicrob Agents Chemother. 1994;38(3):481-486.
-
Centers for Disease Control, Immunization Practices Advisory Committee.
Plague vaccine. MMWR. 1982;31(22):301–304.
-
Bartelloni PJ, Marshall JD, Cavanaugh DC. Clinical and serological
responses to plague vaccine USP. Milit Med. 1973;(11):720-722.
-
Marshall JD Jr, Cavanaugh DC, Bartelloni PJ, Meyer KF. Plague immunization,
III: Serologic response to multiple inoculations of vaccine. J
Infect Dis. 1974;129(suppl):S26-S29.
-
Marshall JD Jr, Baratelloni PJ, Cavanaugh DC, Kadull PJ, Meyer KF.
Plague immunization, II: Relation of adverse clinical reactions to
multiple immunizations with killed vaccine. J Infect Dis.
1974;129(suppl):S19–S25.
-
Williams JE, Cavanaugh DC. Measuring the efficacy of vaccination
in affording protection against plague. Bull WHO. 1979;57(2):309–313.
-
Cavanaugh DC, Elisberg BL, Llewellyn CH, et al. Plague immunization,
V: Indirect evidence for the efficacy of plague vaccine. J Infect
Dis. 1974;129(suppl):S37-S40.
-
Pitt MLM, Estep JE, Welkos SL, Friedlander AM. Efficacy of killed
whole-cell vaccine against a lethal aerosol challenge of plague in
rodents. Annual meeting, American Society for Microbiology; 1994; Las
Vegas, Nev. Abstract E-45.
Source: Plague-Chapter 23.
Medical Aspects of Chemical and Biological Warfare.
Thomas W. McGovern, M.D., FAAD; and
Arthur M. Friedlander, M.D.
Medical NBC Online Information Server
U.S. Army’s Office of the Surgeon General